Universal Recommendations on Planning and Performing the Auditory Brainstem Responses (ABR) with a Focus on Mice and Rats
Abstract
1. Introduction
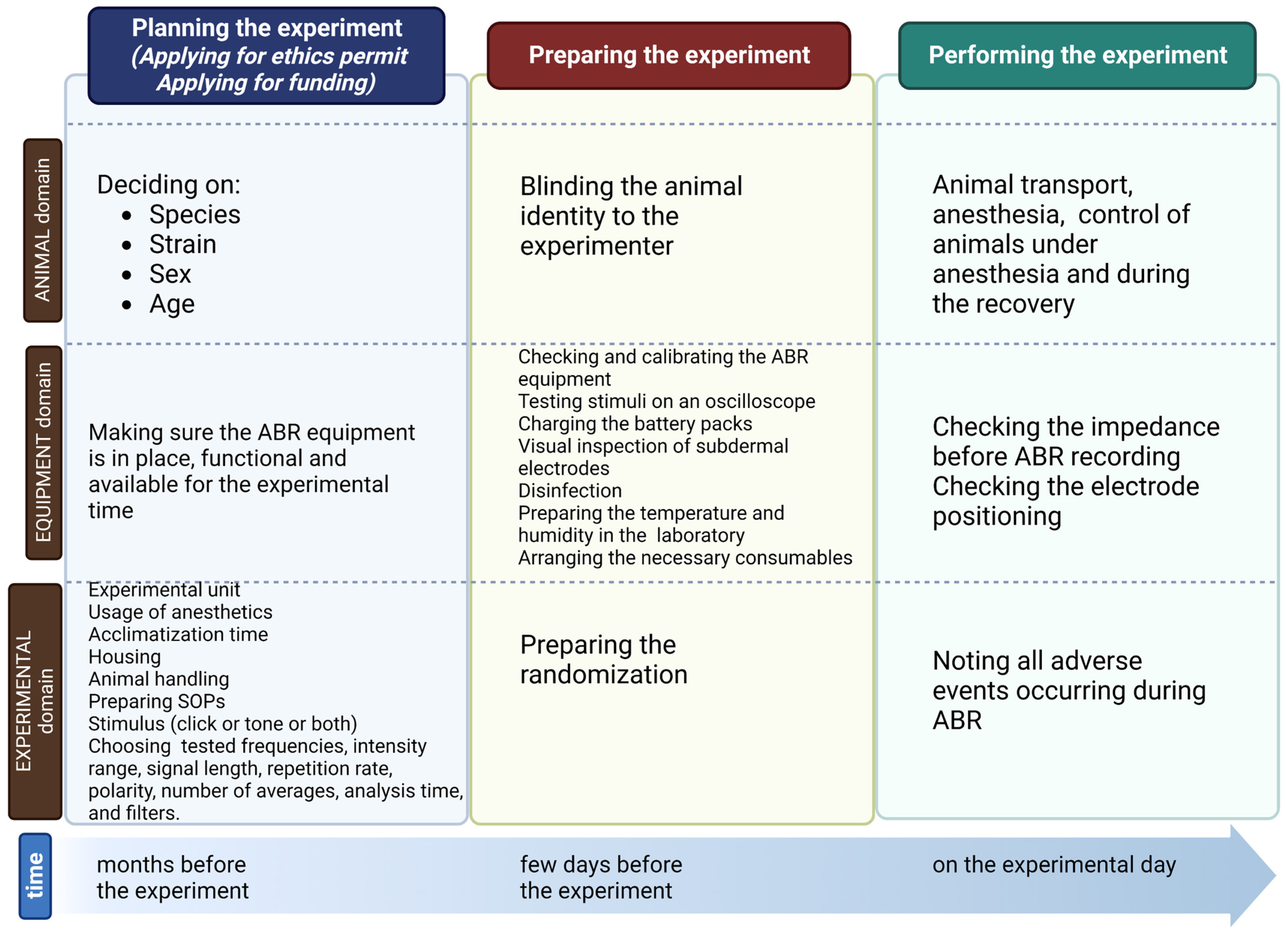
2. Planning the Experiment
2.1. Planning the Experiment: Animals
2.2. Planning the Experiment: Equipment
2.3. Planning the Experiment: Experiment
| Factor | Definition | Influence on ABR Results | Suggestions Based on ABR User Guide |
|---|---|---|---|
| Ramp Number of Cycles (Rise-Plateau-Fall, e.g., 5 ms (2-1-2)) | The number of sinusoidal waves in the rise, plateau, and fall portions of the tone burst’s waveform. Only applicable for tone-burst | An increment in the rise time of the signal stimulus results in elongated absolute latencies [81] | mouse studies: mainly 2.5 ms |
| Repetition rate | Number of stimuli produced per second | Amplitude decrease with an increasing repetition rate of the stimuli—an increase in repetition rate results in an increase in ABR latencies. | 21/s |
| Polarity | Crucial for initial stimulus presentation since it determines the way the sound pressure wave is presented [82] | Three stimulus polarities are used; i.e., rarefaction, condensation, and alternating. The latency of waves I, III, and V are shorter in response to the rarefaction click than the condensation click [83]. | Rarefaction or alternating |
| Number of averages | Impact on the signal-to-noise ratio. The number of averages balances signal quality and minimalization of the time to complete testing. | The typical range of averages: 256–1024 | |
| Analysis time/Recording window | A period following the stimulus is presented to the subject, during which the response is averaged and analyzed | Since decreasing stimulus intensity reduces the amplitude and increases latencies, the analysis time is extended to 15 ms to estimate the hearing threshold. | 10 ms |
| Sampling rate | The average number of samples acquired per second | 12 KHz | |
| Artifact Rejection Threshold | The value defines the lowest level of electrophysiological activity, which contains excessive electric noise. | Clearer ABR response | |
| Filters | Use filters to separate signals based on their frequency, attenuating (reducing in amplitude) the unwanted frequency components and/or emphasizing the features that are important to us [79] | Filters make the presence or absence of the ABR responses more obvious since noise is filtered out. | Highpass filter: 300 Hz Lowpass filter: 3 kHz |
- The SOP describing the handling of animals should be prepared beforehand, and all unnecessary handling should be avoided. A handling tunnel or cupping without restraint in the open hand can minimize the anxiety of mice [102]. It is worth noting that the presence of men in breeding or experimental rooms is stressful for mice [103]. Animal behavior is also influenced by the animals’ familiarity with the personnel involved in the experiment [104]. Importantly, the same breeds of animals purchased from different suppliers may respond to stress in various ways [105].
- Note: Experimenters should not wear scented cosmetics [106].
- Since cage changing is stressful for animals, cleaning cages should be planned in advance [106].
- In addition, social isolation can cause somatic reactions and should be avoided.
- Repeated intraperitoneal injections are also known to stress animals. Attention task performance was similar in rats chronically sham injected and chronically sham injected and restrained [107].
3. Preparing ABR Recordings
3.1. Preparing ABR Recordings: Animals
3.2. Preparing ABR Recordings: Equipment
3.3. Preparing ABR Recordings: Experiment
4. Performing ABR
4.1. Performing ABR: Animals
4.2. Performing ABR: Equipment
4.3. Performing ABR: Experiment
4.4. Protocols
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Szczepek, A.J.; Domarecka, E.; Olze, H. Translational Research in Audiology: Presence in the Literature. Audiol. Res. 2022, 12, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Vlajkovic, S.M.; Chang, H.; Paek, S.Y.; Chi, H.H.; Sreebhavan, S.; Telang, R.S.; Tingle, M.; Housley, G.D.; Thorne, P.R. Adenosine amine congener as a cochlear rescue agent. BioMed Res. Int. 2014, 2014, 841489. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; Chen, K.; Du, X.; Floyd, R.A.; Kopke, R.D. Effects of delayed and extended antioxidant treatment on acute acoustic trauma. Free Radic. Res. 2011, 45, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Ewert, D.; Hu, N.; Du, X.; Li, W.; West, M.B.; Choi, C.H.; Floyd, R.; Kopke, R.D. HPN-07, a free radical spin trapping agent, protects against functional, cellular and electrophysiological changes in the cochlea induced by acute acoustic trauma. PLoS ONE 2017, 12, e0183089. [Google Scholar] [CrossRef]
- Wang, J.; Tian, K.Y.; Fang, Y.; Chang, H.M.; Han, Y.N.; Chen, F.Q. Sulforaphane attenuates cisplatin-induced hearing loss by inhibiting histone deacetylase expression. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211034086. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, J.J. Chapter 30-Auditory brainstem response. In Handbook of Clinical Neurology; Levin, K.H., Chauvel, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 160, pp. 451–464. [Google Scholar]
- Szczepek, A.J.; Dietz, G.P.H.; Reich, U.; Hegend, O.; Olze, H.; Mazurek, B. Differences in Stress-Induced Modulation of the Auditory System Between Wistar and Lewis Rats. Front. Neurosci. 2018, 12, 828. [Google Scholar] [CrossRef]
- Fabiani, M.; Sohmer, H.; Tait, C.; Gafni, M.; Kinarti, R. A functional measure of brain activity: Brain stem transmission time. Electroencephalogr. Clin. Neurophysiol. 1979, 47, 483–491. [Google Scholar] [CrossRef]
- Rupa, V.; Job, A.; George, M.; Rajshekhar, V. Cost-effective initial screening for vestibular schwannoma: Auditory brainstem response or magnetic resonance imaging? Otolaryngol. Head. Neck Surg. 2003, 128, 823–828. [Google Scholar] [CrossRef]
- Ren, W.; Ji, F.; Zeng, J.; Zhao, H. Intra-operative hearing monitoring methods in middle ear surgeries. J. Otol. 2016, 11, 178–184. [Google Scholar] [CrossRef]
- Walsh, E.J.; McGee, J.; Javel, E. Development of auditory-evoked potentials in the cat. I. Onset of response and development of sensitivity. J. Acoust. Soc. Am. 1986, 79, 712–724. [Google Scholar] [CrossRef]
- Walsh, E.J.; McGee, J.; Javel, E. Development of auditory-evoked potentials in the cat. III. Wave amplitudes. J. Acoust. Soc. Am. 1986, 79, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.J.; McGee, J.; Javel, E. Development of auditory-evoked potentials in the cat. II. Wave latencies. J. Acoust. Soc. Am. 1986, 79, 725–744. [Google Scholar] [CrossRef]
- Cederroth, C.R.; Dyhrfjeld-Johnsen, J.; Langguth, B. An update: Emerging drugs for tinnitus. Expert Opin. Emerg. Drugs 2018, 23, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Naert, G.; Pasdelou, M.P.; Le Prell, C.G. Use of the guinea pig in studies on the development and prevention of acquired sensorineural hearing loss, with an emphasis on noise. J. Acoust. Soc. Am. 2019, 146, 3743. [Google Scholar] [CrossRef] [PubMed]
- Ohlemiller, K.K.; Jones, S.M.; Johnson, K.R. Application of Mouse Models to Research in Hearing and Balance. J. Assoc. Res. Otolaryngol. 2016, 17, 493–523. [Google Scholar] [CrossRef]
- Boettcher, F.A. Presbyacusis and the auditory brainstem response. J. Speech Lang. Hear. Res. 2002, 45, 1249–1261. [Google Scholar] [CrossRef]
- Laumen, G.; Ferber, A.T.; Klump, G.M.; Tollin, D.J. The Physiological Basis and Clinical Use of the Binaural Interaction Component of the Auditory Brainstem Response. Ear Hear. 2016, 37, e276–e290. [Google Scholar] [CrossRef]
- Sergeyenko, Y.; Lall, K.; Liberman, M.C.; Kujawa, S.G. Age-related cochlear synaptopathy: An early-onset contributor to auditory functional decline. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 13686–13694. [Google Scholar] [CrossRef]
- Kujawa, S.G.; Liberman, M.C. Adding insult to injury: Cochlear nerve degeneration after “temporary” noise-induced hearing loss. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 14077–14085. [Google Scholar] [CrossRef]
- Bramhall, N.F.; Konrad-Martin, D.; McMillan, G.P.; Griest, S.E. Auditory Brainstem Response Altered in Humans with Noise Exposure Despite Normal Outer Hair Cell Function. Ear Hear. 2017, 38, e1–e12. [Google Scholar] [CrossRef]
- Kujawa, S.G.; Liberman, M.C. Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hear. Res. 2015, 330, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Markand, O.N. Brainstem Auditory Evoked Potentials. In Clinical Evoked Potentials: An. Illustrated Manual; Markand, O.N., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 25–82. [Google Scholar]
- Domarecka, E.; Kalcioglu, M.T.; Mutlu, A.; Özgür, A.; Smit, J.; Olze, H.; Szczepek, A.J. Reporting Data on Auditory Brainstem Responses (ABR) in Rats: Recommendations Based on Review of Experimental Protocols and Literature. Brain Sci. 2021, 11, 1596. [Google Scholar] [CrossRef] [PubMed]
- Domarecka, E.; Olze, H.; Szczepek, A.J. Auditory Brainstem Responses (ABR) of Rats during Experimentally Induced Tinnitus: Literature Review. Brain Sci. 2020, 10, 901. [Google Scholar] [CrossRef]
- Bracken, M.B. Why animal studies are often poor predictors of human reactions to exposure. J. R. Soc. Med. 2009, 102, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Luo, J.; Tan, J.; Yang, L.; Wang, M.; Li, P. Experimental animal models of drug-induced sensorineural hearing loss: A narrative review. Ann. Transl. Med. 2021, 9, 1393. [Google Scholar] [CrossRef] [PubMed]
- Heffner, H.E.; Heffner, R.S. Hearing ranges of laboratory animals. J. Am. Assoc. Lab. Anim. Sci. 2007, 46, 20–22. [Google Scholar]
- Popper, A.N.; Fay, R.R. Comparative Studies of Hearing in Vertebrates; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Koch, L.; Gaese, B.H.; Nowotny, M. Strain Comparison in Rats Differentiates Strain-Specific from More General Correlates of Noise-Induced Hearing Loss and Tinnitus. J. Assoc. Res. Otolaryngol. 2022, 23, 59–73. [Google Scholar] [CrossRef]
- Overbeck, G.W.; Church, M.W. Effects of tone burst frequency and intensity on the auditory brainstem response (ABR) from albino and pigmented rats. Hear. Res. 1992, 59, 129–137. [Google Scholar] [CrossRef]
- Sha, S.H.; Kanicki, A.; Dootz, G.; Talaska, A.E.; Halsey, K.; Dolan, D.; Altschuler, R.; Schacht, J. Age-related auditory pathology in the CBA/J mouse. Hear. Res. 2008, 243, 87–94. [Google Scholar] [CrossRef]
- Zheng, Q.Y.; Johnson, K.R.; Erway, L.C. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear. Res. 1999, 130, 94–107. [Google Scholar] [CrossRef]
- Li, H.S.; Borg, E. Age-related loss of auditory sensitivity in two mouse genotypes. Acta Otolaryngol. 1991, 111, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, H.; Han, X.; Zhao, X.; Hu, F.; Li, P.; Xie, G.; Gao, L.; Cheng, L.; Song, X.; et al. Attenuation of hearing loss in DBA/2J mice by anti-apoptotic treatment. Hear. Res. 2015, 327, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Willott, J.F.; Turner, J.G.; Carlson, S.; Ding, D.; Seegers Bross, L.; Falls, W.A. The BALB/c mouse as an animal model for progressive sensorineural hearing loss. Hear. Res. 1998, 115, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Mannström, P.; Skjönsberg, A.; Järlebark, L.; Ulfendahl, M. Auditory function and cochlear morphology in the German waltzing guinea pig. Hear. Res. 2006, 219, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, T.; Mizuta, K.; Gao, J.; Araki, S.; Araki, K.; Takeshita, T.; Wu, R.; Morita, H. Cochlear findings in the white spotting (Ws) rat. Hear. Res. 2000, 140, 145–156. [Google Scholar] [CrossRef]
- Heid, S.; Hartmann, R.; Klinke, R. A model for prelingual deafness, the congenitally deaf white cat—Population statistics and degenerative changes. Hear. Res. 1998, 115, 101–112. [Google Scholar] [CrossRef]
- Mair, I.W. Hereditary deafness in the dalmatian dog. Arch. Oto-Rhino-Laryngol. 1976, 212, 1–14. [Google Scholar] [CrossRef]
- Xiong, M.; He, Q.; Lai, H.; Wang, J. Oxidative stress in spiral ganglion cells of pigmented and albino guinea pigs exposed to impulse noise. Acta Otolaryngol. 2011, 131, 914–920. [Google Scholar] [CrossRef]
- Murillo-Cuesta, S.; Contreras, J.; Zurita, E.; Cediel, R.; Cantero, M.; Varela-Nieto, I.; Montoliu, L. Melanin precursors prevent premature age-related and noise-induced hearing loss in albino mice. Pigment. Cell Melanoma Res. 2010, 23, 72–83. [Google Scholar] [CrossRef]
- Ohlemiller, K.K.; Rice, M.E.; Lett, J.M.; Gagnon, P.M. Absence of strial melanin coincides with age-associated marginal cell loss and endocochlear potential decline. Hear. Res. 2009, 249, 1–14. [Google Scholar] [CrossRef]
- Loos, M.; Koopmans, B.; Aarts, E.; Maroteaux, G.; van der Sluis, S.; Verhage, M.; Smit, A.B. Sheltering behavior and locomotor activity in 11 genetically diverse common inbred mouse strains using home-cage monitoring. PLoS ONE 2014, 9, e108563. [Google Scholar] [CrossRef] [PubMed]
- Koehl, M.; Battle, S.E.; Turek, F.W. Sleep in female mice: A strain comparison across the estrous cycle. Sleep 2003, 26, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Hamilton, E.; McNamara, E.; Smith, P.F.; Darlington, C.L. The effects of chronic tinnitus caused by acoustic trauma on social behaviour and anxiety in rats. Neuroscience 2011, 193, 143–153. [Google Scholar] [CrossRef]
- Heinla, I.; Åhlgren, J.; Vasar, E.; Voikar, V. Behavioural characterization of C57BL/6N and BALB/c female mice in social home cage—Effect of mixed housing in complex environment. Physiol. Behav. 2018, 188, 32–41. [Google Scholar] [CrossRef]
- Karp, N.A.; Reavey, N. Sex bias in preclinical research and an exploration of how to change the status quo. Br. J. Pharmacol. 2019, 176, 4107–4118. [Google Scholar] [CrossRef]
- Souza, D.D.S.; Luckwu, B.; Andrade, W.T.L.; Pessoa, L.S.F.; Nascimento, J.A.D.; Rosa, M. Variation in the Hearing Threshold in Women during the Menstrual Cycle. Int. Arch. Otorhinolaryngol. 2017, 21, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.D. Sex and racial differences in pharmacological response: Where is the evidence? Pharmacogenetics, pharmacokinetics, and pharmacodynamics. J. Womens Health 2005, 14, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Marcondes, F.K.; Bianchi, F.J.; Tanno, A.P. Determination of the estrous cycle phases of rats: Some helpful considerations. Braz. J. Biol. 2002, 62, 609–614. [Google Scholar] [CrossRef]
- Balogová, Z.; Popelář, J.; Chiumenti, F.; Chumak, T.; Burianová, J.S.; Rybalko, N.; Syka, J. Age-Related Differences in Hearing Function and Cochlear Morphology between Male and Female Fischer 344 Rats. Front. Aging Neurosci. 2017, 9, 428. [Google Scholar] [CrossRef]
- Hunter, K.P.; Willott, J.F. Aging and the auditory brainstem response in mice with severe or minimal presbycusis. Hear. Res. 1987, 30, 207–218. [Google Scholar] [CrossRef]
- Virgen-Ortiz, A.; Apolinar-Iribe, A.; Muñiz, J. Gender-effect on the contractile properties of skeletal muscle in streptozotocin-induced diabetic rats. J. Musculoskelet. Neuronal Interact. 2018, 18, 255–261. [Google Scholar] [PubMed]
- Quirós Cognuck, S.; Reis, W.L.; Silva, M.; Debarba, L.K.; Mecawi, A.S.; de Paula, F.J.A.; Rodrigues Franci, C.; Elias, L.L.K.; Antunes-Rodrigues, J. Sex differences in body composition, metabolism-related hormones, and energy homeostasis during aging in Wistar rats. Physiol. Rep. 2020, 8, e14597. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Gajbhiye, A.; Lyu, A.R.; Kim, T.H.; Shin, S.A.; Kwon, H.C.; Park, Y.H.; Park, M.J. Sex differences in hearing impairment due to diet-induced obesity in CBA/Ca mice. Biol. Sex. Differ. 2023, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Petrofsky, J.; Schwab, E. A re-evaluation of modelling of the current flow between electrodes: Consideration of blood flow and wounds. J. Med. Eng. Technol. 2007, 31, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Henry, K.R. Influence of genotype and age on noise-induced auditory losses. Behav. Genet. 1982, 12, 563–573. [Google Scholar] [CrossRef]
- Henry, K.R. Lifelong susceptibility to acoustic trauma: Changing patterns of cochlear damage over the life span of the mouse. Audiology 1983, 22, 372–383. [Google Scholar] [CrossRef]
- Ohlemiller, K.K.; Wright, J.S.; Heidbreder, A.F. Vulnerability to noise-induced hearing loss in ‘middle-aged’ and young adult mice: A dose-response approach in CBA, C57BL, and BALB inbred strains. Hear. Res. 2000, 149, 239–247. [Google Scholar] [CrossRef]
- Henry, K.R.; Chole, R.A.; McGinn, M.D.; Frush, D.P. Increased ototoxicity in both young and old mice. Arch. Otolaryngol. 1981, 107, 92–95. [Google Scholar] [CrossRef]
- Prieve, B.A.; Yanz, J.L. Age-dependent changes in susceptibility to ototoxic hearing loss. Acta Otolaryngol. 1984, 98, 428–438. [Google Scholar] [CrossRef]
- Bielefeld, E.C.; Gonzalez, A.; DeBacker, J.R. Changing the time intervals between cisplatin cycles alter its ototoxic side effect. Hear. Res. 2021, 404, 108204. [Google Scholar] [CrossRef]
- Soulban, G.; Smolensky, M.H.; Yonovitz, A. Gentamicin-induced chronotoxicity: Use of body temperature as a circadian marker rhythm. Chronobiol. Int. 1990, 7, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Yonovitz, A.; Fisch, J.E. Circadian rhythm dependent kanamycin-induced hearing loss in rodents assessed by auditory brainstem responses. Acta Otolaryngol. 1991, 111, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Festing, M.F.; Altman, D.G. Guidelines for the design and statistical analysis of experiments using laboratory animals. Ilar. J. 2002, 43, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef]
- Mead, R. The Design of Experiments: Statistical Principles for Practical Applications; Cambridge University Press: New York, NY, USA, 1988; 620p. [Google Scholar]
- Ricci, C.; Baumgartner, J.; Malan, L.; Smuts, C.M. Determining sample size adequacy for animal model studies in nutrition research: Limits and ethical challenges of ordinary power calculation procedures. Int. J. Food Sci. Nutr. 2020, 71, 256–264. [Google Scholar] [CrossRef]
- Ordiway, G.; McDonnell, M.; Mohan, S.; Sanchez, J.T. Evaluation of Auditory Brainstem Response in Chicken Hatchlings. J. Vis. Exp. 2022, 182, e63477. [Google Scholar] [CrossRef]
- Kim, Y.H.; Schrode, K.M.; Lauer, A.M. Auditory brainstem response (ABR) measurements in small mammals. In Developmental, Physiological, and Functional Neurobiology of the Inner Ear; Humana: New York, NY, USA, 2022; pp. 357–375. [Google Scholar]
- Lundt, A.; Soos, J.; Henseler, C.; Arshaad, M.I.; Müller, R.; Ehninger, D.; Hescheler, J.; Sachinidis, A.; Broich, K.; Wormuth, C.; et al. Data Acquisition and Analysis in Brainstem Evoked Response Audiometry in Mice. J. Vis. Exp. 2019, 147, e59200. [Google Scholar] [CrossRef]
- Tucker-Davis-Technologies. ABR User Guide. Available online: https://www.tdt.com/files/manuals/ABRGuide.pdf (accessed on 9 February 2023).
- Lanaia, V.; Tziridis, K.; Schulze, H. Salicylate-Induced Changes in Hearing Thresholds in Mongolian Gerbils Are Correlated with Tinnitus Frequency but Not with Tinnitus Strength. Front. Behav. Neurosci. 2021, 15, 698516. [Google Scholar] [CrossRef]
- Moller, A.R. Hearing: Anatomy, Physiology, and Disorders of the Auditory System; Elsevier Science: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Melcher, J.R.; Knudson, I.M.; Fullerton, B.C.; Guinan, J.J., Jr.; Norris, B.E.; Kiang, N.Y. Generators of the brainstem auditory evoked potential in cat. I. An experimental approach to their identification. Hear. Res. 1996, 93, 1–27. [Google Scholar] [CrossRef]
- Canale, A.; Dagna, F.; Lacilla, M.; Piumetto, E.; Albera, R. Relationship between pure tone audiometry and tone burst auditory brainstem response at low frequencies gated with Blackman window. Eur. Arch. Oto-Rhino-Laryngol. 2012, 269, 781–785. [Google Scholar] [CrossRef]
- Burkard, R.; Secor, C. Overview of auditory evoked potentials. In Handbook of Clinical Audiology; Julet, T.L., Ed.; Lippincott Williams and Wilkins: Baltimore, MD, USA, 2002; pp. 233–248. [Google Scholar]
- Burgess, R.C. Filtering of neurophysiologic signals. Handb. Clin. Neurol. 2019, 160, 51–65. [Google Scholar] [CrossRef]
- De Cheveigné, A.; Nelken, I. Filters: When, Why, and How (Not) to Use Them. Neuron 2019, 102, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Fausti, S.A.; Gray, P.S.; Frey, R.H.; Mitchell, C.R. Rise time and center-frequency effects on auditory brainstem responses to high-frequency tone bursts. J. Am. Acad. Audiol. 1991, 2, 24–31. [Google Scholar] [PubMed]
- Hall, J.W. Handbook of Auditory Evoked Responses; Allyn & Bacon: Boston, MA, USA, 1992. [Google Scholar]
- Fowler, C.G. Effects of stimulus phase on the normal auditory brainstem response. J. Speech Hear. Res. 1992, 35, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Singer, W.; Zuccotti, A.; Jaumann, M.; Lee, S.C.; Panford-Walsh, R.; Xiong, H.; Zimmermann, U.; Franz, C.; Geisler, H.S.; Köpschall, I.; et al. Noise-induced inner hair cell ribbon loss disturbs central arc mobilization: A novel molecular paradigm for understanding tinnitus. Mol. Neurobiol. 2013, 47, 261–279. [Google Scholar] [CrossRef]
- Bing, D.; Lee, S.C.; Campanelli, D.; Xiong, H.; Matsumoto, M.; Panford-Walsh, R.; Wolpert, S.; Praetorius, M.; Zimmermann, U.; Chu, H.; et al. Cochlear NMDA receptors as a therapeutic target of noise-induced tinnitus. Cell. Physiol. Biochem. 2015, 35, 1905–1923. [Google Scholar] [CrossRef]
- Cederroth, C.; Gachon, F.; Canlon, B. Time to listen: Circadian impact on auditory research. Curr. Opin. Physiol. 2020, 18, 95–99. [Google Scholar] [CrossRef]
- Greenfield, E.A. Administering Anesthesia to Mice, Rats, and Hamsters. Cold Spring Harb. Protoc. 2019, 2019, 457–459. [Google Scholar] [CrossRef]
- Xu, Q.; Ming, Z.; Dart, A.M.; Du, X.J. Optimizing dosage of ketamine and xylazine in murine echocardiography. Clin. Exp. Pharmacol. Physiol. 2007, 34, 499–507. [Google Scholar] [CrossRef]
- Albrecht, M.; Henke, J.; Tacke, S.; Markert, M.; Guth, B. Effects of isoflurane, ketamine-xylazine and a combination of medetomidine, midazolam and fentanyl on physiological variables continuously measured by telemetry in Wistar rats. BMC Vet. Res. 2014, 10, 198. [Google Scholar] [CrossRef]
- Turner, P.V.; Albassam, M.A. Susceptibility of rats to corneal lesions after injectable anesthesia. Comp. Med. 2005, 55, 175–182. [Google Scholar] [PubMed]
- Turner, J.G.; Larsen, D. Effects of noise exposure on development of tinnitus and hyperacusis: Prevalence rates 12 months after exposure in middle-aged rats. Hear. Res. 2016, 334, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Pace, E.; Lepczyk, L.; Kaufman, M.; Zhang, J.; Perrine, S.A.; Zhang, J. Blast-Induced Tinnitus and Elevated Central Auditory and Limbic Activity in Rats: A Manganese-Enhanced MRI and Behavioral Study. Sci. Rep. 2017, 7, 4852. [Google Scholar] [CrossRef] [PubMed]
- Ritschl, L.M.; Fichter, A.M.; Häberle, S.; von Bomhard, A.; Mitchell, D.A.; Wolff, K.D.; Mücke, T. Ketamine-Xylazine Anesthesia in Rats: Intraperitoneal versus Intravenous Administration Using a Microsurgical Femoral Vein Access. J. Reconstr. Microsurg. 2015, 31, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Gaines Das, R.; North, D. Implications of experimental technique for analysis and interpretation of data from animal experiments: Outliers and increased variability resulting from failure of intraperitoneal injection procedures. Lab. Anim. 2007, 41, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Laferriere, C.A.; Pang, D.S. Review of Intraperitoneal Injection of Sodium Pentobarbital as a Method of Euthanasia in Laboratory Rodents. J. Am. Assoc. Lab. Anim. Sci. 2020, 59, 254–263. [Google Scholar] [CrossRef]
- Gargiulo, S.; Greco, A.; Gramanzini, M.; Esposito, S.; Affuso, A.; Brunetti, A.; Vesce, G. Mice anesthesia, analgesia, and care, Part I: Anesthetic considerations in preclinical research. Ilar. J. 2012, 53, E55–E69. [Google Scholar] [CrossRef]
- Smiler, K.L.; Stein, S.; Hrapkiewicz, K.L.; Hiben, J.R. Tissue response to intramuscular and intraperitoneal injections of ketamine and xylazine in rats. Lab. Anim. Sci. 1990, 40, 60–64. [Google Scholar]
- Vogler, G.A. Chapter 5—Anesthesia Delivery Systems. In Anesthesia and Analgesia in Laboratory Animals, 2nd ed.; Fish, R.E., Brown, M.J., Danneman, P.J., Karas, A.Z., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 127–169. [Google Scholar]
- Obernier, J.A.; Baldwin, R.L. Establishing an appropriate period of acclimatization following transportation of laboratory animals. Ilar. J. 2006, 47, 364–369. [Google Scholar] [CrossRef]
- Milligan, S.R.; Sales, G.D.; Khirnykh, K. Sound levels in rooms housing laboratory animals: An uncontrolled daily variable. Physiol. Behav. 1993, 53, 1067–1076. [Google Scholar] [CrossRef]
- Lauer, A.M.; Larkin, G.; Jones, A.; May, B.J. Behavioral Animal Model of the Emotional Response to Tinnitus and Hearing Loss. J. Assoc. Res. Otolaryngol. 2018, 19, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, K.; Hurst, J.L. Optimising reliability of mouse performance in behavioural testing: The major role of non-aversive handling. Sci. Rep. 2017, 7, 44999. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, P.; Zanos, P.; Mou, T.M.; An, X.; Gerhard, D.M.; Dryanovski, D.I.; Potter, L.E.; Highland, J.N.; Jenne, C.E.; Stewart, B.W.; et al. Experimenters’ sex modulates mouse behaviors and neural responses to ketamine via corticotropin releasing factor. Nat. Neurosci. 2022, 25, 1191–1200. [Google Scholar] [CrossRef]
- Van Driel, K.S.; Talling, J.C. Familiarity increases consistency in animal tests. Behav. Brain Res. 2005, 159, 243–245. [Google Scholar] [CrossRef]
- Theilmann, W.; Kleimann, A.; Rhein, M.; Bleich, S.; Frieling, H.; Löscher, W.; Brandt, C. Behavioral differences of male Wistar rats from different vendors in vulnerability and resilience to chronic mild stress are reflected in epigenetic regulation and expression of p11. Brain Res. 2016, 1642, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Castelhano-Carlos, M.J.; Baumans, V. The impact of light, noise, cage cleaning and in-house transport on welfare and stress of laboratory rats. Lab. Anim. 2009, 43, 311–327. [Google Scholar] [CrossRef]
- Pérez-Valenzuela, C.; Gárate-Pérez, M.F.; Sotomayor-Zárate, R.; Delano, P.H.; Dagnino-Subiabre, A. Reboxetine Improves Auditory Attention and Increases Norepinephrine Levels in the Auditory Cortex of Chronically Stressed Rats. Front. Neural Circuits 2016, 10, 108. [Google Scholar] [CrossRef]
- Karp, N.A.; Pearl, E.J.; Stringer, E.J.; Barkus, C.; Ulrichsen, J.C.; Percie du Sert, N. A qualitative study of the barriers to using blinding in in vivo experiments and suggestions for improvement. PLoS Biol. 2022, 20, e3001873. [Google Scholar] [CrossRef]
- Schrode, K.M.; Dent, M.L.; Lauer, A.M. Sources of variability in auditory brainstem response thresholds in a mouse model of noise-induced hearing loss. J. Acoust. Soc. Am. 2022, 152, 3576. [Google Scholar] [CrossRef]
- Available online: https://www.tdt.com/docs/abr-user-guide/troubleshooting/#determining-the-noise-floor (accessed on 15 April 2023).
- Van Eenige, R.; Verhave, P.S.; Koemans, P.J.; Tiebosch, I.; Rensen, P.C.N.; Kooijman, S. RandoMice, a novel, user-friendly randomization tool in animal research. PLoS ONE 2020, 15, e0237096. [Google Scholar] [CrossRef]
- Available online: https://www.gv-solas.de/wp-content/uploads/2021/08/2020_10Food_withdrawal.pdf (accessed on 24 March 2023).
- Varughese, S.; Ahmed, R. Environmental and Occupational Considerations of Anesthesia: A Narrative Review and Update. Anesth. Analg. 2021, 133, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Akil, O.; Oursler, A.E.; Fan, K.; Lustig, L.R. Mouse Auditory Brainstem Response Testing. Bio Protoc. 2016, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Navarro, K.L.; Huss, M.; Smith, J.C.; Sharp, P.; Marx, J.O.; Pacharinsak, C. Mouse Anesthesia: The Art and Science. Ilar. J. 2021, 62, 238–273. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.T.; Britt, R.H. Effects of hypothermia on the cat brain-stem auditory evoked response. Electroencephalogr. Clin. Neurophysiol. 1984, 57, 143–155. [Google Scholar] [CrossRef]
- Jones, T.A.; Stockard, J.J.; Weidner, W.J. The effects of temperature and acute alcohol intoxication on brain stem auditory evoked potentials in the cat. Electroencephalogr. Clin. Neurophysiol. 1980, 49, 23–30. [Google Scholar] [CrossRef]
- Terkildsen, K.; Osterhammel, P. The influence of reference electrode position on recordings of the auditory brainstem responses. Ear Hear. 1981, 2, 9–14. [Google Scholar] [CrossRef]
- Manta, O.; Sarafidis, M.; Vasileiou, N.; Schlee, W.; Consoulas, C.; Kikidis, D.; Vassou, E.; Matsopoulos, G.K.; Koutsouris, D.D. Development and Evaluation of Automated Tools for Auditory-Brainstem and Middle-Auditory Evoked Potentials Waves Detection and Annotation. Brain Sci. 2022, 12, 1675. [Google Scholar] [CrossRef]
- Bogaerts, S.; Clements, J.D.; Sullivan, J.M.; Oleskevich, S. Automated threshold detection for auditory brainstem responses: Comparison with visual estimation in a stem cell transplantation study. BMC Neurosci. 2009, 10, 104. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A. Room temperature in scientific protocols and experiments should be defined: A reproducibility issue. Biotechniques 2021, 70, 306–308. [Google Scholar] [CrossRef]
- Willott, J.F. Measurement of the auditory brainstem response (ABR) to study auditory sensitivity in mice. Curr. Protoc. Neurosci. 2006, Chapter 8, Unit8.21B. [Google Scholar] [CrossRef]
- Ingham, N.J.; Pearson, S.; Steel, K.P. Using the Auditory Brainstem Response (ABR) to Determine Sensitivity of Hearing in Mutant Mice. Curr. Protoc. Mouse Biol. 2011, 1, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zhou, W.; Bing, D.; Xie, L.; Wang, X.; Zhang, G. Protocol for assessing auditory brainstem response in mice using a four-channel recording system. STAR Protoc. 2022, 3, 101251. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Scudamore, C.L.; Soilleux, E.J.; Karp, N.A.; Smith, K.; Poulsom, R.; Herrington, C.S.; Day, M.J.; Brayton, C.F.; Bolon, B.; Whitelaw, B.; et al. Recommendations for minimum information for publication of experimental pathology data: MINPEPA guidelines. J. Pathol. 2016, 238, 359–367. [Google Scholar] [CrossRef] [PubMed]
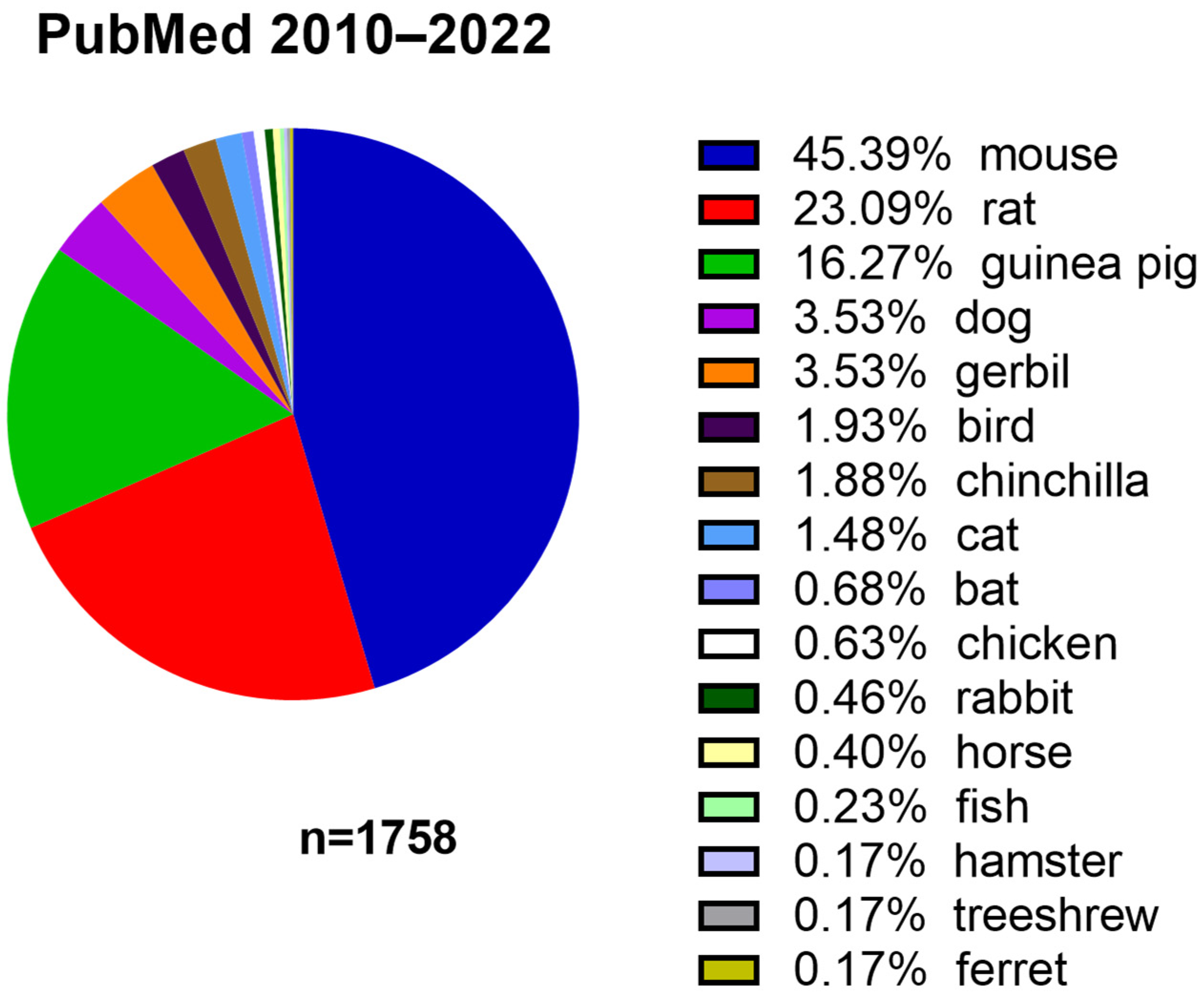

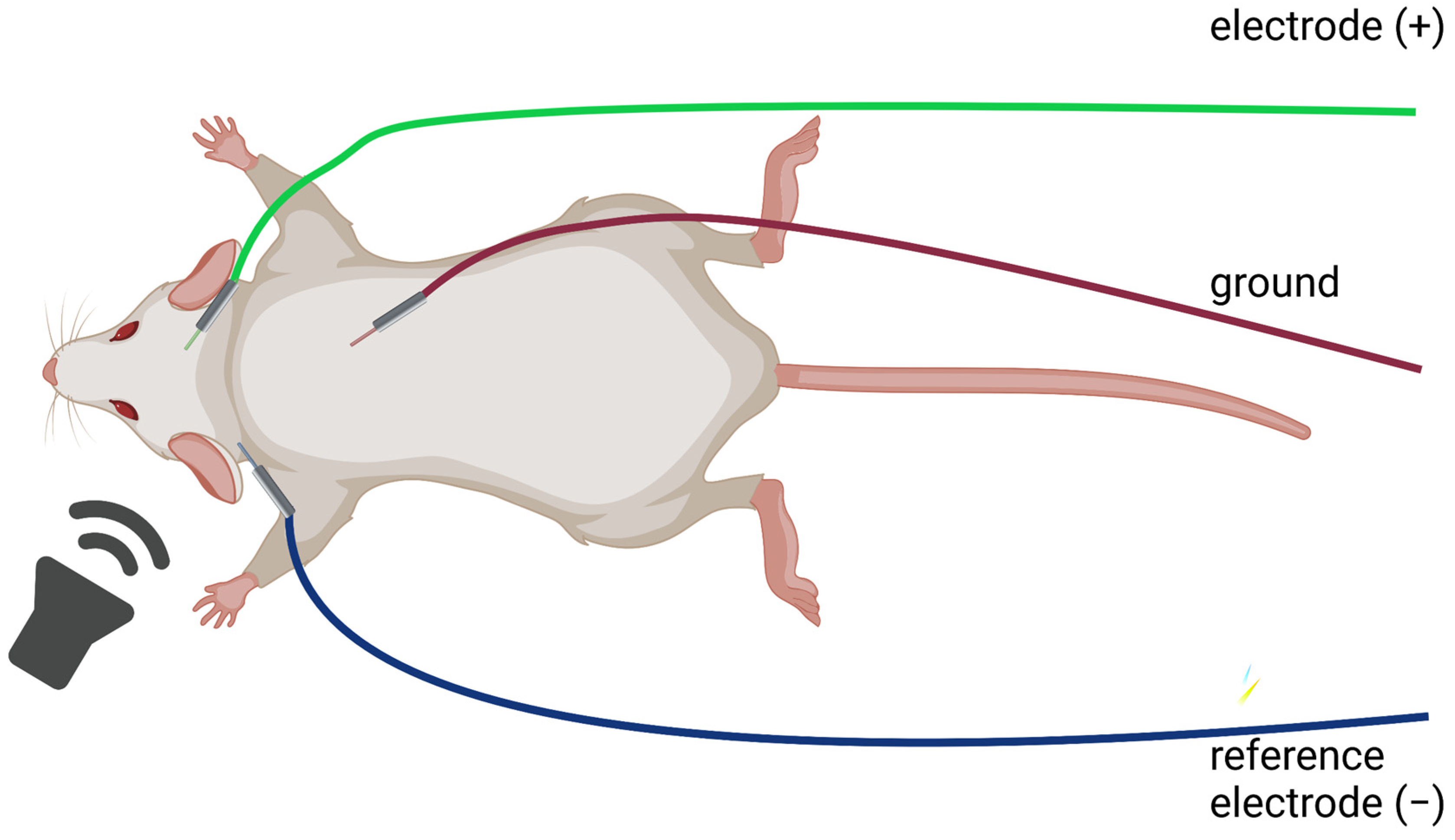
| Mouse Strain | Onset of ARHL |
|---|---|
| C57Bl/6J | 6 months [34] |
| CBA/J | 20 months [32] |
| DBA/2J | 3 weeks [35] |
| Balb/C | 10 months [36] |
| Anesthetic | Sedation [73] | Drawbacks |
|---|---|---|
| Xylazine + Ketamine (i.p., i.m.) | Last ~45 min, the animal is awake after ~90 min from the initial injection. In male Wistar rats, complete sedation occurs in 10 min [89] | Requires proper restraining; rats anesthetized with this drug are more likely to develop corneal lesions than rats anesthetized with isoflurane, which is essential for long-term studies [90]. |
| Isoflurane (inhalation) | Fast-acting, short-acting inhalation agent; the animal is usually fully sedated within 4–5 min. When the gas is removed, the animal wakes up very quickly. | Long-Evans rats anesthetized with isoflurane had higher hearing thresholds than rats anesthetized with ketamine/xylazine. Both click and tone thresholds were elevated, and the ABR response was worse [91,92]. |
| Title | Publication Year | Species | Ref. |
|---|---|---|---|
| Measurement of the auditory brainstem response (ABR) to study auditory sensitivity in mice | 2006 | mice | [122] |
| Using the Auditory Brainstem Response (ABR) to Determine Sensitivity of Hearing in Mutant Mice | 2011 | mice | [123] |
| Mouse Auditory Brainstem Response Testing | 2016 | mice | [114] |
| Data Acquisition and Analysis In Brainstem Evoked Response Audiometry In Mice | 2019 | mice | [72] |
| Protocol for assessing auditory brainstem response in mice using a four-channel recording system | 2022 | mice | [124] |
| Auditory brainstem response (ABR) measurements in small mammals,” in Developmental, Physiological, and Functional Neurobiology of the Inner Ear | 2022 | mice (suggested application also for rats, hamsters, and bats) | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domarecka, E.; Szczepek, A.J. Universal Recommendations on Planning and Performing the Auditory Brainstem Responses (ABR) with a Focus on Mice and Rats. Audiol. Res. 2023, 13, 441-458. https://doi.org/10.3390/audiolres13030039
Domarecka E, Szczepek AJ. Universal Recommendations on Planning and Performing the Auditory Brainstem Responses (ABR) with a Focus on Mice and Rats. Audiology Research. 2023; 13(3):441-458. https://doi.org/10.3390/audiolres13030039
Chicago/Turabian StyleDomarecka, Ewa, and Agnieszka J. Szczepek. 2023. "Universal Recommendations on Planning and Performing the Auditory Brainstem Responses (ABR) with a Focus on Mice and Rats" Audiology Research 13, no. 3: 441-458. https://doi.org/10.3390/audiolres13030039
APA StyleDomarecka, E., & Szczepek, A. J. (2023). Universal Recommendations on Planning and Performing the Auditory Brainstem Responses (ABR) with a Focus on Mice and Rats. Audiology Research, 13(3), 441-458. https://doi.org/10.3390/audiolres13030039







