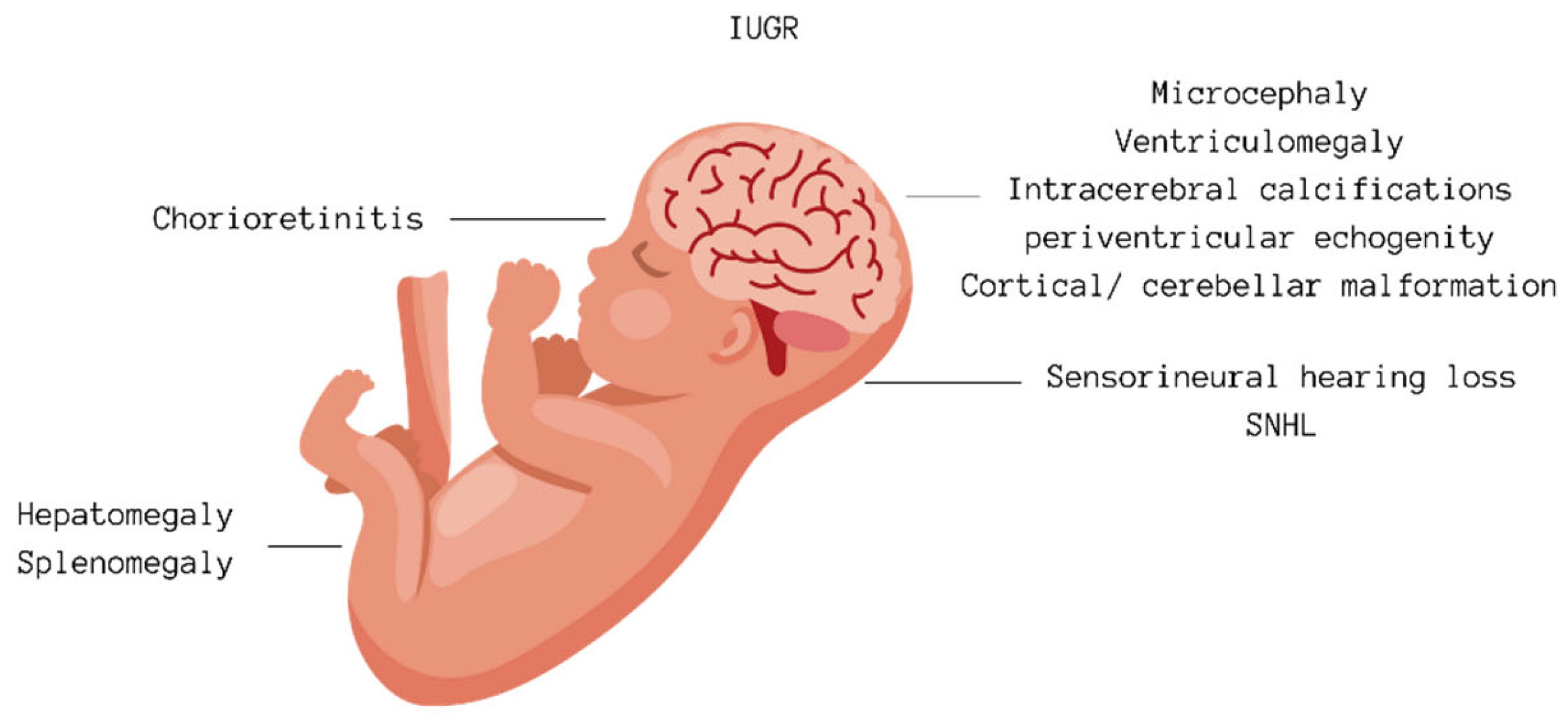
Congenital Cytomegalovirus-Related Hearing Loss
Abstract
1. Introduction
2. Materials and Methods
3. CMV Infection during Pregnancy
| Study | Conclusion |
|---|---|
| Lanzieri et al., 2017 [22] | At age 18, SNHL prevalence was 25% among case patients and 8% among controls. The risk of delayed-onset SNHL was not significantly greater for case patients than for controls. For case patients, the risk of delayed-onset SNHL was significantly greater among those with unilateral congenital/early-onset hearing loss than those without. The prevalence of severe to profound bilateral SNHL among case patients was 2%. |
| Palma et al., 2019 [23] |
Urinary CMV testing was carried out in 2966 children, representing 3.9% of total live births, between 2004 and 2014. CMV infection was confirmed in 339 children, and information on hearing loss was available in 250 (73.8%), of which 45/250 were cCMV, while 205/250 were acquired. A few children (n = 6/250-13%) with cCMV infection had confirmed hearing impairment. Among them, two were diagnosed after 2012 through the neonatal hearing screening program and were positive. The prevalence of symptomatic cCMV after the introduction of newborn hearing screening (2/10) was 20%, while the proportion of symptomatic cCMV with hearing loss before the screening was 11%. Among the 205 children (82%) with acquired CMV, 6 (2.9%) had moderate to severe hearing impairment. The remaining three cases were attributed to a delayed diagnosis of cCMV. All six cases with acquired CMV were born before the implementation of the newborn hearing screening. |
| Forner et al., 2014 [24] | The study aimed to analyze the kinetics of CMV viremia and viruria clearance in postnatal life after primary CMV intrauterine infection. All of the 33 newborns included were born full-term. Ten of thirty-tree infants (30%) developed postnatal sequelae during the first months or years of life. Eight children developed unilateral or bilateral hearing loss, where four infants developed severe bilateral hearing loss (average tone loss, 71–90 dB hearing level) four children presented moderate unilateral hearing loss (average tone loss, 41–70 dB hearing level). Two children developed psychomotor retardation, and one baby developed progressive right-side hemiparesis. The time of appearance of clinical abnormalities ranged from 3 months to 5 years of age. The remaining 23 infants (70%) presented no symptoms when the follow-up was concluded at 6 years of age. |
| Bradford et al., 2015 [25] | In the study, among 50 infants with serum samples, 37 tested positive for CMV DNA at enrollment, indicating viremia. These viremic infants were more prone to developing hearing loss at both the initial assessment and the 6-month follow-up. Additionally, they displayed other markers of active CMV disease, including elevated alanine aminotransferase levels, petechial rash, and organomegaly. |
| Picone et al., 2018 [15] | Following a retrospective analysis of 238 patients with maternal primary CMV infection identified during routine screening, the cohort underwent monitoring with serial ultrasound scans. The rate of intrauterine transmission was 24.9%, varying across different pregnancy periods. Maternal infections during the preconception or periconceptional period and the first trimester were associated with a significantly higher risk of ultrasound abnormalities compared with later periods. Among the infected newborns, three were symptomatic, all previously flagged during prenatal ultrasounds. Interestingly, no symptomatic fetal infections were observed when maternal infection occurred after the 14th week of gestation. Overall, 5.5% of clinically asymptomatic cases later developed hearing loss. |
4. Advanced Diagnostic Approaches for Congenital Cytomegalovirus Infection
5. Congenital CMV Infection Treatment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korver, A.M.; Smith, R.J.; Van Camp, G.; Schleiss, M.R.; Bitner-Glindzicz, M.A.; Lustig, L.R.; Usami, S.I.; Boudewyns, A.N. Congenital hearing loss. Nat. Rev. Dis. Primers 2017, 3, 16094. [Google Scholar] [CrossRef] [PubMed]
- Lieu, J.E.C.; Kenna, M.; Anne, S.; Davidson, L. Hearing Loss in Children: A Review. JAMA 2020, 324, 2195–2205. [Google Scholar] [CrossRef] [PubMed]
- Tanaka-Ouyang, L.; Marlin, S.; Nevoux, J. Les surdités d’origine génétique [Genetic hearing loss]. Presse Med. 2017, 46, 1089–1096. (In French) [Google Scholar] [CrossRef] [PubMed]
- Wiwanitkit, V. Hearing loss in congenital Zika virus. Braz. J. Otorhinolaryngol. 2017, 83, 239. [Google Scholar] [CrossRef] [PubMed]
- Goderis, J.; De Leenheer, E.; Smets, K.; Van Hoecke, H.; Keymeulen, A.; Dhooge, I. Hearing Loss and Congenital CMV Infection: A Systematic Review. Pediatrics 2014, 134, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Dollard, S.C.; Grosse, S.D.; Ross, D.S. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev. Med. Virol. 2007, 17, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Kenneson, A.; Cannon, M.J. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 2007, 17, 253–276. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.C.; Nance, W.E. Newborn hearing screening—A silent revolution. N. Engl. J. Med. 2006, 354, 2151–2164. [Google Scholar] [CrossRef] [PubMed]
- Andronaco, D.W. Congenital Cytomegalovirus and Hearing Loss. J. Obstet. Gynecol. Neonatal Nurs. 2020, 49, 293–304. [Google Scholar] [CrossRef]
- Enders, G.; Daiminger, A.; Bader, U.; Exler, S.; Enders, M. Intrauterine transmission and clinical outcome of 248 pregnancies with primary cytomegalovirus infection in relation to gestational age. J. Clin. Virol. 2011, 52, 244–246. [Google Scholar] [CrossRef]
- Khalil, A.; Sotiriadis, A.; Chaoui, R.; da Silva Costa, F.; D’Antonio, F.; Heath, P.T.; Jones, C.; Malinger, G.; Odibo, A.; Prefumo, F.; et al. ISUOG Practice Guidelines: Role of ultrasound in congenital infection. Ultrasound Obstet. Gynecol. 2020, 56, 128–151. [Google Scholar] [CrossRef]
- Townsend, C.L.; Forsgren, M.; Ahlfors, K.; Ivarsson, S.A.; Tookey, P.A.; Peckham, C.S. Long-term outcomes of congenital cytomegalovirus infection in Sweden and the United Kingdom. Clin. Infect. Dis. 2013, 56, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Boppana, S.B.; Rivera, L.B.; Fowler, K.B.; Mach, M.; Britt, W.J. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N. Engl. J. Med. 2001, 344, 1366–1371. [Google Scholar] [CrossRef] [PubMed]
- Pesch, M.H.; Saunders, N.A.; Abdelnabi, S. Cytomegalovirus Infection in Pregnancy: Prevention, Presentation, Management and Neonatal Outcomes. J. Midwifery Womens Health 2021, 66, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Picone, O.; Vauloup-Fellous, C.; Cordier, A.G.; Guitton, S.; Senat, M.V.; Fuchs, F.; Ayoubi, J.M.; Grangeot Keros, L.; Benachi, A. A series of 238 cytomegalovirus primary infections during pregnancy: Description and outcome. Prenat. Diagn. 2013, 33, 751–758. [Google Scholar] [CrossRef]
- Pass, R.F.; Fowler, K.B.; Boppana, S.B.; Britt, W.J.; Stagno, S. Congenital cytomegalovirus infection following first trimester maternal infection: Symptoms at birth and outcome. J. Clin. Virol. 2006, 35, 216–220. [Google Scholar] [CrossRef]
- Liesnard, C.; Donner, C.; Brancart, F.; Gosselin, F.; Delforge, M.L.; Rodesch, F. Prenatal diagnosis of congenital cytomegalovirus infection: Prospective study of 237 pregnancies at risk. Obstet. Gynecol. 2000, 95, 881–888. [Google Scholar] [CrossRef]
- Nicloux, M.; Peterman, L.; Parodi, M.; Magny, J.F. Outcome and management of newborns with congenital cytomegalovirus infection. Arch. Pediatr. 2020, 27, 160–165. [Google Scholar] [CrossRef]
- Schleiss, M.R. Cytomegalovirus in the neonate: Immune correlates of infection and protection. Clin. Dev. Immunol. 2013, 2013, 501801. [Google Scholar] [CrossRef]
- Ville, Y. Advocating for cytomegalovirus maternal serologic screening in the first trimester of pregnancy: If you do not know where you are going, you will wind up somewhere else. Am. J. Obstet. Gynecol. MFM. 2021, 3, 100356. [Google Scholar] [CrossRef]
- Denoyelle, F.; Rouillon, I.; Alvin, F.; Parodi, M.; Couloigner, V.; Loundon, N.; Garabédian, N. Le dépistage néonatal de la surdité [Neonatal hearing screening]. Med. Sci. 2021, 37, 519–527. (In French) [Google Scholar]
- Lanzieri, T.M.; Chung, W.; Flores, M.; Blum, P.; Caviness, A.C.; Bialek, S.R.; Congenital Cytomegalovirus Group. Hearing loss in children with asymptomatic congenital cytomegalovirus infection. Pediatrics 2017, 139, e20162610. [Google Scholar] [CrossRef] [PubMed]
- Palma, S.; Roversi, M.F.; Bettini, M.; Mazzoni, S.; Pietrosemoli, P.; Lucaccioni, L.; Berardi, A.; Genovese, E. Hearing loss in children with congenital cytomegalovirus infection: An 11-year retrospective study based on laboratory database of a tertiary paediatric hospital. Acta Otorhinolaryngol. Ital. 2019, 39, 40–45. [Google Scholar] [CrossRef]
- Forner, G.; Abate, D.; Mengoli, C.; Palù, G.; Gussetti, N. High Cytomegalovirus (CMV) DNAemia Predicts CMV Sequelae in Asymptomatic Congenitally Infected Newborns Born to Women with Primary Infection During Pregnancy. J. Infect. Dis. 2015, 212, 67–71. [Google Scholar] [CrossRef]
- Bradford, R.D.; Cloud, G.; Lakeman, A.D.; Boppana, S.; Kimberlin, D.W.; Jacobs, R.; Demmler, G.; Sanchez, P.; Britt, W.; Soong, S.; et al. Detection of cytomegalovirus (CMV) DNA by polymerase chain reaction is associated with hearing loss in newborns with symptomatic congenital CMV infection involving the central nervous system. J. Infect. Dis. 2005, 191, 227–233. [Google Scholar] [CrossRef]
- Clemens, C.J.; Davis, S.A. Minimizing false-positives in universal newborn hearing screening: A simple solution. Pediatrics 2001, 107, E29. [Google Scholar] [CrossRef]
- Caluraud, S.; Marcolla-Bouchetemblé, A.; de Barros, A.; Moreau-Lenoir, F.; de Sevin, E.; Rerolle, S.; Charrière, E.; Lecler-Scarcella, V.; Billet, F.; Obstoy, M.-F.; et al. Newborn hearing screening: Analysis and outcomes after 100,000 births in Upper-Normandy French region. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Joint Committee of Infant Hearing (JCIH). Year, 2007 position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics 2007, 120, 898–921. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Maheshwari, A.; Boppana, S. CMV-induced Hearing Loss. Newborn 2023, 2, 249–262. [Google Scholar]
- Ciodaro, F.; Freni, F.; Alberti, G.; Forelli, M.; Gazia, F.; Bruno, R.; Sherdell, E.P.; Galletti, B.; Galletti, F. Application of Cervical Vestibular-Evoked Myogenic Potentials in Adults with Moderate to Profound Sensorineural Hearing Loss: A Preliminary Study. Int. Arch. Otorhinolaryngol. 2020, 24, e5–e10. [Google Scholar] [CrossRef]
- Dollard, S.C.; Schleiss, M.R.; Grosse, S.D. Public health and laboratory considerations regarding newborn screening for congenital cytomegalovirus. J. Inherit. Metab. Dis. 2010, 33, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Leruez-Ville, M.; Ghout, I.; Bussières, L.; Stirnemann, J.; Magny, J.F.; Couderc, S.; Salomon, L.J.; Guilleminot, T.; Aegerter, P.; Benoist, G.; et al. In utero treatment of congenital cytomegalovirus infection with valacyclovir in a multicenter, open-label, phase II study. Am. J. Obstet. Gynecol. 2016, 215, 462.e1–462.e10. [Google Scholar] [CrossRef] [PubMed]
- Desveaux, C.; Klein, J.; Leruez-Ville, M.; Ramirez-Torres, A.; Lacroix, C.; Breuil, B.; Froment, C.; Bascands, J.-L.; Schanstra, J.P.; Ville, Y. Identification of Symptomatic Fetuses Infected with Cytomegalovirus Using Amniotic Fluid Peptide Biomarkers. PLoS Pathog. 2016, 12, e1005395. [Google Scholar] [CrossRef]
- Vande Walle, C.; Keymeulen, A.; Oostra, A.; Schiettecatte, E.; Dhooge, I.; Smets, K.; Herregods, N. Apparent diffusion coefficient values of the white matter in magnetic resonance imaging of the neonatal brain may help predict outcome in congenital cytomegalovirus infection. Pediatr. Radiol. 2024, 54, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Robles, V.; Weimer, K.E.D.; Gehtland, L.M.; Kucera, K.S. Improved Dried Blood Spot PCR Assay for Universal Congenital Cytomegalovirus Screening in Newborns. Microbiol. Spectr. 2023, 11, e0404122. [Google Scholar] [CrossRef]
- Kimberlin, D.W.; Acosta, E.P.; Sánchez, P.J.; Sood, S.; Agrawal, V.; Homans, J.; Jacobs, R.F.; Lang, D.; Romero, J.R.; Griffin, J.; et al. Pharmacokinetic and pharmacodynamic assessment of oral valganciclovir in the treatment of symptomatic congenital cytomegalovirus disease. J. Infect. Dis. 2008, 197, 836–845. [Google Scholar] [CrossRef]
- Oliver, S.E.; Cloud, G.A.; Sánchez, P.J.; Demmler, G.J.; Dankner, W.; Shelton, M.; Jacobs, R.F.; Vaudry, W.; Pass, R.F.; Soong, S.J.; et al. Neurodevelopmental outcomes following ganciclovir therapy in symptomatic congenital cytomegalovirus infections involving the central nervous system. J. Clin. Virol. 2009, 46, S22–S26. [Google Scholar] [CrossRef]
- Faure Bardon, V.; Peytavin, G.; Lê, M.P.; Guilleminot, T.; Elefant, E.; Stirnemann, J.; Leruez-Ville, M.; Ville, Y. Placental transfer of Letermovir & Maribavir in the ex vivo human cotyledon perfusion model. New perspectives for in utero treatment of congenital cytomegalovirus infection. PLoS ONE 2020, 15, e0232140. [Google Scholar]
- Tanna, R.J.; Lin, J.W.; De Jesus, O. Sensorineural Hearing Loss. [Updated 23 August 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Gazia, F.; Abita, P.; Alberti, G.; Loteta, S.; Longo, P.; Caminiti, F.; GarGano, R. NICU Infants & SNHL: Experience of a western Sicily tertiary care centre. Acta Med. Mediterr. 2019, 35, 1001. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gana, N.; Huluță, I.; Cătănescu, M.-Ș.; Apostol, L.-M.; Nedelea, F.M.; Sima, R.-M.; Botezatu, R.; Panaitescu, A.M.; Gică, N. Congenital Cytomegalovirus-Related Hearing Loss. Audiol. Res. 2024, 14, 507-517. https://doi.org/10.3390/audiolres14030043
Gana N, Huluță I, Cătănescu M-Ș, Apostol L-M, Nedelea FM, Sima R-M, Botezatu R, Panaitescu AM, Gică N. Congenital Cytomegalovirus-Related Hearing Loss. Audiology Research. 2024; 14(3):507-517. https://doi.org/10.3390/audiolres14030043
Chicago/Turabian StyleGana, Nicoleta, Iulia Huluță, Mihai-Ștefan Cătănescu, Livia-Mihaela Apostol, Florina Mihaela Nedelea, Romina-Marina Sima, Radu Botezatu, Anca Maria Panaitescu, and Nicolae Gică. 2024. "Congenital Cytomegalovirus-Related Hearing Loss" Audiology Research 14, no. 3: 507-517. https://doi.org/10.3390/audiolres14030043
APA StyleGana, N., Huluță, I., Cătănescu, M.-Ș., Apostol, L.-M., Nedelea, F. M., Sima, R.-M., Botezatu, R., Panaitescu, A. M., & Gică, N. (2024). Congenital Cytomegalovirus-Related Hearing Loss. Audiology Research, 14(3), 507-517. https://doi.org/10.3390/audiolres14030043