Psychometric Properties of the European Evaluation of Vertigo Scale (EEV) for a Spanish-Speaking Population: A Validation Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Cross-Cultural Adaptation
2.3. Measurements
2.4. Statistical Analysis
3. Results
3.1. Test–Retest Reliability
3.2. Concurrent Validity
3.3. Discriminant Validity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karatas, M. Central Vertigo and Dizziness: Epidemiology, Differential Diagnosis, and Common Causes. Neurologist 2008, 14, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Della-Morte, D.; Rundek, T. Dizziness and Vertigo. Front. Neurol. Neurosci. 2012, 30, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Hackenberg, B.; O’Brien, K.; Döge, J.; Lackner, K.J.; Beutel, M.E.; Münzel, T.; Wild, P.S.; Pfeiffer, N.; Chalabi, J.; Matthias, C.; et al. Vertigo and Its Burden of Disease—Results from a Population-based Cohort Study. Laryngoscope Investig. Otolaryngol. 2023, 8, 1624. [Google Scholar] [CrossRef] [PubMed]
- Ruthberg, J.S.; Rasendran, C.; Kocharyan, A.; Mowry, S.E.; Otteson, T.D. The Economic Burden of Vertigo and Dizziness in the United States. J. Vestib. Res. 2021, 31, 81–90. [Google Scholar] [CrossRef]
- Kovacs, E.; Wang, X.; Grill, E. Economic Burden of Vertigo: A Systematic Review. Health Econ. Rev. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Chen, X.; Wei, D.; Fang, F.; Song, H.; Yin, L.; Kaijser, M.; Gurholt, T.P.; Andreassen, O.A.; Valdimarsdóttir, U.; Hu, K.; et al. Peripheral Vertigo and Subsequent Risk of Depression and Anxiety Disorders: A Prospective Cohort Study Using the UK Biobank. BMC Med. 2024, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Yardley, L.; Redfern, M.S. Psychological Factors Influencing Recovery from Balance Disorders. J. Anxiety Disord. 2001, 15, 107–119. [Google Scholar] [CrossRef]
- Yardley, L. Contribution of Symptoms and Beliefs to Handicap in People with Vertigo: A Longitudinal Study. Br. J. Clin. Psychol. 1994, 33, 101–113. [Google Scholar] [CrossRef]
- Yardley, L.; Putman, J. Quantitative Analysis of Factors Contributing to Handicap and Distress in Vertiginous Patients: A Questionnaire Study. Clin. Otolaryngol. Allied Sci. 1992, 17, 231–236. [Google Scholar] [CrossRef]
- Yardley, L.; Masson, E.; Verschuur, C.; Haacke, N.; Luxon, L. Symptoms, Anxiety and Handicap in Dizzy Patients: Development of the Vertigo Symptom Scale. J. Psychosom. Res. 1992, 36, 731–741. [Google Scholar] [CrossRef]
- Jacobson, G.P.; Newman, C.W. The Development of the Dizziness Handicap Inventory. Arch. Otolaryngol. Head Neck Surg. 1990, 116, 424–427. [Google Scholar] [CrossRef]
- Powell, L.E.; Myers, A.M. The Activities-Specific Balance Confidence (ABC) Scale. J. Gerontol. Ser. A 1995, 50A, M28–M34. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.S.; Kimball, K.T.; Adams, A.S. Application of the Vestibular Disorders Activities of Daily Living Scale. Laryngoscope 2000, 110, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Megnigbeto, C.A.; Sauvage, J.-P.; Launois, R. The European Evaluation of Vertigo Scale (EEV): A Clinical Validation Study. Rev. Laryngol. Otol. Rhinol. 2001, 122, 95–102. [Google Scholar]
- Hobart, J.C.; Cano, S.J.; Warner, T.T.; Thompson, A.J. What Sample Sizes for Reliability and Validity Studies in Neurology? J. Neurol. 2012, 259, 2681–2694. [Google Scholar] [CrossRef]
- Montilla-Ibáñez, A.; Martínez-Amat, A.; Lomas-Vega, R.; Cruz-Díaz, D.; Torre-Cruz, M.J.D.l.; Casuso-Pérez, R.; Hita-Contreras, F. The Activities-Specific Balance Confidence Scale: Reliability and Validity in Spanish Patients with Vestibular Disorders. Disabil. Rehabil. 2017, 39, 697–703. [Google Scholar] [CrossRef]
- Castillejos-Carrasco-Muñoz, R.; Ibáñez-Vera, A.J.; Peinado-Rubia, A.B.; Tapia-Toca, M.C.; Paez-Mantilla, D.; Lomas-Vega, R. Psychometric Properties of the Short Version of the Activities-Specific Balance Confidence Scale in Vestibular Patients. Otol. Neurotol. 2023, 44, e188–e193. [Google Scholar] [CrossRef]
- Pérez, N.; Garmendia, I.; Martín, E.; García-Tapia, R. Cultural Adaptation of 2 Questionnaires for Health Measurement in Patients with Vertigo. Acta Otorrinolaringol. Esp. 2000, 51, 572–580. [Google Scholar]
- Brenner, H.; Kliebsch, U. Dependence of Weighted Kappa Coefficients on the Number of Categories. Epidemiology 1996, 7, 199–202. [Google Scholar] [CrossRef]
- Kvålseth, T.O. An Alternative Interpretation of the Linearly Weighted Kappa Coefficients for Ordinal Data. Psychometrika 2018, 83, 618–627. [Google Scholar] [CrossRef]
- Landis, J.; Koch, G.G. The Measurement of the Observer Agreement for Categorial Data. Biometrics 1977, 33, 159. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Hillsdale, N., Ed.; Lawrence Erlbaum Associates: New York, NY, USA, 1998. [Google Scholar]
- Lajoie, Y.; Gallagher, S.P. Predicting Falls within the Elderly Community: Comparison of Postural Sway, Reaction Time, the Berg Balance Scale and the Activities-Specific Balance Confidence (ABC) Scale for Comparing Fallers and Non-Fallers. Arch. Gerontol. Geriatr. 2004, 38, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Zweig, M.H.; Campbell, G. Receiver-Operating Characteristic (ROC) Plots: A Fundamental Evaluation Tool in Clinical Medicine. Clin. Chem. 1993, 39, 561–577. [Google Scholar] [CrossRef] [PubMed]
- Swets, J. Measuring the Accuracy of Diagnostic Systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Mutlu, B.; Mutlu, A.; Bakici, B. The Reliability and Validity of the Turkish Version of the European Evaluation of Vertigo Scale (EEV-TR). Kulak Burun Boğaz Baş Boyun Cerrahisi Derg. 2022, 30, 139–145. [Google Scholar] [CrossRef]
- Eryaman, E.; Gökcan, G.; Parmaksiz, E.; Acar, N.O.; Ozlüoǧlu, L.N. Are Thiazides Effective on Hypertensive Vertigo? A Preliminary Study. Kulak Burun Bogaz Ihtis Derg 2012, 22, 219–224. [Google Scholar] [CrossRef]
- Tan, M.; Cengiz, D.U.; Demir, İ.; Demirel, S.; Çolak, S.C.; Karakaş, O.; Bayındır, T. Effects of COVID-19 on the Audio-Vestibular System. Am. J. Otolaryngol. 2022, 43, 103173. [Google Scholar] [CrossRef]
- Fernandez-Cascon, S.; Rettig-Infante, I.; Morales-Medina, G.; Buendia-Pajares, C. Evaluación del Paciente Previa a La Rehabilitación Vestibular. Revista ORL 2019, 11, 29–42. [Google Scholar] [CrossRef]

| Count | Mean | Standard Deviation | ||
|---|---|---|---|---|
| Diagnostic | Ménière’s disease | 54 | ||
| Vestibular migraine | 16 | |||
| BPPV | 30 | |||
| Gender | Male | 58 | ||
| Female | 42 | |||
| Age (years) | 57 | 8 | ||
| Weight (kilograms) | 70.5 | 9.4 | ||
| Height (metres) | 1.71 | 0.06 | ||
| Body mass index | 23.94 | 2.09 | ||
| DHI_Total_Score | 45.56 | 5.83 | ||
| ABC_16 | 63.29 | 14.98 | ||
| EEV_Score | 11.74 | 2.16 | ||
| Variable | Spearman’s Rho Coefficient | Correlation | p-Value |
|---|---|---|---|
| DHI_Emotional | −0.422 | Moderate | <0.001 *** |
| DHI_Physical | 0.257 | Poor | 0.010 * |
| DHI_Functional | −0.284 | Poor | 0.004 ** |
| DHI_Total | −0.179 | Poor | 0.075 |
| ABC_16 items | −0.538 | Strong | <0.001 *** |
| ABC_6 items | −0.501 | Strong | <0.001 *** |
| EEV Cut-Off | Sen | 95% CI | Spe | 95% CI | +LR | 95% CI | −LR | 95% CI | +PV | 95% CI | −PV | 95% CI | Criterion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤12 | 84.00 | 70.9–92.8 | 68.00 | 53.3–80.5 | 2.63 | 1.72–4.00 | 0.24 | 0.12–0.46 | 72.4 | 63.3–80.0 | 81.0 | 68.7–89.2 | ABC-16 ≤ 67 |
| ≤12 | 95.65 | 85.2–99.5 | 74.07 | 60.3–85.0 | 3.69 | 2.34–5.82 | 0.059 | 0.015–0.23 | 75.9 | 66.6–83.2 | 95.2 | 83.6–98.7 | ABC-6 ≤ 55 |
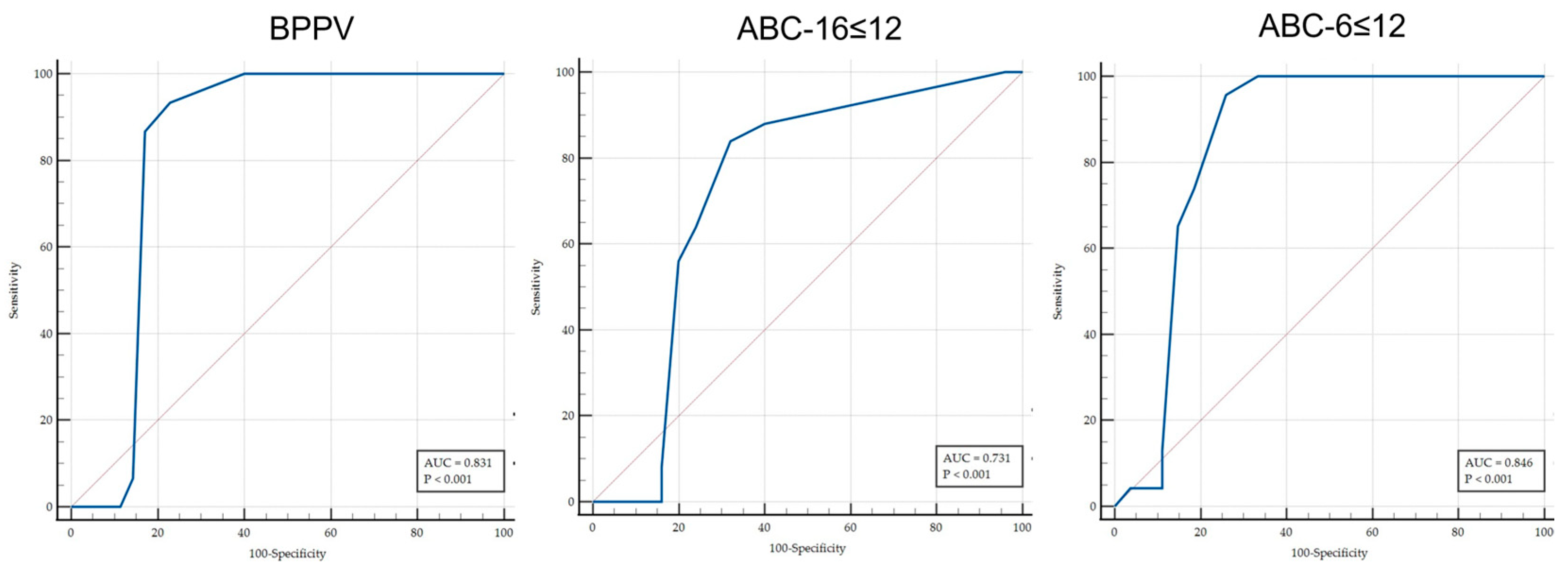
| ≤11 | 93.33 | 77.9–99.2 | 77.14 | 65.6–86.3 | 4.08 | 2.63–6.35 | 0.086 | 0.023–0.33 | 63.6 | 53.0–73.1 | 96.4 | 87.6–99.0 | D = BPPV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montilla-Ibáñez, M.A.; Lomas-Vega, R.; López-Ruiz, M.d.C.; Díaz-Fernández, Á.; Ibáñez-Vera, A.J.; Peinado-Rubia, A.B.; Obrero-Gaitán, E.; Sedeño-Vidal, A. Psychometric Properties of the European Evaluation of Vertigo Scale (EEV) for a Spanish-Speaking Population: A Validation Study. Audiol. Res. 2025, 15, 84. https://doi.org/10.3390/audiolres15040084
Montilla-Ibáñez MA, Lomas-Vega R, López-Ruiz MdC, Díaz-Fernández Á, Ibáñez-Vera AJ, Peinado-Rubia AB, Obrero-Gaitán E, Sedeño-Vidal A. Psychometric Properties of the European Evaluation of Vertigo Scale (EEV) for a Spanish-Speaking Population: A Validation Study. Audiology Research. 2025; 15(4):84. https://doi.org/10.3390/audiolres15040084
Chicago/Turabian StyleMontilla-Ibáñez, María Alharilla, Rafael Lomas-Vega, María del Carmen López-Ruiz, Ángeles Díaz-Fernández, Alfonso Javier Ibáñez-Vera, Ana Belén Peinado-Rubia, Esteban Obrero-Gaitán, and Ana Sedeño-Vidal. 2025. "Psychometric Properties of the European Evaluation of Vertigo Scale (EEV) for a Spanish-Speaking Population: A Validation Study" Audiology Research 15, no. 4: 84. https://doi.org/10.3390/audiolres15040084
APA StyleMontilla-Ibáñez, M. A., Lomas-Vega, R., López-Ruiz, M. d. C., Díaz-Fernández, Á., Ibáñez-Vera, A. J., Peinado-Rubia, A. B., Obrero-Gaitán, E., & Sedeño-Vidal, A. (2025). Psychometric Properties of the European Evaluation of Vertigo Scale (EEV) for a Spanish-Speaking Population: A Validation Study. Audiology Research, 15(4), 84. https://doi.org/10.3390/audiolres15040084










