Instrumental Assessment and Pharmacological Treatment of Migraine-Related Vertigo in Pediatric Age
Abstract
1. Introduction
2. Materials and Methods
3. Results
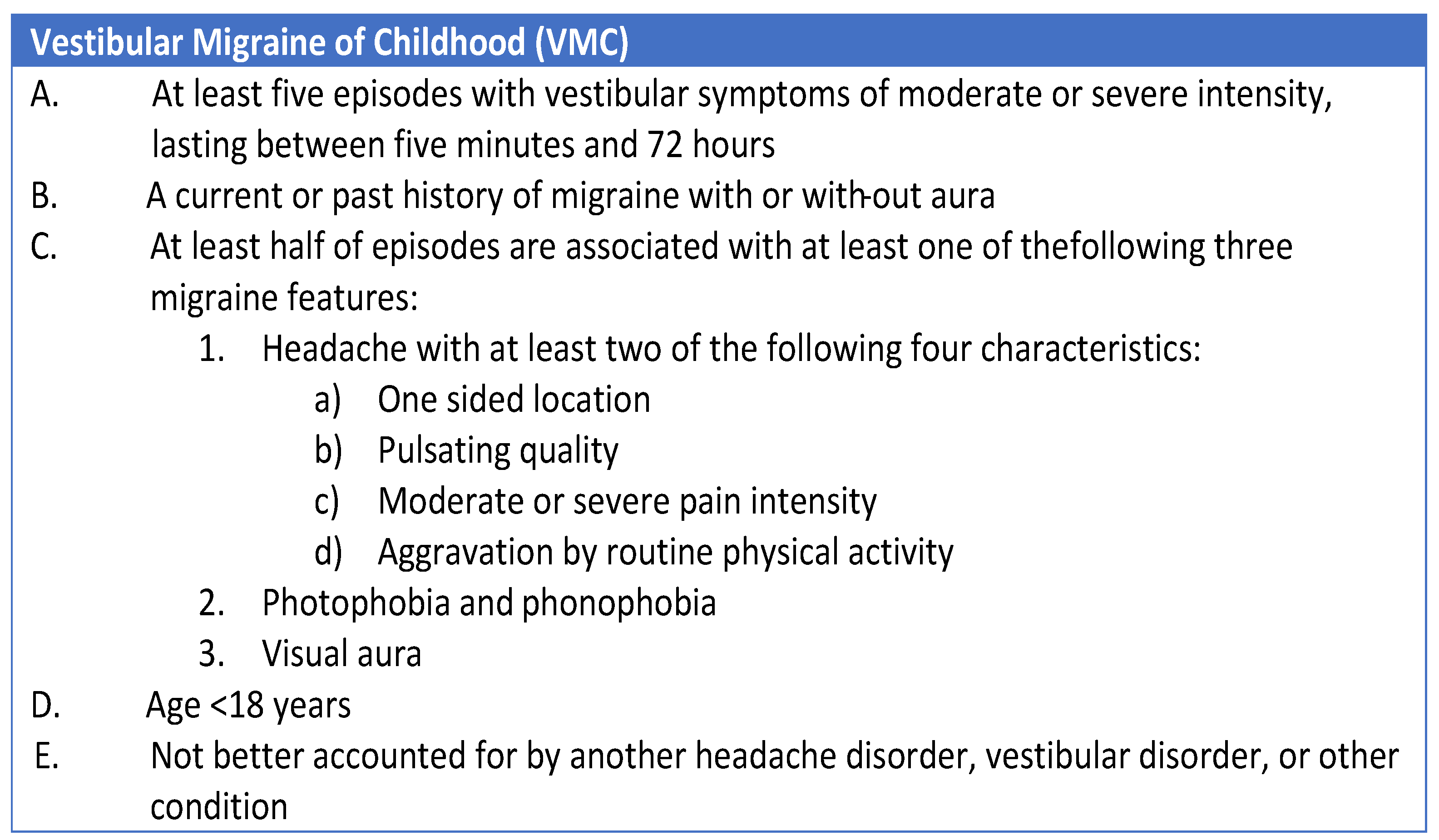
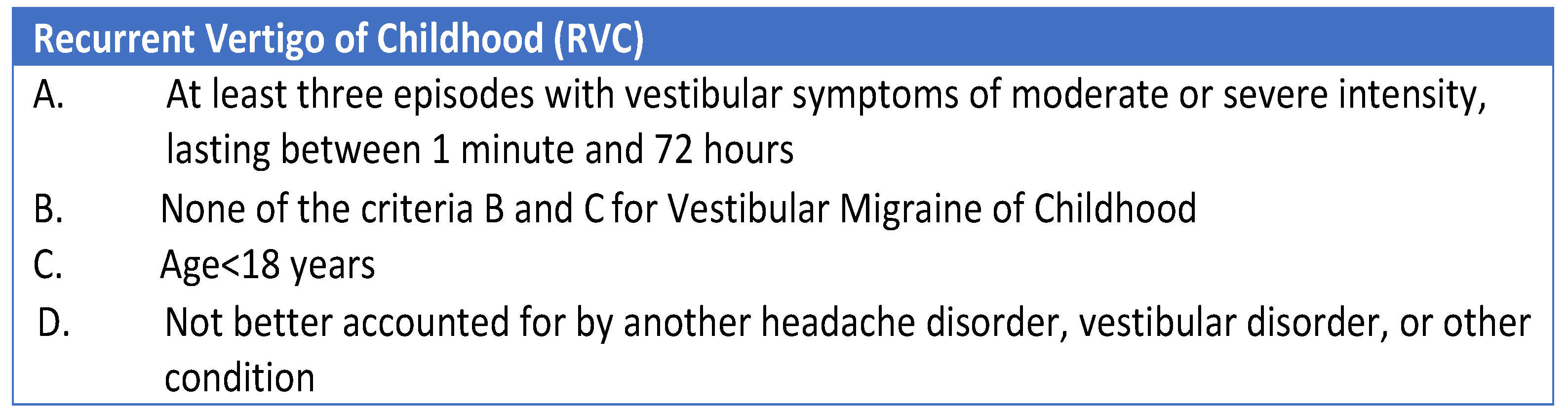
3.1. Recent Attempts to Perform an Etiological Classification
3.2. Vestibular Function Test in the Diagnosis of Children Vertigo
3.3. Better Options in the Pharmacological Treatment for Specific Pediatric Vertigo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riina, N.; Ilmari, P.; Kentala, E. Vertigo and imbalance in children: A retrospective study in a Helsinki University otorhinolaryngology clinic. Arch. Otolaryngol. Head Neck Surg. 2005, 131, 996–1000. [Google Scholar] [CrossRef]
- Fancello, V.; Palma, S.; Monzani, D.; Pelucchi, S.; Genovese, E.; Ciorba, A. Vertigo and Dizziness in Children: An Update. Children 2021, 8, 1025. [Google Scholar] [CrossRef]
- Casani, A.P.; Dallan, I.; Navari, E.; Sellari Franceschini, S.; Cerchiai, N. Vertigo in childhood: Proposal for a diagnostic algorithm based upon clinical experience. Acta Otorhinolaryngol. Ital. 2015, 35, 180–185. [Google Scholar]
- Gruber, M.; Cohen-Kerem, R.; Kaminer, M.; Shupak, A. Vertigo in children and adolescents: Characteristics and outcome. Sci. World J. 2012, 2012, 109624. [Google Scholar] [CrossRef] [PubMed]
- Chiarella, G.; Viola, P. The Challenge of Pediatric Vertigo. J. Ear. Nose Throat Disord. 2017, 2, 1027. [Google Scholar]
- Balatsouras, D.G.; Kaberos, A.; Assimakopoulos, D.; Katotomichelakis, M.; Economou, N.C.; Korres, S.G. Etiology of vertigo in children. Int. J. Pediatr. Otorhinolaryngol. 2007, 71, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Haripriya, G.R.; Lepcha, A.; Augustine, A.M.; John, M.; Philip, A.; Mammen, M.D. Prevalence, clinical profile, and diagnosis of pediatric dizziness in a tertiary care hospital. Int. J. Pediatr. Otorhinolaryngol. 2021, 146, 110761. [Google Scholar] [CrossRef] [PubMed]
- Gedik-Soyuyuce, O.; Gence-Gumus, Z.; Ozdilek, A.; Ada, M.; Korkut, N. Vestibular disorders in children: A retrospective analysis of vestibular function test findings. Int. J. Pediatr. Otorhinolaryngol. 2021, 146, 110751. [Google Scholar] [CrossRef]
- Wang, R.; Chao, X.; Luo, J.; Zhang, D.; Xu, J.; Liu, X.; Fan, Z.; Wang, H.; Xu, L. Objective vestibular function changes in children following cochlear implantation. J. Vestib. Res. 2022, 32, 29–37. [Google Scholar] [CrossRef]
- Licameli, G.; Zhou, G.; Kenna, M.A. Disturbance of vestibular function attributable to cochlear implantation in children. Laryngoscope 2009, 119, 740–745. [Google Scholar] [CrossRef]
- Vibert, D.; Kompis, M.; Caversaccio, M.; Mantokoudis, G. Vestibular function, subjective complaints, perceived disability in daily life, and sports activities in patients with cochlear implants performed during childhood: A prospective cross-section study. Acta Oto-Laryngol. 2023, 143, 735–741. [Google Scholar] [CrossRef]
- van de Berg, R.; Widdershoven, J.; Bisdorff, A.; Evers, S.; Wiener-Vacher, S.; Cushing, S.L.; Mack, K.J.; Kim, J.S.; Jahn, K.; Strupp, M.; et al. Vestibular Migraine of Childhood and Recurrent Vertigo of Childhood: Diagnostic criteria Consensus document of the Committee for the Classification of Vestibular Disorders of the Bárány Society and the International Headache Society. J. Vestib. Res. 2021, 31, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Gioacchini, F.M.; Alicandri-Ciufelli, M.; Kaleci, S.; Magliulo, G.; Re, M. Prevalence and diagnosis of vestibular disorders in children: A review. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 718–724. [Google Scholar] [CrossRef]
- Dunker, K.; Schnabel, L.; Grill, E.; Filippopulos, F.M.; Huppert, D. Recurrent Vertigo of Childhood: Clinical features and prognosis. Front. Neurol. 2022, 13, 1022395. [Google Scholar] [CrossRef]
- Davitt, M.; Delvecchio, M.T.; Aronoff, S.C. The Differential Diagnosis of Vertigo in Children: A Systematic Review of 2726 Cases. Pediatr. Emerg. Care 2020, 36, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Zhou, G.; Lipson, S.; Kawai, K.; Corcoran, M.; Brodsky, J.R. Multifactorial Characteristics of Pediatric Dizziness and Imbalance. Laryngoscope 2021, 131, E1308–E1314. [Google Scholar] [CrossRef] [PubMed]
- Yates, T. Benign paroxysmal torticollis. Handb. Clin. Neurol. 2023, 198, 241–247. [Google Scholar] [CrossRef]
- Baloh, R.W. Episodic ataxias 1 and 2. Handb. Clin. Neurol. 2012, 103, 595–602. [Google Scholar] [CrossRef]
- Božanić Urbančič, N.; Vozel, D.; Kordiš, Š.; Hribar, M.; Urbančič, J.; Battelino, S. Indicators of pediatric peripheral vestibular disorder: A retrospective study in a tertiary referral center. Int. J. Pediatr. Otorhinolaryngol. 2022, 159, 111221. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Q.; Shen, J.; Chen, J.; Jin, Y.; Zhang, Q.; Duan, M.; Yang, J. Etiological classification and management of dizziness in children: A systematic review and meta-analysis. Front. Neurol. 2023, 14, 1125488. [Google Scholar] [CrossRef]
- Jahn, K.; Langhagen, T.; Heinen, F. Vertigo and dizziness in children. Curr. Opin. Neurol. 2015, 28, 78–82. [Google Scholar] [CrossRef]
- Scarpa, A.; Gioacchini, F.M.; Cassandro, E.; Tulli, M.; Ralli, M.; Re, M.; Cassandro, C. Clinical application of cVEMPs and oVEMPs in patients affected by Ménière’s disease, vestibular neuritis and benign paroxysmal positional vertigo: A systematic review. Acta Otorhinolaryngol. Ital. 2019, 39, 298–307. [Google Scholar] [CrossRef]
- Perez, N.; Rama-Lopez, J. Head-impulse and caloric tests in patients with dizziness. Otol. Neurotol. 2003, 24, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Ornitz, E.M.; Atwell, C.W.; Walter, D.O.; Hartmann, E.E.; Kaplan, A.R. The maturation of vestibular nystagmus in infancy and childhood. Acta Oto-Laryngol. 1979, 88, 244–256. [Google Scholar] [CrossRef]
- Nandi, R.; Luxon, L.M. Development and assessment of the vestibular system. Int. J. Audiol. 2008, 47, 566–577. [Google Scholar] [CrossRef]
- Janky, K.L.; Rodriguez, A.I. Quantitative Vestibular Function Testing in the Pediatric Population. Semin. Hear. 2018, 39, 257–274. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, R.C.; Morlet, T.; Nicholas, B.D.; Josephson, G.; Horlbeck, D.; Lundy, L.; Mercado, A. Prevalence of vestibular and balance disorders in children. Otol. Neurotol. 2010, 31, 1441–1444. [Google Scholar] [CrossRef]
- Wang, S.J.; Chen, C.N.; Hsieh, W.S.; Young, Y.H. Development of vestibular evoked myogenic potentials in preterm neonates. Audiol. Neurotol. 2008, 13, 145–152. [Google Scholar] [CrossRef]
- Ecevit, A.; Anuk-Ince, D.; Erbek, S.; Ozkiraz, S.; Kurt, A.; Erbek, S.S.; Tarcan, A. Comparison of cervical vestibular evoked myogenic potentials between late preterm and term infants. Turk. J. Pediatr. 2012, 54, 509–514. [Google Scholar] [PubMed]
- Chen, C.N.; Wang, S.J.; Wang, C.T.; Hsieh, W.S.; Young, Y.H. Vestibular evoked myogenic potentials in newborns. Audiol. Neurotol. 2007, 12, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Valente, L.M.; Goebel, J.A.; Sinks, B. Pediatric vestibular evaluation: Two children with sensorineural hearing loss. J. Am. Acad. Audiol. 2012, 23, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Duarte, D.S.B.; Cabral, A.M.L.; Britto, D.B.L.A. Vestibular assessment in children aged zero to twelve years: An integrative review. Braz. J. Otorhinolaryngol. 2022, 88 (Suppl. S3), S212–S224. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Q.; Chen, J.; Wang, X.; Zhang, Y.; Liu, S.; Wang, L.; Shen, J.; Shen, M.; Tang, X.; et al. Characteristics of vestibular migraine, probable vestibular migraine, and recurrent vertigo of childhood in caloric and video head impulse tests. Front. Neurol. 2022, 13, 1050282. [Google Scholar] [CrossRef]
- El Bouhmadi, K.; Loudghiri, M.; Oukessou, Y.; Rouadi, S.; Abada, R.; Roubal, M.; Mahtar, M. Correlation between caloric test results and VHIT VOR gains in unilateral horizontal canal deficits: A cross-sectional study. Ann. Med. Surg. 2023, 85, 1614–1618. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kwon, E.; Kim, H.J.; Choi, J.Y.; Oh, H.J.; Koo, J.W.; Kim, J.S. Dissociated Results between Caloric and Video Head Impulse Tests in Dizziness: Prevalence, Pattern, Lesion Location, and Etiology. J. Clin. Neurol. 2020, 16, 277–284. [Google Scholar] [CrossRef] [PubMed]
- McCaslin, D.L.; Rivas, A.; Jacobson, G.P.; Bennett, M.L. The dissociation of video head impulse test (vHIT) and bithermal caloric test results provide topological localization of vestibular system impairment in patients with “definite” Ménière’s disease. Am. J. Audiol. 2015, 24, 1–10. [Google Scholar] [CrossRef]
- Langhagen, T.; Lehrer, N.; Borggraefe, I.; Heinen, F.; Jahn, K. Vestibular migraine in children and adolescents: Clinical findings and laboratory tests. Front. Neurol. 2015, 5, 292. [Google Scholar] [CrossRef]
- Marcelli, V.; Furia, T.; Marciano, E. Vestibular pathways involvement in children with migraine: A neuro-otological study. Headache J. Head Face Pain 2010, 50, 71–76. [Google Scholar] [CrossRef]
- Chen, J.Y.; Yang, J.; Zhang, Q.; Wang, W.; Ma, X.B.; Mei, L.; Shen, J.L.; Shen, M.; Chen, X.P. An analysis of the results of video head impulse test in benign paroxysmal vertigo of childhood. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2019, 33, 232–236. (In Chinese) [Google Scholar] [CrossRef]
- Ertugrul, G. Clinical use of child-friendly video head impulse test in dizzy children. Am. J. Otolaryngol. 2022, 43, 103432. [Google Scholar] [CrossRef]
- Wiener-Vacher, S.R.; Wiener, S.I. Video head impulse tests with a remote camera system: Normative values of semicircular canal vestibulo-ocular reflex gain in infants and children. Front. Neurol. 2017, 8, 434. [Google Scholar] [CrossRef] [PubMed]
- Retamal, S.R.; Díaz, P.O.; Fernández, A.M.; Muñoz, C.G.; Espinoza, M.R.; Araya, V.S.; Rivera, J.T. Assessment protocol and reference values of vestibulo-ocular reflex (VOR) gain in the horizontal plane recorded with video-Head Impulse Test (vHIT) in a pediatric population. CoDAS 2021, 33, e20200076. [Google Scholar] [CrossRef]
- Lehnen, N.; Ramaioli, C.; Todd, N.S.; Bartl, K.; Kohlbecher, S.; Jahn, K.; Schneider, E. Clinical and video head impulses: A simple bedside test in children. J. Neurol. 2017, 264, 1002–1004. [Google Scholar] [CrossRef]
- Hamilton, S.S.; Zhou, G.; Brodsky, J.R. Video head impulse testing (VHIT) in the pediatric population. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 1283–1287. [Google Scholar] [CrossRef]
- Ross, L.M.; Helminski, J.O. Test-retest and interrater reliability of the video head impulse test in the pediatric population. Otol. Neurotol. 2016, 37, 558–563. [Google Scholar] [CrossRef]
- Kim, K.S.; Jung, Y.K.; Hyun, K.J.; Kim, M.J.; Kim, H.J. Usefulness and practical insights of the pediatric video head impulse test. Int. J. Pediatr. Otorhinolaryngol. 2020, 139, 110424. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.N.; Chen, S.; Janky, K.L. Stability of Vestibular Testing in Children With Hearing Loss. Am J. Audiol. 2022, 31, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Eviatar, L.; Eviatar, A. The normal nystagmic response of infants to caloric and per rotatory stimulation. Laryngoscope 1979, 89 Pt 1, 1036–1045. [Google Scholar] [CrossRef]
- Beretti, T.; Desnous, B. Vertigo and dizziness in children: When to consider a neurological cause. Arch. Pediatr. 2023, 30, 505–509. [Google Scholar] [CrossRef]
- Saniasiaya, J.; Salim, R. Characteristics and diagnostic approach of vestibular migraine in children and adolescents: A systematic review. Auris Nasus Larynx 2023, 50, 218–227. [Google Scholar] [CrossRef]
- Brodsky, J.R.; Cusick, B.A.; Zhou, G. Evaluation and management of vestibular migraine in children: Experience from a pediatric vestibular clinic. Eur. J. Paediatr. Neurol. 2016, 20, 85–92. [Google Scholar] [CrossRef]
- Viola, P.; Marcianò, G.; Casarella, A.; Pisani, D.; Astorina, A.; Scarpa, A.; Siccardi, E.; Basile, E.; De Sarro, G.; Gallelli, L.; et al. The Pharmacological Treatment of Pediatric Vertigo. Children 2022, 9, 584. [Google Scholar] [CrossRef]
- Langhagen, T.; Landgraf, M.N.; Huppert, D.; Heinen, F.; Jahn, K. Vestibular Migraine in Children and Adolescents. Curr. Pain Headache Rep. 2016, 20, 67. [Google Scholar] [CrossRef]
- Tekin, H.; Edem, P. Effects and side effects of migraine prophylaxis in children. Pediatr. Int. 2022, 64, e15094. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.L.; Shimeall, W.; Sessums, L.; Dezee, K.J.; Becher, D.; Diemer, M.; Berbano, E.; O’Malley, P.G. Tricyclic antidepressants and headaches: Systematic review and meta-analysis. BMJ 2010, 341, c5222. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Byun, S.H.; Kim, J.S.; Lim, B.C.; Chae, J.H.; Choi, J.; Kim, K.J.; Hwang, Y.S.; Hwang, H. Comparison of flunarizine and topiramate for the prophylaxis of pediatric migraines. Eur. J. Paediatr. Neurol. 2013, 17, 45–49. [Google Scholar] [CrossRef]
- Vitkovic, J.; Winoto, A.; Rance, G.; Dowell, R.; Paine, M. Vestibular rehabilitation outcomes in patients with and without vestibular migraine. J. Neurol. 2013, 260, 3039–3048. [Google Scholar] [CrossRef]
- Devaraja, K. Vertigo in children; a narrative review of the various causes and their management. Int. J. Pediatr. Otorhinolaryngol. 2018, 111, 32–38. [Google Scholar] [CrossRef]
- Kacperski, J. Prophylaxis of migraine in children and adolescents. Pediatr. Drugs 2015, 17, 217–226. [Google Scholar] [CrossRef]
- Martín Sanz, E.; Barona de Guzmán, R. Benign paroxysmal vertigo of childhood: Categorization and comparison with benign positional paroxysmal vertigo in adult. Acta Otorrinolaringol. Esp. 2007, 58, 296–301. [Google Scholar] [CrossRef] [PubMed]

| Age | cVEMP | oVEMP | Caloric | vHIT |
|---|---|---|---|---|
| 0–2 | X | X | ||
| 3–7 | X | X | X | |
| >8 | X | X | X | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viola, P.; Scarpa, A.; Chiarella, G.; Pisani, D.; Astorina, A.; Ricciardiello, F.; De Luca, P.; Re, M.; Gioacchini, F.M. Instrumental Assessment and Pharmacological Treatment of Migraine-Related Vertigo in Pediatric Age. Audiol. Res. 2024, 14, 129-138. https://doi.org/10.3390/audiolres14010011
Viola P, Scarpa A, Chiarella G, Pisani D, Astorina A, Ricciardiello F, De Luca P, Re M, Gioacchini FM. Instrumental Assessment and Pharmacological Treatment of Migraine-Related Vertigo in Pediatric Age. Audiology Research. 2024; 14(1):129-138. https://doi.org/10.3390/audiolres14010011
Chicago/Turabian StyleViola, Pasquale, Alfonso Scarpa, Giuseppe Chiarella, Davide Pisani, Alessia Astorina, Filippo Ricciardiello, Pietro De Luca, Massimo Re, and Federico Maria Gioacchini. 2024. "Instrumental Assessment and Pharmacological Treatment of Migraine-Related Vertigo in Pediatric Age" Audiology Research 14, no. 1: 129-138. https://doi.org/10.3390/audiolres14010011
APA StyleViola, P., Scarpa, A., Chiarella, G., Pisani, D., Astorina, A., Ricciardiello, F., De Luca, P., Re, M., & Gioacchini, F. M. (2024). Instrumental Assessment and Pharmacological Treatment of Migraine-Related Vertigo in Pediatric Age. Audiology Research, 14(1), 129-138. https://doi.org/10.3390/audiolres14010011







