The Link Between Anxiety and Depression, and Balance in Young Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.2.1. Evaluation of Postural Stability
2.2.2. Statistical Analysis
3. Results
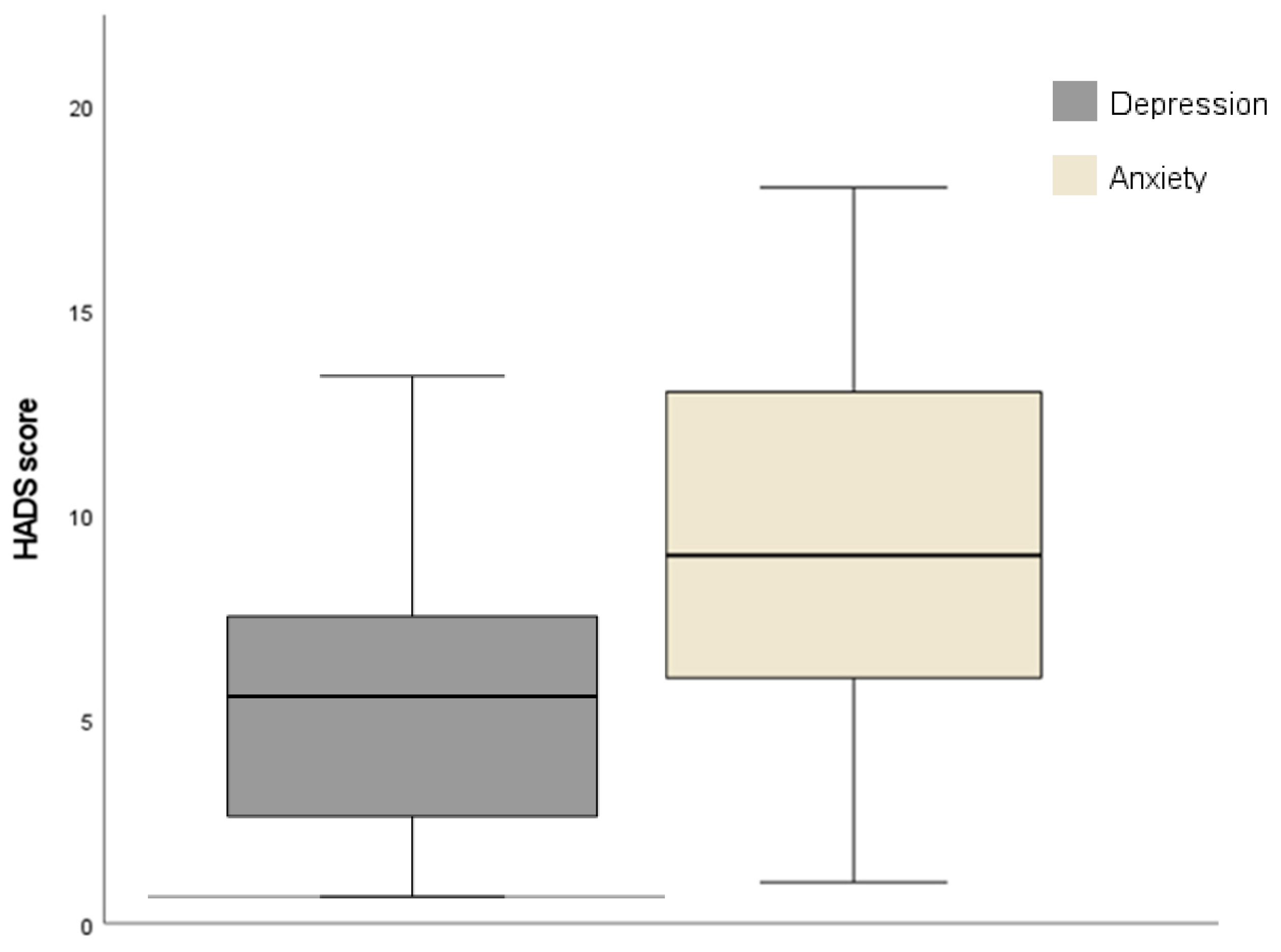
3.1. Participants’ Demographic and Clinical Characteristics
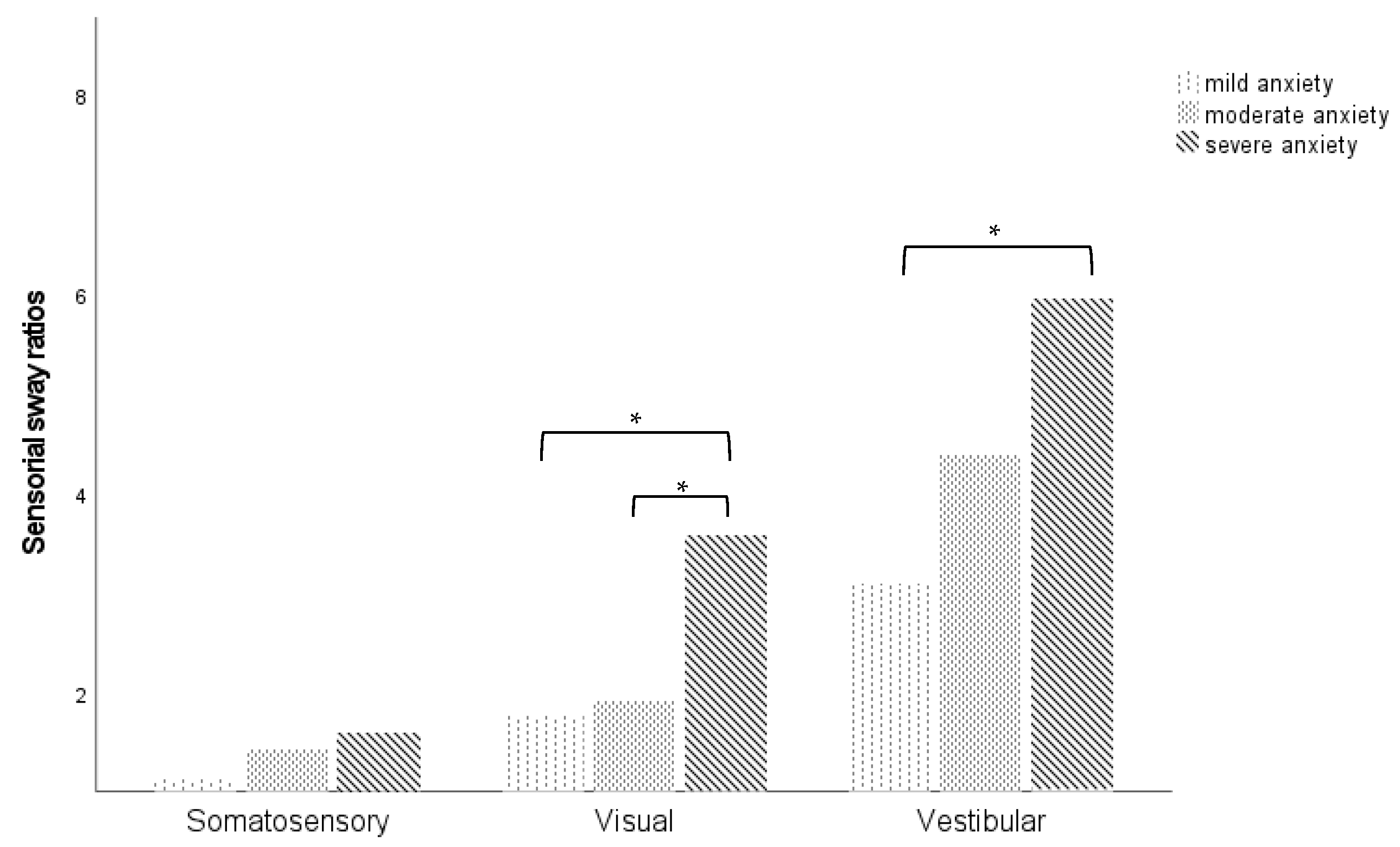
3.2. Postural Stability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| F/EO | Firm/eyes open |
| F/EC | Firm/eyes closed |
| FO/EO | Foam/eyes open |
| FO/EC | Foam/eyes closed |
| HADS | Hospital Anxiety and Depression Scale |
| mCTSIB | Modified Clinical Test for the Sensory Interaction on Balance |
| SD | Standard deviation |
References
- Hegeman, J.M.; Kok, R.M.; van der Mast, R.C.; Giltay, E.J. Phenomenology of depression in older compared with younger adults: Meta-analysis. Br. J. Psychiatry 2012, 200, 275–281. [Google Scholar] [CrossRef]
- Nutt, D. Anxiety and depression: Individual entities or two sides of the same coin? Int. J. Psychiatry Clin. Pract. 2004, 8, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.A.; Doan, J.B.; McKenzie, N.C.; Cooper, S.A. Anxiety-mediated gait adaptations reduce errors of obstacle negotiation among younger and older adults: Implications for fall risk. Gait Posture 2006, 24, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Villaume, S.C.; Chen, S.; Adam, E.K. Age disparities in prevalence of anxiety and depression among US adults during the COVID-19 pandemic. JAMA Netw. Open 2023, 6, e2345073. [Google Scholar]
- Cruz, I.B.M.; Barreto, D.C.M.; Fronza, A.B.; Jung, I.E.C.; Krewer, E.C.; Rocha, M.I.U.M.; Silveira, A.F. Dinamic balance, lifestyle, and emotional states in young adults. Braz. J. Otorhinolaryngol. 2010, 76, 392–398. [Google Scholar] [CrossRef]
- Maywald, M.; Pogarell, O.; Levai, S.; Paolini, M.; Ischentscher, N.; Rauschmann, B.S.; Krause, D.; Stöcklein, S.; Goreigk, S.; Röll, L.; et al. Neurofunctional differences and similarities between persistent-postural dizziness and anxiety disorder. NeuroImage Clin. 2023, 37, 103330. [Google Scholar] [CrossRef]
- Jáuregui-Renaud, K.; Cabrera-Pereyra, R.; Miguel-Rega, J.A. Graviception uncertainty, spatial anxiety and derealization in patients with Persistent Postural-Perceptual Dizziness. J. Clin. Med. 2024, 13, 6665. [Google Scholar] [CrossRef] [PubMed]
- Nashner, L.M.; Shepard, N.T. Computerized Dynamic Posturography: Methodology and Interpretations. In Balance Function Assessment and Management, 3rd ed.; Jacobson, G.P., Shepard, N.T., Eds.; Plural Publishing: San Diego, CA, USA, 2021. [Google Scholar]
- Peterka, R.J. Sensorimotor integration in human postural control. J. Neurophysiol. 2002, 88, 1097–1118. [Google Scholar] [CrossRef]
- Ivanenko, Y.; Gurfinkel, V.S. Human Postural Control. Front. Neurosci. 2018, 12, 171. [Google Scholar] [CrossRef]
- Khaya, M.; Liao, L.; Gustafson, K.M.; Akinwuntan, A.E.; Manor, B. Cortical Correlates of Increased Postural Task Difficulty in Young Adults: A Combined Pupillometry and EEG Study. Sensors 2022, 22, 5594. [Google Scholar] [CrossRef]
- Park, J.; Lee, C.; Nam, Y.E.; Lee, H. Association between depressive symptoms and dynamic balance among young adults in the community. Helyon 2024, 10, e24093. [Google Scholar] [CrossRef] [PubMed]
- Kahya, M.; Wood, T.A.; Sosnoff, J.J.; Devos, H. Increased postural demand is associated with greater cognitive workload in healthy young adults: A pupillometry study. Front. Hum. Neurosci. 2018, 12, 288. [Google Scholar] [CrossRef]
- Al-Momani, M.; Al-Momani, F.; Alghadir, A.H.; Alharethy, S.; Gabr, S.A. Factors related to gait and balance deficits in older adults. Clin. Interv. Aging 2016, 11, 1043–1049. [Google Scholar] [PubMed]
- Salihu, A.T.; Usman, J.S.; Hill, K.D.; Zoghi, M.; Jaberzadeh, S. Mental fatigue does not affect static balance under both single and dual task conditions in young adults. Exp. Brain Res. 2023, 241, 1769–1784. [Google Scholar] [CrossRef]
- Redfern, M.S.; Furman, M.S.; Jacob, R.G. Visually induced postural sway in anxiety disorders. J. Anxiety Disord. 2007, 21, 704–716. [Google Scholar] [CrossRef]
- Johnson, K.J.; Zaback, M.; Tokuno, C.D.; Carpenter, M.G.; Adkin, A.L. Exploring the relationship between threat-related changes in anxiety, attention focus, and postural control. Psychol. Res. 2019, 83, 445–558. [Google Scholar] [CrossRef]
- Stins, J.F.; Roerdink, M.; Peek, P.J. To freeze or not to freeze? Affective and cognitive perturbations have markedly different effects on postural control. Hum. Mov. Sci. 2011, 30, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Hauck, L.J.; Carpenter, M.G.; Frank, J.S. Task-specific measures of balance efficacy, anxiety, and stability and their relationship to clinical balance performance. Gait Posture 2008, 27, 676–682. [Google Scholar] [CrossRef]
- Adkin, A.L.; Carpenter, M.G. New insights on emotional contributions to human postural control. Front. Neurol. 2018, 9, 789. [Google Scholar] [CrossRef]
- Indovina, I.; Riccelli, R.; Staab, J.P.; Lacquaniti, F.; Passamonti, L. Personality traits modulate subcortical and cortical vestibular and anxiety responses to sound-evoked otolithic receptor stimulation. J. Psychosom. Res. 2014, 77, 391–400. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Pais-Ribeiro, J.; Silva, I.; Ferreira, T.; Martins, A.; Meneses, R.; Baltar, M. Validation study of a Portuguese version of the Hospital Anxiety and Depression Scale. Psychol. Health Med. 2007, 12, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C. International Experiences with the Hospital Anxiety and Depression Scale—A review of validation data and clinical results. J. Psychosom. Res. 1997, 42, 17–41. [Google Scholar] [CrossRef]
- Palm, H.G.; Strobel, J.; Achatz, G.; von Luebken, F.; Friemert, B. The role and interaction of visual and auditory afferents in postural stability. Gait Posture 2009, 30, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Scoppa, F.; Capra, R.; Gallamini, M.; Shiffer, R. Clinical stabilometry standardization: Basic definitions--acquisition interval--sampling frequency. Gait Posture 2013, 37, 290–292. [Google Scholar] [CrossRef]
- Goto, F.; Kabeya, M.; Kushiro, K.; Ttsutsumi, T.; Hayashi, K. Effect of anxiety on antero-posterior postural stability in patients with dizziness. Neurosci. Lett. 2011, 487, 204–206. [Google Scholar] [CrossRef]
- Sarigiannidis, I.; Kirk, P.A.; Roiser, J.P.; Robinson, O.J. Does overloading cognitive resources mimic the impact of anxiety on temporal cognition? J. Exp. Psychol. Learn. Mem. Cogn. 2020, 46, 1828–1835. [Google Scholar] [CrossRef]
- Hacohen-Brown, S.; Gilboa-Scechtman, E.; Zaidel, A. Modality-specific effects on threat on self-motion perception. BMC Biol. 2024, 22, 120. [Google Scholar] [CrossRef]
- Arrighi, L.; Hausmann, M. Spatial anxiety and self-confidence mediate sex/gender differences in mental rotation. Learn. Mem. 2022, 29, 312–320. [Google Scholar] [CrossRef]
- Alahmari, K.A.; Alshehri, S. Evaluating the efficacy of vestibular rehabilitation therapy on quality of life in persistent postural-perceptual dizziness: The role of anxiety and depression in treatment outcomes. Front. Neurol. 2025, 16, 1524324. [Google Scholar] [CrossRef]
- Yılmazer, E.; Hamamci, Z.; Türk, F. Effects of mindfulness on test anxiety: A meta-analysis. Front. Psychol. 2024, 15, 1401467. [Google Scholar] [CrossRef] [PubMed]
| N (%) or Mean (SD) | Min | Max | ||
| Age | 21.86 (2.63) | 18 | 32 | |
| Gender | Male | 13 (26%) | - | - |
| Female | 37 (74%) | - | - | |
| Employment status | Student | 40 (80%) | - | - |
| Employed | 10 (20%) | - | - | |
| Physical activity | No | 31 (62%) | - | - |
| Yes | 19 (38%) | - | - | |
| Smoke | No | 37 (74%) | - | - |
| Yes | 13 (26%) | - | - | |
| N (%) or Mean (SD) | ||
| Anxiety levels (N = 31) | Mild | 10 (32.3%) |
| Moderate | 15 (48.4%) | |
| Severe | 6 (19.4%) | |
| Depression levels (N = 12) | Mild | 7 (58.3%) |
| Moderate | 5 (41.7%) | |
| Severe | - | |
| Mean (SD) | Min | Max | DP | |
|---|---|---|---|---|
| F/EO | 0.17 | 0.10 | 0.40 | 0.08 |
| F/EC | 0.19 | 0.10 | 0.40 | 0.07 |
| FO/EO | 0.33 | 0.20 | 0.80 | 0.17 |
| FO/EC | 0.61 | 0.20 | 1.20 | 0.23 |
| Sway index | 0.33 | 0.20 | 0.80 | 0.15 |
| Anxiety | Depression | |
|---|---|---|
| F/EO | −0.017 | −0.053 |
| F/EC | 0.210 | −0.044 |
| FO/EO | 0.199 | 0.43 |
| FO/EC | 0.160 | 0.016 |
| Sway index | 0.259 * | −0.026 |
| Somatosensory | 0.281 | 0.137 |
| Visual | 0.276 * | 0.083 |
| Vestibular | 0.186 | 0.064 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, T.; Bernardo, P.; Serrano, M. The Link Between Anxiety and Depression, and Balance in Young Adults. Audiol. Res. 2025, 15, 57. https://doi.org/10.3390/audiolres15030057
Marques T, Bernardo P, Serrano M. The Link Between Anxiety and Depression, and Balance in Young Adults. Audiology Research. 2025; 15(3):57. https://doi.org/10.3390/audiolres15030057
Chicago/Turabian StyleMarques, Tatiana, Patrícia Bernardo, and Margarida Serrano. 2025. "The Link Between Anxiety and Depression, and Balance in Young Adults" Audiology Research 15, no. 3: 57. https://doi.org/10.3390/audiolres15030057
APA StyleMarques, T., Bernardo, P., & Serrano, M. (2025). The Link Between Anxiety and Depression, and Balance in Young Adults. Audiology Research, 15(3), 57. https://doi.org/10.3390/audiolres15030057







