Identifying Health-Related Conditions Associated with Tinnitus in Young Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Questionnaire
2.3. Statistical Analyses
3. Results
3.1. Demographic Details of the Study Sample
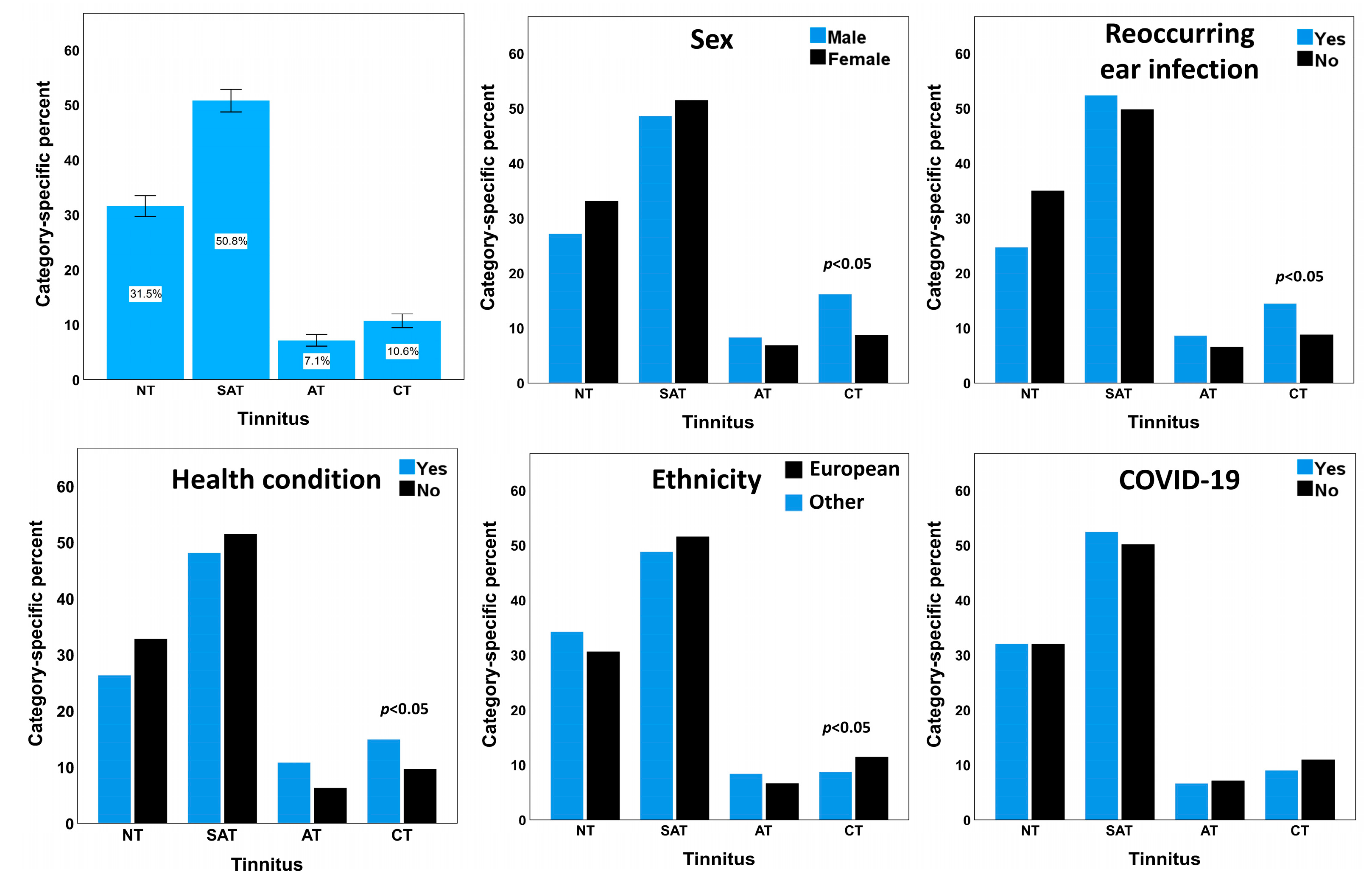
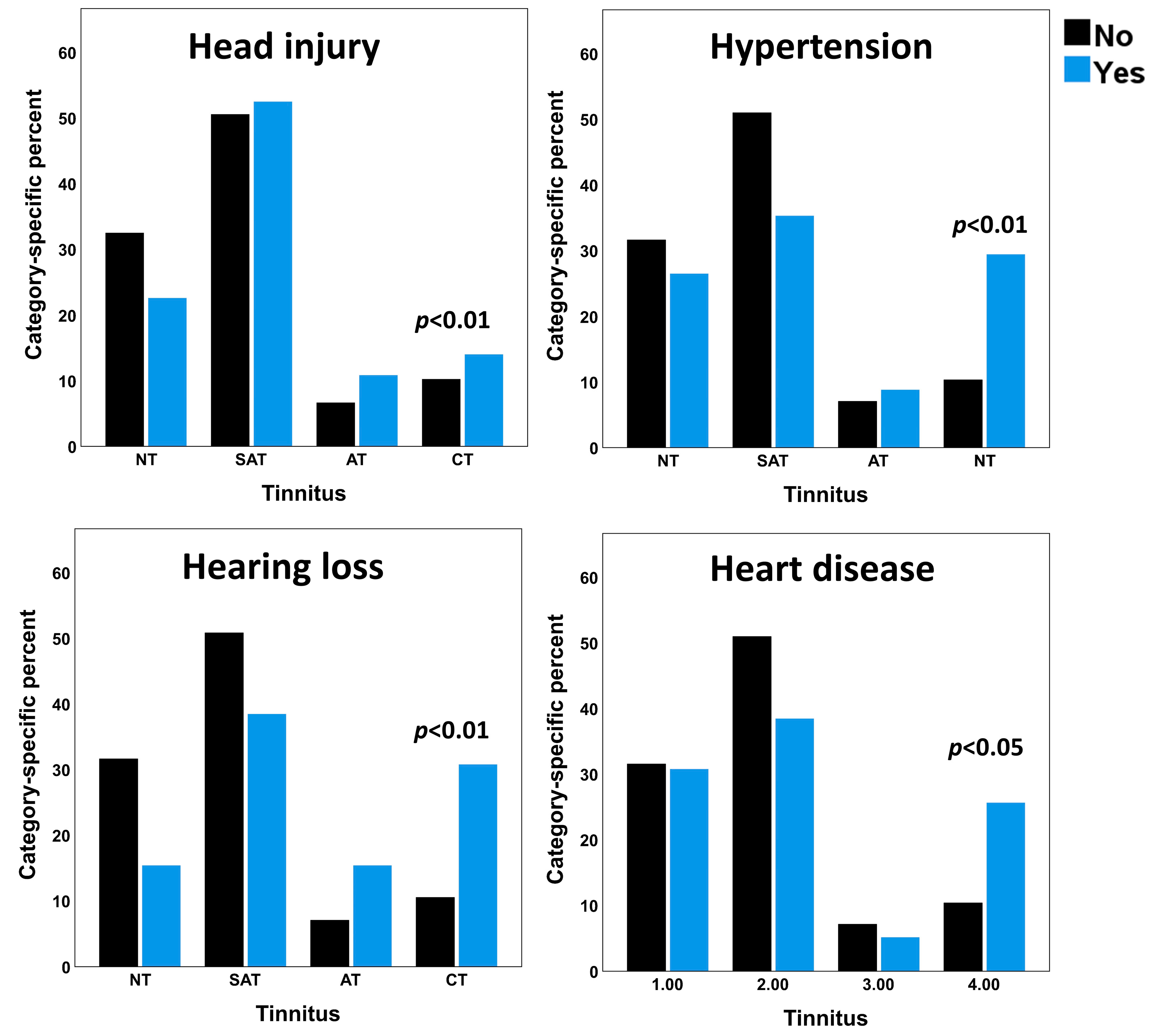
3.2. Health Conditions Associated with Tinnitus
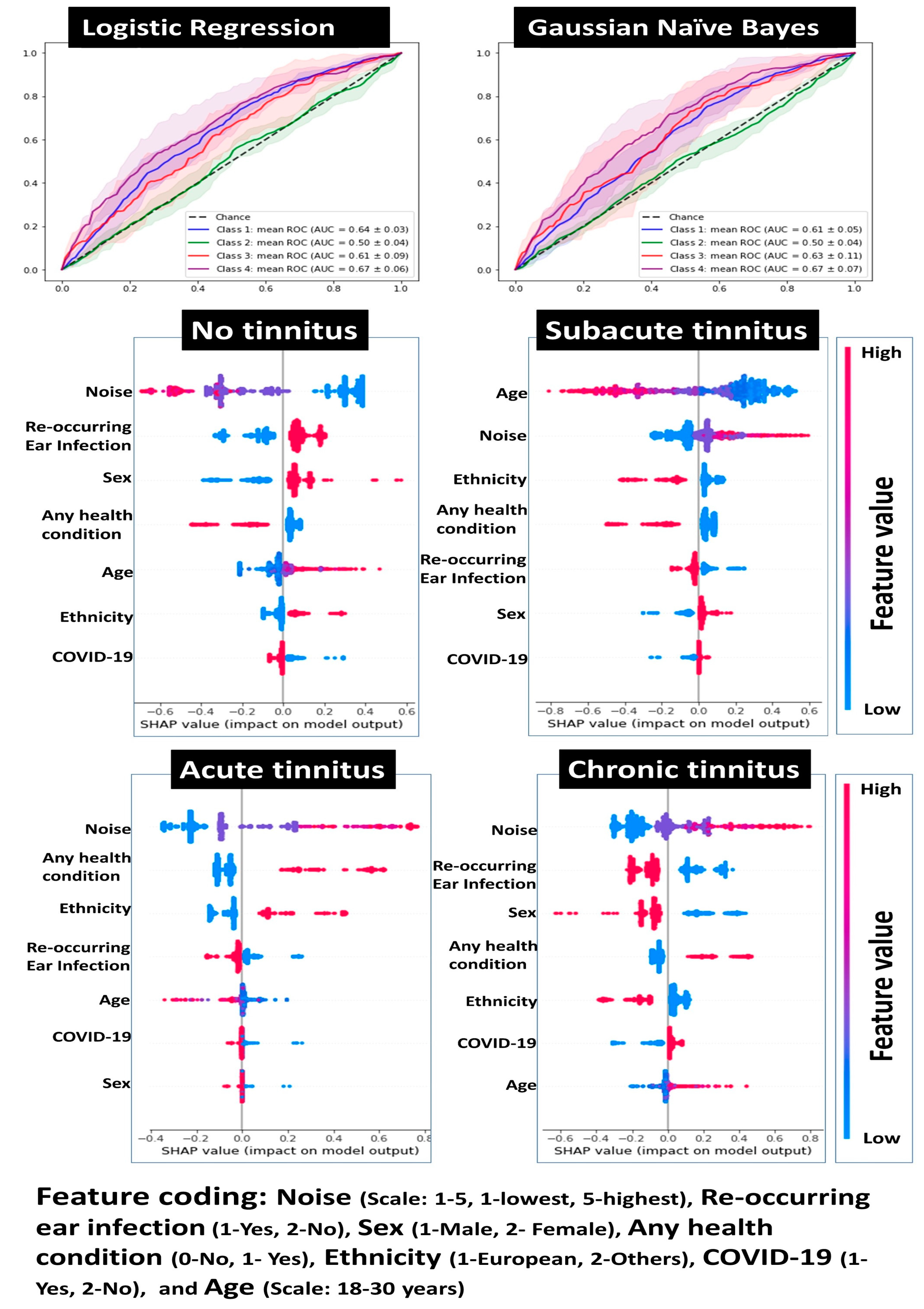
3.3. Results of the Regression Analyses
3.4. Association between COVID-19 and Reoccurring Middle Ear Infections
3.5. Risk Modeling for Tinnitus
4. Discussion
4.1. Prevalence of Tinnitus in Youth
4.2. Health-Related Factors Associated with Tinnitus in Young Adults
4.3. Noise as a Major Risk Factor for Tinnitus in Young Adults
4.4. Demographic and Health-Related Risk Factors of Tinnitus in Young Adults
4.5. Reoccurring Ear Infections as a Risk Factor for Tinnitus
4.6. Tinnitus and COVID-19
4.7. Reoccurring Ear Infection and COVID-19
4.8. Association between Mental Health Conditions and Tinnitus in Young Adults
4.9. Experimental Caveats
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| No Tinnitus | Subacute Tinnitus | Acute Tinnitus | Chronic Tinnitus | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Count | Row % | Count | Row % | Count | Row % | Count | Row % | ||
| Meningitis ++ | No | 709 | 31.5 | 1145 | 50.9 | 159 | 7.1 | 236 | 10.5 |
| Yes | 3 | 33.3 | 1 | 11.1 | 1 | 11.1 | 4 | 44.4 | |
| Hearing loss +++ | No | 710 | 31.6 | 1141 | 50.8 | 158 | 7.0 | 236 | 10.5 |
| Yes | 2 | 15.4 | 5 | 38.5 | 2 | 15.4 | 4 | 30.8 | |
| Hypertension ** | No | 703 | 31.6 | 1134 | 51.0 | 157 | 7.1 | 230 | 10.3 |
| Yes | 9 | 26.5 | 12 | 35.3 | 3 | 8.8 | 10 | 29.4 | |
| Low blood pressure | No | 712 | 31.6 | 1143 | 50.7 | 160 | 7.1 | 240 | 10.6 |
| Yes | 0 | 0.0 | 3 | 100.0 | 0 | 0.0 | 0 | 0.0 | |
| Head injury ** | No | 662 | 32.5 | 1030 | 50.6 | 136 | 6.7 | 209 | 10.3 |
| Yes | 50 | 22.6 | 116 | 52.5 | 24 | 10.9 | 31 | 14.0 | |
| Heart disease * | No | 700 | 31.5 | 1131 | 51.0 | 158 | 7.1 | 230 | 10.4 |
| Yes | 12 | 30.8 | 15 | 38.5 | 2 | 5.1 | 10 | 25.6 | |
| Malaria * | No | 708 | 31.6 | 1143 | 51.0 | 157 | 7.0 | 235 | 10.5 |
| Yes | 4 | 26.7 | 3 | 20.0 | 3 | 20.0 | 5 | 33.3 | |
| Scarlet fever * | No | 709 | 31.6 | 1141 | 50.8 | 159 | 7.1 | 235 | 10.5 |
| Yes | 3 | 21.4 | 5 | 35.7 | 1 | 7.1 | 5 | 35.7 | |
| Diabetes | No | 707 | 31.5 | 1144 | 50.9 | 160 | 7.1 | 237 | 10.5 |
| Yes | 5 | 50.0 | 2 | 20.0 | 0 | 0.0 | 3 | 30.0 | |
| Mumps | No | 708 | 31.6 | 1139 | 50.8 | 157 | 7.0 | 238 | 10.6 |
| Yes | 4 | 25.0 | 7 | 43.8 | 3 | 18.8 | 2 | 12.5 | |
| Temporomandibular joint disorder | No | 712 | 31.5 | 1146 | 50.8 | 159 | 7.0 | 240 | 10.6 |
| Yes | 0 | 0.0 | 0 | 0.0 | 1 | 100.0 | 0 | 0.0 | |
| Any health condition ** | No | 597 | 32.8 | 936 | 51.4 | 113 | 6.2 | 175 | 9.6 |
| Yes | 115 | 26.3 | 210 | 48.1 | 47 | 10.8 | 65 | 14.9 | |
Appendix B
| Variables | Intermittent Tinnitus | Continuous Tinnitus |
|---|---|---|
| Pseudo R-square (Cox and Snell) | 0.05 | 0.12 |
| Age (covariate) | 0.97 (0.94–1.01) | 0.96 (0.92–1.01) |
| Sex | ||
| Male | 1.24 (0.94–1.01) | 1.63 (1.22–2.17) ** |
| Female | 1 | 1 |
| Ethnicity | ||
| Europeans | 1.18 (0.93–1.49) | 1.00 (0.76–1.31) |
| Non-Europeans | 1 | 1 |
| Reoccurring ear infections | ||
| Yes | 1.46 (1.15–1.85) * | 1.66 (1.26–2.19) ** |
| No | 1 | 1 |
| Health history | ||
| Present | 1.17 (0.89–1.53) | 1.41 (1.04–1.92) * |
| Absent | 1 | 1 |
| Noise exposure | ||
| Always | 3.14 (1.32–7.45) * | 6.25 (2.58–15.11) *** |
| Most of the time | 3.89 (2.23–6.78) *** | 6.62 (3.70–11.83) *** |
| About half the time | 1.88 (1.21–2.90) * | 3.46 (2.18–5.49) *** |
| Sometimes | 2.00 (1.60–2.51) *** | 2.31 (1.76–3.03) *** |
| Never | 1 | 1 |
| COVID-19 | ||
| Yes | 1.07 (0.81–1.42) | 1.07 (0.81–1.42) |
| No | 1 | 1 |
References
- Shargorodsky, J.; Curhan, G.C.; Farwell, W.R. Prevalence and Characteristics of Tinnitus among US Adults. Am. J. Med. 2010, 123, 711–718. [Google Scholar] [CrossRef] [PubMed]
- American Tinnitus Association. Understanding the Facts; American Tinnitus Association: Vienna, Austria, 2020; Available online: https://www.ata.org/understanding-facts#nhnes (accessed on 9 August 2022).
- Alterman, T.; Steege, A.L.; Li, J.; Petersen, M.R.; Muntaner, C. Ethnic, Racial, and Gender Variations in Health among Farm Operators in the United States. Ann. Epidemiol. 2008, 18, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Masterson, E.A.; Sweeney, M.H.; Deddens, J.A.; Themann, C.L.; Wall, D.K. Prevalence of Workers with Shifts in Hearing by Industry: A Comparison of OSHA and NIOSH Hearing Shift Criteria. J. Occup. Environ. Med. 2014, 56, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, J.M.; Lin, H.W.; Bhattacharyya, N. Prevalence, Severity, Exposures, and Treatment Patterns of Tinnitus in the United States. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 959–965. [Google Scholar] [CrossRef]
- Bhatt, J.M.; Bhattacharyya, N.; Lin, H.W. Relationships between Tinnitus and the Prevalence of Anxiety and Depression. Laryngoscope 2017, 127, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Crönlein, T.; Langguth, B.; Pregler, M.; Kreuzer, P.M.; Wetter, T.C.; Schecklmann, M. Insomnia in Patients with Chronic Tinnitus: Cognitive and Emotional Distress as Moderator Variables. J. Psychosom. Res. 2016, 83, 65–68. [Google Scholar] [CrossRef]
- Pinto, P.C.L.; Marcelos, C.M.; Mezzasalma, M.A.; Osterne, F.J.V.; de Melo Tavares de Lima, M.A.; Nardi, A.E. Tinnitus and Its Association with Psychiatric Disorders: Systematic Review. J. Laryngol. Otol. 2014, 128, 660–664. [Google Scholar] [CrossRef]
- Jafari, Z.; Kolb, B.E.; Mohajerani, M.H. Age-Related Hearing Loss and Tinnitus, Dementia Risk, and Auditory Amplification Outcomes. Ageing Res. Rev. 2019, 56, 100963. [Google Scholar] [CrossRef]
- Langguth, B.; Landgrebe, M.; Kleinjung, T.; Sand, G.P.; Hajak, G. Tinnitus and Depression. World J. Biol. Psychiatry Off. J. World Fed. Soc. Biol. Psychiatry 2011, 12, 489–500. [Google Scholar] [CrossRef]
- Pattyn, T.; Van Den Eede, F.; Vanneste, S.; Cassiers, L.; Veltman, D.J.; Van De Heyning, P.; Sabbe, B.C.G. Tinnitus and Anxiety Disorders: A Review. Hear. Res. 2016, 333, 255–265. [Google Scholar] [CrossRef]
- Bhatt, I.S. Prevalence of and Risk Factors for Tinnitus and Tinnitus-Related Handicap in a College-Aged Population. Ear Hear. 2018, 39, 517–526. [Google Scholar] [CrossRef]
- Mahboubi, H.; Oliaei, S.; Kiumehr, S.; Dwabe, S.; Djalilian, H.R. The Prevalence and Characteristics of Tinnitus in the Youth Population of the United States. Laryngoscope 2013, 123, 2001–2008. [Google Scholar] [CrossRef] [PubMed]
- Savastano, M.; Marioni, G.; de Filippis, C. Tinnitus in Children without Hearing Impairment. Int. J. Pediatr. Otorhinolaryngol. 2009, 73 (Suppl. S1), S13–S15. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.L.; Serpanos, Y.C. High Frequency Hearing Sensitivity in Adolescent Females of a Lower Socioeconomic Status over a Period of 24 Years (1985–2008). J. Adolesc. Health 2011, 48, 203–208. [Google Scholar] [CrossRef]
- Weilnhammer, V.; Gerstner, D.; Huß, J.; Schreiber, F.; Alvarez, C.; Steffens, T.; Herr, C.; Heinze, S. Exposure to Leisure Noise and Intermittent Tinnitus among Young Adults in Bavaria: Longitudinal Data from a Prospective Cohort Study. Int. J. Audiol. 2022, 61, 89–96. [Google Scholar] [CrossRef]
- Breinbauer, H.A.; Anabalón, J.L.; Gutierrez, D.; Cárcamo, R.; Olivares, C.; Caro, J. Output Capabilities of Personal Music Players and Assessment of Preferred Listening Levels of Test Subjects: Outlining Recommendations for Preventing Music-Induced Hearing Loss. Laryngoscope 2012, 122, 2549–2556. [Google Scholar] [CrossRef] [PubMed]
- Vogel, I.; van de Looij-Jansen, P.M.; Mieloo, C.L.; Burdorf, A.; de Waart, F. Risky Music Listening, Permanent Tinnitus and Depression, Anxiety, Thoughts about Suicide and Adverse General Health. PLoS ONE 2014, 9, e98912. [Google Scholar] [CrossRef]
- Vogel, I.; Verschuure, H.; van der Ploeg, C.P.B.; Brug, J.; Raat, H. Adolescents and MP3 Players: Too Many Risks, Too Few Precautions. Pediatrics 2009, 123, e953–e958. [Google Scholar] [CrossRef]
- Rawool, V.W.; Colligon-Wayne, L.A. Auditory Lifestyles and Beliefs Related to Hearing Loss among College Students in the USA. Noise Health 2008, 10, 1–10. [Google Scholar] [CrossRef]
- Gilles, A.; Van Hal, G.; De Ridder, D.; Wouters, K.; Van de Heyning, P. Epidemiology of Noise-Induced Tinnitus and the Attitudes and Beliefs towards Noise and Hearing Protection in Adolescents. PLoS ONE 2013, 8, e70297. [Google Scholar] [CrossRef]
- Bhagat, S.P.; Davis, A.M. Modification of Otoacoustic Emissions Following Ear-Level Exposure to MP3 Player Music. Int. J. Audiol. 2008, 47, 751–760. [Google Scholar] [CrossRef]
- Keppler, H.; Dhooge, I.; Maes, L.; D’haenens, W.; Bockstael, A.; Philips, B.; Swinnen, F.; Vinck, B. Short-Term Auditory Effects of Listening to an MP3 Player. Arch. Otolaryngol. Head Neck Surg. 2010, 136, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, S.G.; Liberman, M.C. Adding Insult to Injury: Cochlear Nerve Degeneration after “Temporary” Noise-Induced Hearing Loss. J. Neurosci. 2009, 29, 14077–14085. [Google Scholar] [CrossRef] [PubMed]
- Hickox, A.E.; Liberman, M.C. Is Noise-Induced Cochlear Neuropathy Key to the Generation of Hyperacusis or Tinnitus? J. Neurophysiol. 2014, 111, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhao, F.; Mahafza, N.; Lu, W. Detecting Noise-Induced Cochlear Synaptopathy by Auditory Brainstem Response in Tinnitus Patients With Normal Hearing Thresholds: A Meta-Analysis. Front. Neurosci. 2021, 15, 778197. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Ma, W.; Zheng, Y.; Yang, H.; Lin, H. A Systematic Review and Meta-Analysis on the Association between Hypertension and Tinnitus. Int. J. Hypertens. 2015, 2015, 583493. [Google Scholar] [CrossRef]
- Chávez-Delgado, M.E.; Vázquez-Granados, I.; Rosales-Cortés, M.; Velasco-Rodríguez, V. Cochleovestibular dysfunction in patients with diabetes mellitus, hypertension and dyslipidemia. Acta Otorrinolaringol. Esp. 2012, 63, 93–101. [Google Scholar] [CrossRef]
- Borghi, C.; Brandolini, C.; Prandin, M.G.; Dormi, A.; Modugno, G.C.; Pirodda, A. Prevalence of Tinnitus in Patients Withhypertension and the Impact of Different Anti Hypertensive Drugs on the Incidence of Tinnitus: A Prospective, Single-Blind, Observational Study. Curr. Ther. Res. Clin. Exp. 2005, 66, 420–432. [Google Scholar] [CrossRef]
- Sogebi, O.A. Characterization of Tinnitus in Nigeria. Auris. Nasus. Larynx 2013, 40, 356–360. [Google Scholar] [CrossRef]
- Nowak, K.; Banaszewski, J.; Dabrowski, P.; Szymiec, E.; Szyfter, W. Tinnitus in systemic diseases. Otolaryngol. Pol. 2002, 56, 213–216. [Google Scholar]
- Baguley, D.M.; Bartnik, G.; Kleinjung, T.; Savastano, M.; Hough, E.A. Troublesome Tinnitus in Childhood and Adolescence: Data from Expert Centres. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Degeest, S.; Corthals, P.; Dhooge, I.; Keppler, H. The Impact of Tinnitus Characteristics and Associated Variables on Tinnitus-Related Handicap. J. Laryngol. Otol. 2016, 130, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Choi, H.G.; Lee, H.-J.; An, S.-Y.; Kim, S.W.; Lee, J.S.; Hong, S.K.; Kim, H.-J. Analysis of the Prevalence of and Risk Factors for Tinnitus in a Young Population. Otol. Neurotol. 2014, 35, 1218–1222. [Google Scholar] [CrossRef] [PubMed]
- Almufarrij, I.; Munro, K.J. One Year on: An Updated Systematic Review of SARS-CoV-2, COVID-19 and Audio-Vestibular Symptoms. Int. J. Audiol. 2021, 60, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Beukes, E.W.; Baguley, D.M.; Jacquemin, L.; Lourenco, M.P.C.G.; Allen, P.M.; Onozuka, J.; Stockdale, D.; Kaldo, V.; Andersson, G.; Manchaiah, V. Changes in Tinnitus Experiences During the COVID-19 Pandemic. Front. Public Health 2020, 8, 592878. [Google Scholar] [CrossRef]
- Savtale, S.; Hippargekar, P.; Bhise, S.; Kothule, S. Prevalence of Otorhinolaryngological Symptoms in Covid 19 Patients. Indian J. Otolaryngol. Head Neck Surg. 2022, 74, 3378–3384. [Google Scholar] [CrossRef]
- Fidan, V.; Akin, O.; Koyuncu, H. Rised Sudden Sensorineural Hearing Loss during COVID-19 Widespread. Am. J. Otolaryngol. 2021, 42, 102996. [Google Scholar] [CrossRef]
- Rhman, S.; Abdel Wahid, A. COVID-19 and Sudden Sensorineural Hearing Loss, a Case Report. Otolaryngol. Case Rep. 2020, 16, 100198. [Google Scholar] [CrossRef]
- Degen, C.; Lenarz, T.; Willenborg, K. Acute Profound Sensorineural Hearing Loss After COVID-19 Pneumonia. Mayo Clin. Proc. 2020, 95, 1801–1803. [Google Scholar] [CrossRef]
- Goshtasbi, K.; Abouzari, M.; Risbud, A.; Mostaghni, N.; Muhonen, E.G.; Martin, E.; Djalilian, H.R. Tinnitus and Subjective Hearing Loss Are More Common in Migraine: A Cross-Sectional NHANES Analysis. Otol. Neurotol. 2021, 42, 1329–1333. [Google Scholar] [CrossRef]
- Böhning, D. Multinomial Logistic Regression Algorithm. Ann. Inst. Stat. Math. 1992, 44, 197–200. [Google Scholar] [CrossRef]
- Noble, W.S. What Is a Support Vector Machine? Nat. Biotechnol. 2006, 24, 1565–1567. [Google Scholar] [CrossRef] [PubMed]
- Biau, G.; Scornet, E. A Random Forest Guided Tour. TEST 2016, 25, 197–227. [Google Scholar] [CrossRef]
- Kataria, A.; Singh, M. A Review of Data Classification Using K-Nearest Neighbour Algorithm. Int. J. Emerg. Technol. Adv. Eng. 2013, 3, 354–360. [Google Scholar]
- Wickramasinghe, I.; Kalutarage, H. Naive Bayes: Applications, Variations and Vulnerabilities: A Review of Literature with Code Snippets for Implementation. Soft Comput. 2021, 25, 2277–2293. [Google Scholar] [CrossRef]
- Chen, T.; He, T.; Benesty, M.; Khotilovich, V.; Tang, Y.; Cho, H.; Chen, K.; Mitchell, R.; Cano, I.; Zhou, T. Xgboost: Extreme Gradient Boosting; R Packag Version 0.4-2; 2015; Volume 1, pp. 1–4. Available online: https://cran.r-project.org/web/packages/xgboost/vignettes/xgboost.pdf (accessed on 27 June 2023).
- Refaeilzadeh, P.; Tang, L.; Liu, H. Cross-Validation. In Encyclopedia of Database Systems; liu, L., Özsu, M.T., Eds.; Springer: Boston, MA, USA, 2009; pp. 532–538. ISBN 978-0-387-39940-9. [Google Scholar]
- Mandrekar, J.N. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [PubMed]
- Bradley, A.P. The Use of the Area under the ROC Curve in the Evaluation of Machine Learning Algorithms. Pattern Recognit. 1997, 30, 1145–1159. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Lee, S.I. A unified approach to interpreting model predictions. In Advances in Neural Information Processing Systems; Curran Associates Inc.: Red Hook, NY, USA, 2017; Volume 30. [Google Scholar]
- Rosing, S.N.; Schmidt, J.H.; Wedderkopp, N.; Baguley, D.M. Prevalence of Tinnitus and Hyperacusis in Children and Adolescents: A Systematic Review. BMJ Open 2016, 6, e010596. [Google Scholar] [CrossRef]
- Nemholt, S.; Schmidt, J.H.; Wedderkopp, N.; Baguley, D.M. A Cross-Sectional Study of the Prevalence and Factors Associated With Tinnitus and/or Hyperacusis in Children. Ear Hear. 2020, 41, 344–355. [Google Scholar] [CrossRef]
- Narayanan, S.S.; Murali, M.; Lucas, J.C.; Sykes, K.J. Micronutrients in Tinnitus: A National Health and Nutrition Examination Survey Analysis. Am. J. Otolaryngol. 2022, 43, 103460. [Google Scholar] [CrossRef]
- McCormack, A.; Edmondson-Jones, M.; Somerset, S.; Hall, D. A Systematic Review of the Reporting of Tinnitus Prevalence and Severity. Hear. Res. 2016, 337, 70–79. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Lee, H.; Gilham, B.; Cardinal, B.J. Association between Accelerometer-Assessed Physical Activity and Tinnitus, NHANES 2005–2006. Res. Q. Exerc. Sport 2013, 84, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Maskalick, S.; Brown, K.; Gilham, B. Association between Depression and Tinnitus in a Nationally Representative Sample of US Older Adults. Aging Ment. Health 2013, 17, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Folmer, R.L.; McMillan, G.P.; Austin, D.F.; Henry, J.A. Audiometric Thresholds and Prevalence of Tinnitus among Male Veterans in the United States: Data from the National Health and Nutrition Examination Survey, 1999–2006. J. Rehabil. Res. Dev. 2011, 48, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Spankovich, C.; Gonzalez, V.B.; Su, D.; Bishop, C.E. Self Reported Hearing Difficulty, Tinnitus, and Normal Audiometric Thresholds, the National Health and Nutrition Examination Survey 1999–2002. Hear. Res. 2018, 358, 30–36. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Cardinal, B.J.; Gilham, B. NHANES: No significant link between cardiorespiratory fitness and tinnitus. Hear. J. 2011, 64, 34–36. [Google Scholar] [CrossRef]
- Reavis, K.M.; Henry, J.A.; Marshall, L.M.; Carlson, K.F. Prevalence of Self-Reported Depression Symptoms and Perceived Anxiety Among Community-Dwelling U.S. Adults Reporting Tinnitus. Perspect. ASHA Spec. Interes. Groups 2020, 5, 959–970. [Google Scholar] [CrossRef]
- Nondahl, D.M.; Cruickshanks, K.J.; Huang, G.-H.; Klein, B.E.K.; Klein, R.; Nieto, F.J.; Tweed, T.S. Tinnitus and Its Risk Factors in the Beaver Dam Offspring Study. Int. J. Audiol. 2011, 50, 313–320. [Google Scholar] [CrossRef]
- Jury, M.A.; Flynn, M.C. Auditory and Vestibular Sequelae to Traumatic Brain Injury: A Pilot Study. N. Z. Med. J. 2001, 114, 286–288. [Google Scholar]
- Kreuzer, P.M.; Landgrebe, M.; Vielsmeier, V.; Kleinjung, T.; De Ridder, D.; Langguth, B. Trauma-Associated Tinnitus. J. Head Trauma Rehabil. 2014, 29, 432–442. [Google Scholar] [CrossRef]
- Ceranic, B.J.; Prasher, D.K.; Raglan, E.; Luxon, L.M. Tinnitus after Head Injury: Evidence from Otoacoustic Emissions. J. Neurol. Neurosurg. Psychiatry 1998, 65, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Kreuzer, P.M.; Landgrebe, M.; Schecklmann, M.; Staudinger, S.; Langguth, B. Trauma-Associated Tinnitus: Audiological, Demographic and Clinical Characteristics. PLoS ONE 2012, 7, e45599. [Google Scholar] [CrossRef] [PubMed]
- Vernon, J.A.; Press, L.S. Characteristics of Tinnitus Induced by Head Injury. Arch. Otolaryngol. Head. Neck Surg. 1994, 120, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Negrila-Mezei, A.; Enache, R.; Sarafoleanu, C. Tinnitus in Elderly Population: Clinic Correlations and Impact upon QoL. J. Med. Life 2011, 4, 412–416. [Google Scholar] [PubMed]
- Patel, S.D.; Patel, S.; Finberg, A.; Shah, V.N.; Mittal, R.; Eshraghi, A.A. Association Between Tinnitus and Hypertension: A Cross-Sectional Analysis of the National Health and Nutrition Examination Survey. Otol. Neurotol. 2022, 43, 766–772. [Google Scholar] [CrossRef]
- Wang, L.Y.; Young, T.-H. Hepatitis, Gallbladder Hydrops, Splenomegaly, and Ascites in a Child with Scarlet Fever. Pediatr. Emerg. Care 2012, 28, 1215–1217. [Google Scholar] [CrossRef]
- Blake, C.J. On the Etiology of Acquired Deaf-Mutism, Having Especial Reference to the Effects of Scarlet Fever. Bost. Med. Surg. J. 1870, 83, 405–408. [Google Scholar] [CrossRef]
- Williams, H.J. Otitis Media and Orbital Cellulitis Complicating Scarlet Fever: Preliminary Report on Loss of Hearing from This Disease. Arch. Otolaryngol. 1939, 29, 82–89. [Google Scholar] [CrossRef]
- Fraser, G.R. Epidemiology of Profound Childhood Deafness. Audiology 1974, 13, 335–341. [Google Scholar] [CrossRef]
- Jozefowicz-Korczynska, M.; Pajor, A.; Lucas Grzelczyk, W. The Ototoxicity of Antimalarial Drugs-A State of the Art Review. Front. Neurol. 2021, 12, 661740. [Google Scholar] [CrossRef]
- Sindhusake, D.; Golding, M.; Wigney, D.; Newall, P.; Jakobsen, K.; Mitchell, P. Factors Predicting Severity of Tinnitus: A Population-Based Assessment. J. Am. Acad. Audiol. 2004, 15, 269–280. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Hearing Loss Due to Recreational Exposure to Loud Sounds: A Review; World Health Organization: Geneva, SwitTzerland, 2015; Available online: https://apps.who.int/iris/handle/10665/154589 (accessed on 27 June 2023).
- Jiang, W.; Zhao, F.; Guderley, N.; Manchaiah, V. Daily Music Exposure Dose and Hearing Problems Using Personal Listening Devices in Adolescents and Young Adults: A Systematic Review. Int. J. Audiol. 2016, 55, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Johnson, O.; Andrew, B.; Walker, D.; Morgan, S.; Aldren, A. British University Students’ Attitudes towards Noise-Induced Hearing Loss Caused by Nightclub Attendance. J. Laryngol. Otol. 2014, 128, 24–29. [Google Scholar] [CrossRef]
- Hunter, A. Attitudes, Risk Behavior, and Noise Exposure among Young Adults with Hearing Problems: Identifying a Typology. Semin. Hear. 2017, 38, 332–347. [Google Scholar] [CrossRef]
- NIOSH Criteria for a Recommended Standard: Occupational Noise Exposure: Revised Criteria, 1998; U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication: Cincinnati, OH, USA, 1998; pp. 98–126. Available online: https://www.cdc.gov/niosh/docs/98-126/pdfs/98-126.pdf (accessed on 18 May 2022).
- Fink, D.; Mayes, J. Unsafe at Any Sound: Hearing Loss and Tinnitus in Personal Audio System Users. Proc. Meet. Acoust. 2021, 43, 40003. [Google Scholar] [CrossRef]
- Rabinowitz, P.; Cantley, L.F.; Galusha, D.; Trufan, S.; Swersey, A.; Dixon-Ernst, C.; Ramirez, V.; Neitzel, R. Assessing Hearing Conservation Program Effectiveness: Results of a Multisite Assessment. J. Occup. Environ. Med. 2018, 60, 29–35. [Google Scholar] [CrossRef]
- Eldred, K.; Gannon, W.; Von Gierke, H. Criteria for Short Time Exposure of Personnel to High Intensity Jet Aircraft Noise; WADC Technical Note 55-355; Wright-Patterson Air Force Base: Fairborn, OH, USA, 1955. [Google Scholar]
- Liberman, M.C.; Kujawa, S.G. Cochlear Synaptopathy in Acquired Sensorineural Hearing Loss: Manifestations and Mechanisms. Hear. Res. 2017, 349, 138–147. [Google Scholar] [CrossRef]
- Fernandez, K.A.; Guo, D.; Micucci, S.; De Gruttola, V.; Liberman, M.C.; Kujawa, S.G. Noise-Induced Cochlear Synaptopathy with and Without Sensory Cell Loss. Neuroscience 2020, 427, 43–57. [Google Scholar] [CrossRef]
- Engdahl, B.; Krog, N.H.; Kvestad, E.; Hoffman, H.J.; Tambs, K. Occupation and the Risk of Bothersome Tinnitus: Results from a Prospective Cohort Study (HUNT). BMJ Open 2012, 2, e000512. [Google Scholar] [CrossRef]
- Gopinath, B.; McMahon, C.M.; Rochtchina, E.; Karpa, M.J.; Mitchell, P. Incidence, Persistence, and Progression of Tinnitus Symptoms in Older Adults: The Blue Mountains Hearing Study. Ear Hear. 2010, 31, 407–412. [Google Scholar] [CrossRef]
- Henderson, E.; Testa, M.A.; Hartnick, C. Prevalence of Noise-Induced Hearing-Threshold Shifts and Hearing Loss among US Youths. Pediatrics 2011, 127, e39–e46. [Google Scholar] [CrossRef] [PubMed]
- Khedr, E.M.; Ahmed, M.A.; Shawky, O.A.; Mohamed, E.S.; El Attar, G.S.; Mohammad, K.A. Epidemiological Study of Chronic Tinnitus in Assiut, Egypt. Neuroepidemiology 2010, 35, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Sindhusake, D.; Golding, M.; Newall, P.; Rubin, G.; Jakobsen, K.; Mitchell, P. Risk Factors for Tinnitus in a Population of Older Adults: The Blue Mountains Hearing Study. Ear Hear. 2003, 24, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Bu, X.; Zhou, L.; Xing, G.; Liu, C.; Wang, D. An Epidemiologic Study of Tinnitus in a Population in Jiangsu Province, China. J. Am. Acad. Audiol. 2011, 22, 578–585. [Google Scholar] [CrossRef]
- Amali, A.; Hosseinzadeh, N.; Samadi, S.; Nasiri, S.; Zebardast, J. Sensorineural Hearing Loss in Patients with Chronic Suppurative Otitis Media: Is There a Significant Correlation? Electron. Physician 2017, 9, 3823–3827. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, R.M.; Shin, J.J.; Schwartz, S.R.; Coggins, R.; Gagnon, L.; Hackell, J.M.; Hoelting, D.; Hunter, L.L.; Kummer, A.W.; Payne, S.C.; et al. Clinical Practice Guideline: Otitis Media with Effusion (Update). Otolaryngol. Neck Surg. 2016, 154, S1–S41. [Google Scholar] [CrossRef]
- Harris, A.S.; Elhassan, H.A.; Flook, E.P. Why Are Ototopical Aminoglycosides Still First-Line Therapy for Chronic Suppurative Otitis Media? A Systematic Review and Discussion of Aminoglycosides versus Quinolones. J. Laryngol. Otol. 2016, 130, 2–7. [Google Scholar] [CrossRef]
- Parrino, D.; Frosolini, A.; Gallo, C.; De Siati, R.D.; Spinato, G.; de Filippis, C. Tinnitus Following COVID-19 Vaccination: Report of Three Cases. Int. J. Audiol. 2022, 61, 526–529. [Google Scholar] [CrossRef]
- Chapman, S.J.; Hill, A.V.S. Human Genetic Susceptibility to Infectious Disease. Nat. Rev. Genet. 2012, 13, 175–188. [Google Scholar] [CrossRef]
- Rundle, C.W.; Presley, C.L.; Militello, M.; Barber, C.; Powell, D.L.; Jacob, S.E.; Atwater, A.R.; Watsky, K.L.; Yu, J.; Dunnick, C.A. Hand Hygiene during COVID-19: Recommendations from the American Contact Dermatitis Society. J. Am. Acad. Dermatol. 2020, 83, 1730–1737. [Google Scholar] [CrossRef]
- Chen, X.; Ren, L.; Xue, X.; Yu, N.; Liu, P.; Shen, W.; Zhou, H.; Wang, B.; Zhou, J.; Yang, S.; et al. The Comorbidity of Depression and Anxiety Symptoms in Tinnitus Sufferers: A Network Analysis. Brain Sci. 2023, 13, 583. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, I.S.; Wilson, N.; Dias, R.; Torkamani, A. A Genome-Wide Association Study of Tinnitus Reveals Shared Genetic Links to Neuropsychiatric Disorders. Sci. Rep. 2022, 12, 22511. [Google Scholar] [CrossRef] [PubMed]
- Clifford, R.E.; Maihofer, A.X.; Stein, M.B.; Ryan, A.F.; Nievergelt, C.M. Novel Risk Loci in Tinnitus and Causal Inference With Neuropsychiatric Disorders Among Adults of European Ancestry. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Radez, J.; Reardon, T.; Creswell, C.; Lawrence, P.J.; Evdoka-Burton, G.; Waite, P. Why Do Children and Adolescents (Not) Seek and Access Professional Help for Their Mental Health Problems? A Systematic Review of Quantitative and Qualitative Studies. Eur. Child Adolesc. Psychiatry 2021, 30, 183–211. [Google Scholar] [CrossRef]
| Variables | Tinnitus (Frequency (%)) | |||
|---|---|---|---|---|
| NT | ST | AT | CT | |
| Sex | ||||
| Male | 148 (6.7) | 265 (11.7) | 45 (2.0) | 88 (3.9) |
| Female | 559 (24.7) | 868 (38.5) | 115 (5.1) | 147 (6.5) |
| Non-binary | 3 (0.1) | 7 (0.3) | 0 (0) | 5 (0.2) |
| No disclosure | 2 (0.08) | 6 (0.2) | 0 (0) | 0 (0) |
| Ethnicity | ||||
| Europeans | 503 (22.3) | 848 (37.6) | 109 (4.8) | 187 (8.3) |
| Others | 209 (9.3) | 298 (13.2) | 51 (2.3) | 53 (2.3) |
| Reoccurring ear infections | ||||
| Yes | 152 (6.7) | 323 (14.3) | 53 (2.3) | 89 (3.9) |
| No | 560 (24.8) | 823 (36.4) | 107 (4.7) | 151 (6.7) |
| Health history | ||||
| Present | 115 (5.1) | 210 (9.3) | 47 (2.1) | 65 (2.9) |
| Absent | 597 (26.4) | 936 (41.5) | 113 (5.0) | 175 (7.8) |
| Noise | ||||
| Always | 7 (0.3) | 24 (1.1) | 11 (0.5) | 10 (0.5) |
| Most of the time | 18 (0.8) | 79 (3.7) | 13 (0.6) | 28 (1.3) |
| About half the time | 35 (1.7) | 81 (3.8) | 26 (1.2) | 29 (1.4) |
| Sometimes | 197 (9.3) | 441 (20.8) | 57 (2.7) | 100 (4.7) |
| Never | 405 (19.1) | 456 (21.5) | 45 (2.1) | 59 (2.8) |
| COVID-19 | ||||
| Yes | 164 (7.3) | 269 (11.9) | 47 (2.1) | 65 (2.9) |
| No | 548 (24.3) | 877 (38.8) | 126 (5.6) | 194 (8.6) |
| No Tinnitus | Subacute Tinnitus | Acute Tinnitus | Chronic Tinnitus | Chi-Square | p-Value | ||
|---|---|---|---|---|---|---|---|
| Count (Row %) | Count (Row %) | Count (Row %) | Count Row % | ||||
| Head injury | No | 662 (32.5) | 1030 (50.6) | 136 (6.7) | 209 (10.3) | 8.01 | 0.005 |
| Yes | 50 (22.6) | 116 (52.5) | 24 (10.9) | 31 (14.0) | |||
| Hypertension | No | 703 (31.6) | 1134 (51.0) | 157 (7.1) | 230 (10.3) | 7.73 | 0.005 |
| Yes | 9 (26.5) | 12 (35.3) | 3 (8.8) | 10 (29.4) | |||
| Hearing loss | No | 710 (31.6) | 1141 (50.8) | 158 (7) | 236 (10.5) | 7.25 | 0.007 |
| Yes | 2 (15.4) | 5 (38.5) | 2 (15.4) | 4 (30.8) | |||
| Heart disease | No | 700 (31.5) | 1131 (51.0) | 158 (7.1) | 230 (10.4) | 4.89 | 0.027 |
| Yes | 12 (30.8) | 15 (38.5) | 2 (5.1) | 10 (25.6) | |||
| Malaria | No | 708 (31.6) | 1143 (51.0) | 157 (7.0) | 235 (10.5) | 4.43 | 0.035 |
| Yes | 4 (26.7) | 3 (20.0) | 3 (20.0) | 5 (33.3) | |||
| Scarlet fever | No | 709 (31.6) | 1141 (50.8) | 159 (7.1) | 235 (10.5) | 5.95 | 0.01 |
| Yes | 3 (21.4) | 5 (35.7) | 1 (7.1) | 5 (35.7) | |||
| Any health condition | No | 597 (32.8) | 936 (51.4) | 113 (6.2) | 175 (9.6) | 7.37 | 0.007 |
| Yes | 115 (26.3) | 210 (48.1) | 47 (10.8) | 65 (14.9) | |||
| Variables | Subacute Tinnitus | Acute Tinnitus | Chronic Tinnitus | Any Tinnitus |
|---|---|---|---|---|
| Pseudo R-square (Cox and Snell) | 0.04 | 0.10 | 0.16 | 0.05 |
| Age (covariate) | 0.96 (0.92–1.01) | 0.97 (0.93–0.99) * | 1.02 (0.96–1.08) | 0.97 (0.93–1.00) |
| Sex | ||||
| Male | 1.23 (0.98–1.58) | 1.37 (0.89–2.09) | 2.26 (1.58–3.24) ** | 1.37 (1.08–1.73) * |
| Female | 1 | 1 | 1 | 1 |
| Ethnicity | ||||
| Europeans | 1.10 (0.87–1.38) | 0.81 (0.54–1.21) | 1.58 (1.07–2.34) * | 1.11 (0.89–1.39) |
| Non-Europeans | 1 | 1 | 1 | 1 |
| Reoccurring ear infections | ||||
| Yes | 1.40 (1.11–1.77) * | 1.66 (1.11–2.47) * | 2.28 (1.61–3.23) ** | 1.53 (1.22–1.91) ** |
| No | 1 | 1 | 1 | 1 |
| Any health condition | ||||
| Present | 1.11 (0.85–1.45) | 1.94 (1.28–2.95) * | 1.58 (1.08–2.32) * | 1.25 (0.97–1.61) |
| Absent | 1 | 1 | 1 | 1 |
| Noise exposure | ||||
| Always | 2.72 (1.15–6.44) * | 12.86 (4.69–35.24) *** | 8.22 (2.95–22.89) ** | 4.10 (1.82–9.25) ** |
| Most of the time | 3.91 (2.26–6.74) *** | 6.10 (2.77–13.49) *** | 10.9 (5.53–21.5) *** | 4.75 (2.80–8.06) *** |
| About half the time | 1.75 (1.14–2.68) * | 5.71 (3.12–10.46) *** | 4.87 (2.71–8.76) *** | 2.38 (1.59–3.55) ** |
| Sometimes | 1.90 (1.52–2.36) *** | 2.43 (1.58–3.75) ** | 3.51 (2.40–5.14) *** | 2.09 (1.70–2.59) *** |
| Never | 1 | 1 | 1 | 1 |
| COVID-19 | ||||
| Yes | 0.95 (0.75–1.20) | 0.81 (0.52–1.27) | 0.73 (0.49–1.09) | 0.91 (0.72–1.14) |
| No | 1 | 1 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatt, I.S.; Washnik, N.J.; Kingsbury, S.; Deshpande, A.K.; Kingsbury, H.; Bhagavan, S.G.; Michel, K.; Dias, R.; Torkamani, A. Identifying Health-Related Conditions Associated with Tinnitus in Young Adults. Audiol. Res. 2023, 13, 546-562. https://doi.org/10.3390/audiolres13040048
Bhatt IS, Washnik NJ, Kingsbury S, Deshpande AK, Kingsbury H, Bhagavan SG, Michel K, Dias R, Torkamani A. Identifying Health-Related Conditions Associated with Tinnitus in Young Adults. Audiology Research. 2023; 13(4):546-562. https://doi.org/10.3390/audiolres13040048
Chicago/Turabian StyleBhatt, Ishan Sunilkumar, Nilesh J. Washnik, Sarah Kingsbury, Aniruddha K. Deshpande, Hailey Kingsbury, Srividya Grama Bhagavan, Klayre Michel, Raquel Dias, and Ali Torkamani. 2023. "Identifying Health-Related Conditions Associated with Tinnitus in Young Adults" Audiology Research 13, no. 4: 546-562. https://doi.org/10.3390/audiolres13040048
APA StyleBhatt, I. S., Washnik, N. J., Kingsbury, S., Deshpande, A. K., Kingsbury, H., Bhagavan, S. G., Michel, K., Dias, R., & Torkamani, A. (2023). Identifying Health-Related Conditions Associated with Tinnitus in Young Adults. Audiology Research, 13(4), 546-562. https://doi.org/10.3390/audiolres13040048








