Cochlear Aqueduct Morphology in Superior Canal Dehiscence Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Source
2.2. Superior Canal Pathology and SCDS Symptom Classification
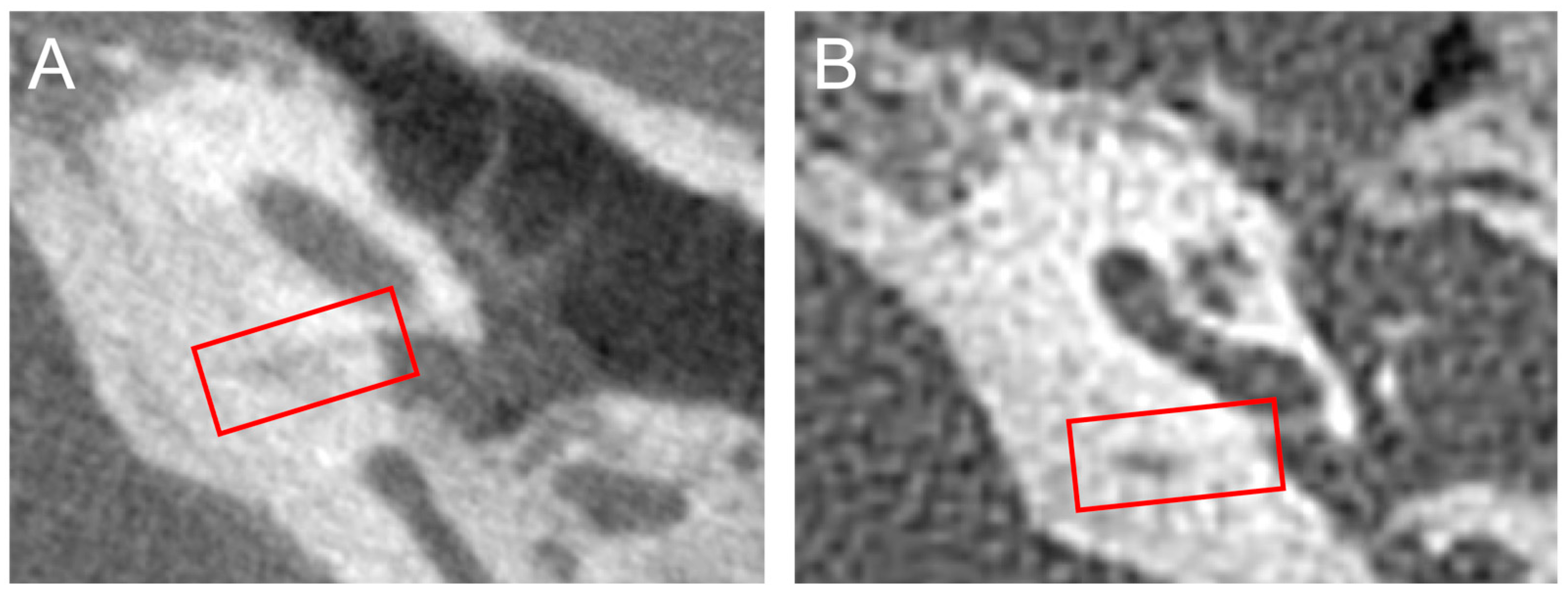
2.3. Cochlear Aqueduct Morphology
2.4. Statistical Methods
3. Results
3.1. Demographics
3.2. CA Morphology and Superior Canal Pathology Classification
3.3. CA Morphology and SCDS Symptoms in SCDS Ears
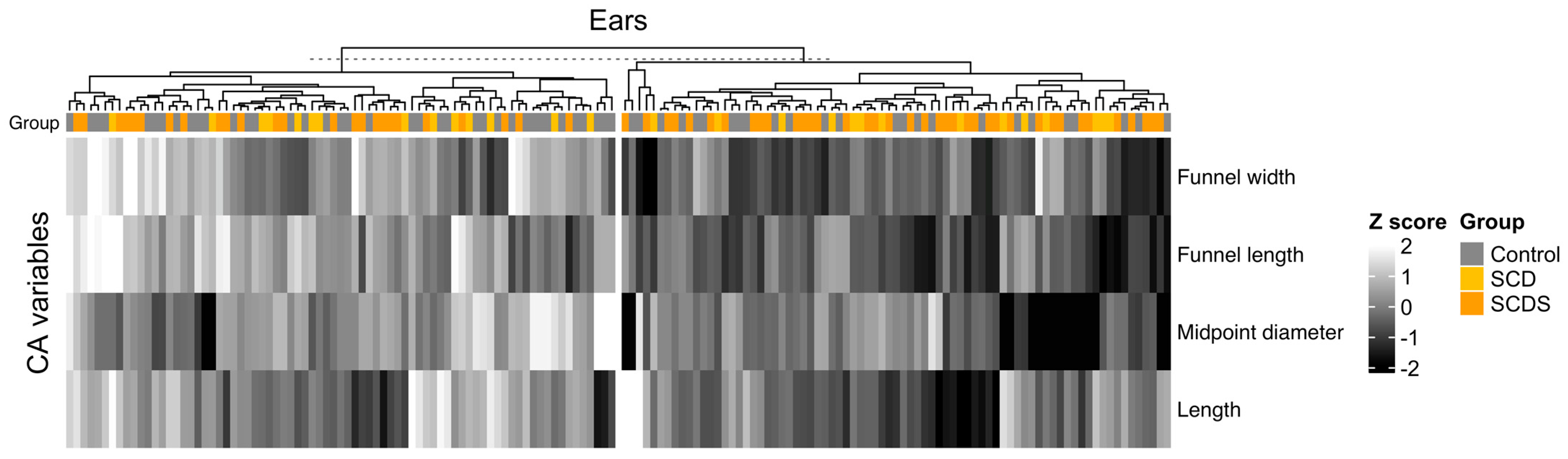
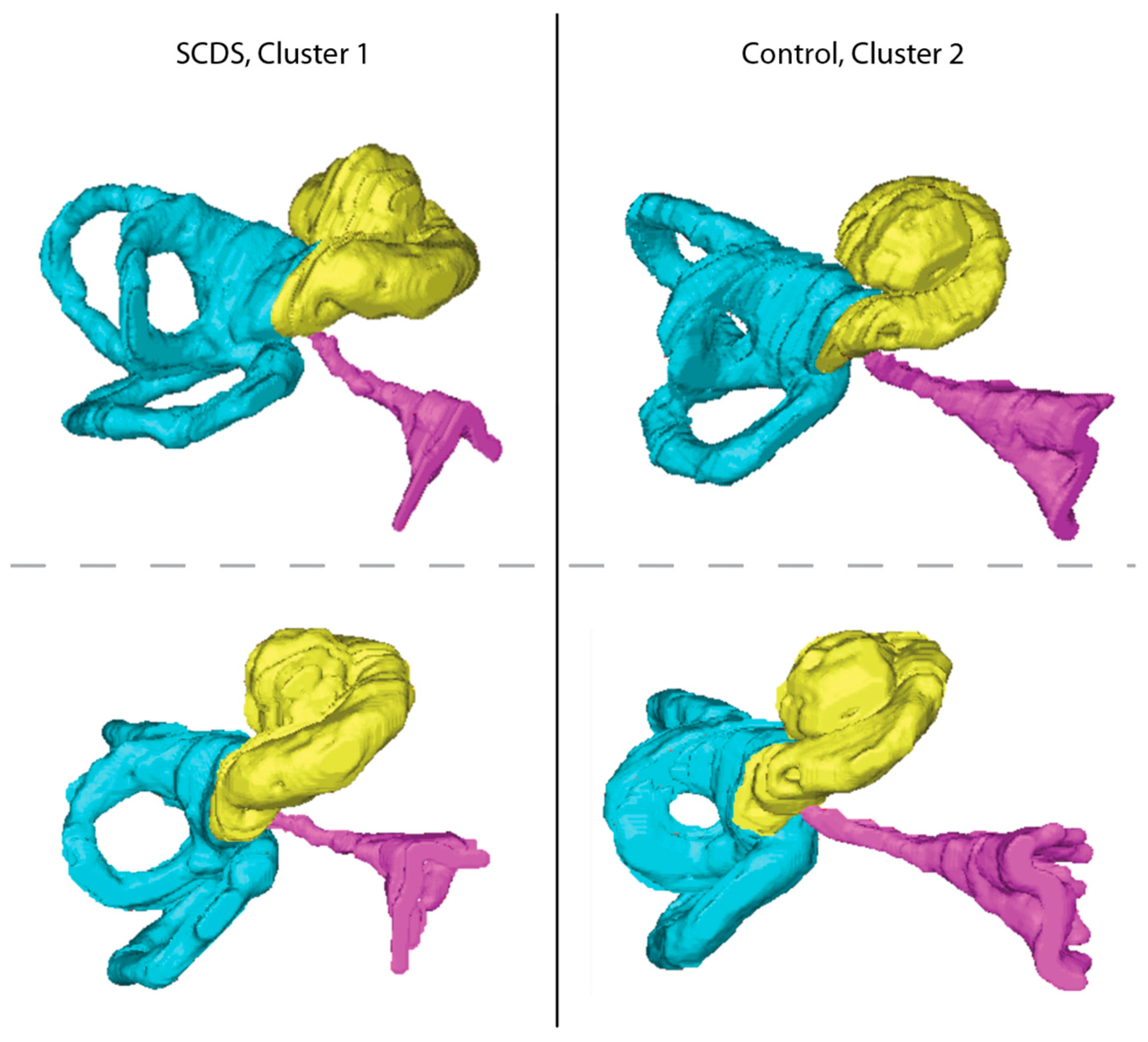
3.4. Clustering of Ears by CA Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lempert, J.; Meltzer, P.E.; Wever, E.G.; Lawrence, M.; Rambo, J.H.T. Structure and Function of the Cochlear Aqueduct. AMA Arch. Otolaryngol. 1952, 55, 134–145. [Google Scholar] [CrossRef]
- Carlborg, B.I.; Farmer, J.C. Transmission of Cerebrospinal Fluid Pressure via the Cochlear Aqueduct and Endolymphatic Sac. Am. J. Otolaryngol. 1983, 4, 273–282. [Google Scholar] [CrossRef]
- Gopen, Q.; Rosowski, J.J.; Merchant, S.N. Anatomy of the Normal Human Cochlear Aqueduct with Functional Implications. Hear. Res. 1997, 107, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Stenfelt, S. Inner Ear Contribution to Bone Conduction Hearing in the Human. Hear. Res. 2015, 329, 41–51. [Google Scholar] [CrossRef]
- Ward, B.K.; Carey, J.P.; Minor, L.B. Superior Canal Dehiscence Syndrome: Lessons from the First 20 Years. Front. Neurol. 2017, 8, 177. [Google Scholar] [CrossRef] [PubMed]
- Minor, L.B.; Solomon, D.; Zinreich, J.S.; Zee, D.S. Sound- and/or Pressure-Induced Vertigo Due to Bone Dehiscence of the Superior Semicircular Canal. Arch. Otolaryngol. Head Neck Surg. 1998, 124, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Naert, L.; Van de Berg, R.; Van de Heyning, P.; Bisdorff, A.; Sharon, J.D.; Ward, B.K.; Van Rompaey, V. Aggregating the Symptoms of Superior Semicircular Canal Dehiscence Syndrome. Laryngoscope 2018, 128, 1932–1938. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.K.; van de Berg, R.; van Rompaey, V.; Bisdorff, A.; Hullar, T.E.; Welgampola, M.S.; Carey, J.P. Superior Semicircular Canal Dehiscence Syndrome: Diagnostic Criteria Consensus Document of the Committee for the Classification of Vestibular Disorders of the Bárány Society. J. Vestib. Res. 2021, 31, 131–141. [Google Scholar] [CrossRef]
- Carey, J.P.; Minor, L.B.; Nager, G.T. Dehiscence or Thinning of Bone Overlying the Superior Semicircular Canal in a Temporal Bone Survey. Arch. Otolaryngol. Head Neck Surg. 2000, 126, 137–147. [Google Scholar] [CrossRef]
- Hegemann, S.C.A.; Carey, J.P. Is Superior Canal Dehiscence Congenital or Acquired? A Case Report and Review of the Literature. Otolaryngol. Clin. N. Am. 2011, 44, 377–382. [Google Scholar] [CrossRef]
- Tunkel, A.E.; Carey, J.P.; Pearl, M. Flat Panel Computed Tomography in the Diagnosis of Superior Semicircular Canal Dehiscence Syndrome. Otol. Neurotol. 2019, 40, 213. [Google Scholar] [CrossRef]
- Wichova, H.; Alvi, S.; Boatright, C.; Ledbetter, L.; Staecker, H.; Lin, J. High-Resolution Computed Tomography of the Inner Ear: Effect of Otosclerosis on Cochlear Aqueduct Dimensions. Ann. Otol. Rhinol. Laryngol. 2019, 128, 749–754. [Google Scholar] [CrossRef]
- Migirov, L.; Kronenberg, J. Radiology of the Cochlear Aqueduct. Ann. Otol. Rhinol. Laryngol. 2005, 114, 863–866. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Pearson, K. On the Criterion That a given System of Deviations from the Probable in the Case of a Correlated System of Variables Is Such That It Can Be Reasonably Supposed to Have Arisen from Random Sampling. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1900, 50, 157–175. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Fisher, R.A. Statistical Methods for Research Workers. In Breakthroughs in Statistics: Methodology and Distribution; Kotz, S., Johnson, N.L., Eds.; Springer Series in Statistics; Springer: New York, NY, USA, 1992; pp. 66–70. ISBN 978-1-4612-4380-9. [Google Scholar]
- Zar, J.H. Multisample Hypotheses and the Analysis of Variance. In Biostatistical Analysis; Prentice-Hall/Pearson: Upper Saddle River, NJ, USA, 2010; pp. 189–224. ISBN 978-0-275-96740-6. [Google Scholar]
- Köhn, H.-F.; Hubert, L.J. Hierarchical Cluster Analysis. In Wiley StatsRef: Statistics Reference Online; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 1–13. ISBN 978-1-118-44511-2. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Zar, J.H. Logistic Regression. In Biostatistical Analysis; Prentice-Hall/Pearson: Upper Saddle River, NJ, USA, 2010; pp. 577–584. ISBN 978-0-275-96740-6. [Google Scholar]
- Jackler, R.K.; Hwang, P.H. Enlargement of the Cochlear Aqueduct: Fact or Fiction? Otolaryngol. Head Neck Surg. 1993, 109, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Stenfelt, S. Investigation of Mechanisms in Bone Conduction Hyperacusis With Third Window Pathologies Based on Model Predictions. Front. Neurol. 2020, 11, 966. [Google Scholar] [CrossRef]
- Stenfelt, S. Model Predictions for Bone Conduction Perception in the Human. Hear. Res. 2016, 340, 135–143. [Google Scholar] [CrossRef]
- Baxter, M.; McCorkle, C.; Trevino Guajardo, C.; Geraldine Zuniga, M.; Carter, A.; Della Santina, C.C.; Minor, L.B.; Carey, J.P.; Ward, B.K. Clinical and Physiologic Predictors and Post Operative Outcomes of Near Dehiscence Syndrome. Otol. Neurotol. 2019, 40, 204–212. [Google Scholar] [CrossRef]
- Iversen, M.M.; Rabbitt, R.D. Biomechanics of Third Window Syndrome. Front. Neurol. 2020, 11, 891. [Google Scholar] [CrossRef] [PubMed]
- Iversen, M.M.; Zhu, H.; Zhou, W.; Della Santina, C.C.; Carey, J.P.; Rabbitt, R.D. Sound Abnormally Stimulates the Vestibular System in Canal Dehiscence Syndrome by Generating Pathological Fluid-Mechanical Waves. Sci. Rep. 2018, 8, 10257. [Google Scholar] [CrossRef] [PubMed]
- Yew, A.; Zarinkhou, G.; Spasic, M.; Trang, A.; Gopen, Q.; Yang, I. Characteristics and Management of Superior Semicircular Canal Dehiscence. J. Neurol. Surg. B Skull Base 2012, 73, 365–370. [Google Scholar] [CrossRef]
- Minor, L.B. Clinical Manifestations of Superior Semicircular Canal Dehiscence. Laryngoscope 2005, 115, 1717–1727. [Google Scholar] [CrossRef]
- Chien, W.W.; Janky, K.; Minor, L.B.; Carey, J.P. Superior Canal Dehiscence Size: Multivariate Assessment of Clinical Impact. Otol. Neurotol. 2012, 33, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Pfammatter, A.; Darrouzet, V.; Gärtner, M.; Somers, T.; Van Dinther, J.; Trabalzini, F.; Ayache, D.; Linder, T. A Superior Semicircular Canal Dehiscence Syndrome Multicenter Study: Is There an Association between Size and Symptoms? Otol. Neurotol. 2010, 31, 447–454. [Google Scholar] [CrossRef]
- Niesten, M.E.F.; Hamberg, L.M.; Silverman, J.B.; Lou, K.V.; McCall, A.A.; Windsor, A.; Curtin, H.D.; Herrmann, B.S.; Grolman, W.; Nakajima, H.H.; et al. Superior Canal Dehiscence Length and Location Influences Clinical Presentation and Audiometric and Cervical Vestibular-Evoked Myogenic Potential Testing. Audiol. Neurootol. 2014, 19, 97–105. [Google Scholar] [CrossRef]

| Variable | Overall, N = 156 1 | Control, N = 64 1 | SCD, N = 28 1 | SCDS, N = 64 1 | p-Value 2 |
|---|---|---|---|---|---|
| Age, years | 49.5 ± 13.8 | 49.7 ± 15.6 | 51.5 ± 13.0 | 48.3 ± 12.2 | 0.60 |
| BMI, kg/m2 | 26.9 (22.6, 31.1) | 26.0 (23.2, 29.3) | 28.7 (21.5, 32.2) | 27.1 (22.0, 31.0) | 0.74 |
| Sex (Female) | 95/156 (61%) | 36/64 (56%) | 17/28 (61%) | 42/64 (66%) | 0.55 |
| Race | 0.009 | ||||
| White | 140/156 (90%) | 51/64 (80%) | 26/28 (93%) | 63/64 (98%) | |
| Black | 8/156 (5.1%) | 6/64 (9.4%) | 2/28 (7.1%) | 0/64 (0%) | |
| DTA | 2/156 (1.3%) | 2/64 (3.1%) | 0/28 (0%) | 0/64 (0%) | |
| Other | 6/156 (3.8%) | 5/64 (7.8%) | 0/28 (0%) | 1/64 (1.6%) |
| Variable | Control, N = 64 1 | SCD, N = 28 1 | SCDS, N = 64 1 | p-Value 2 |
|---|---|---|---|---|
| CA Funnel width (mm) * | 5.31 ± 1.50 | 5.03 ± 1.25 | 5.15 ± 1.51 | 0.67 |
| CA Funnel length (mm) * | 4.86 ± 1.08 | 4.83 ± 1.19 | 4.55 ± 1.05 | 0.23 |
| CA Midpoint diameter (mm) * | 0.37 (0.28, 0.47) | 0.33 (0.24, 0.44) | 0.35 (0.25, 0.44) | 0.31 |
| CA Length (mm) * | 13.35 ± 1.95 | 12.97 ± 1.90 | 12.32 ± 2.11 | 0.018 |
| Migirov-Kronenberg Classification | 0.98 | |||
| Type 1 | 37/64 (58%) | 18/28 (64%) | 41/64 (64%) | |
| Type 2 | 14/64 (22%) | 5/28 (18%) | 10/64 (16%) | |
| Type 3 | 12/64 (19%) | 5/28 (18%) | 12/64 (19%) | |
| Type 4 | 1/64 (1.6%) | 0/28 (0%) | 1/64 (1.6%) |
| Variable | Auditory and Vestibular Symptoms, N = 49 1 | Auditory Symptoms Only, N = 15 1 | p-Value 2 |
|---|---|---|---|
| Age, years | 48.4 ± 11.9 | 48.3 ± 13.3 | 0.98 |
| BMI, kg/m2 | 27.3 (22.0, 31.0) | 25.6 (23.3, 28.4) | 0.55 |
| Sex (Female) | 32/49 (65%) | 10/15 (67%) | 0.92 |
| Race | >0.99 | ||
| White | 48/49 (98%) | 15/15 (100%) | |
| Other | 1/49 (2.0%) | 0/15 (0%) | |
| CA Funnel width (mm) * | 5.35 ± 1.61 | 4.54 ± 0.90 | 0.069 |
| CA Funnel length (mm) * | 4.59 ± 1.03 | 4.42 ± 1.12 | 0.60 |
| CA Midpoint diameter (mm) * | 0.36 (0.25, 0.46) | 0.30 (0.25, 0.37) | 0.26 |
| CA Length (mm) * | 12.12 (11.14, 13.73) | 11.59 (10.53, 13.11) | 0.54 |
| Migirov-Kronenberg Classification | 0.71 | ||
| Type 1 | 30/49 (61%) | 11/15 (73%) | |
| Type 2 | 9/49 (18%) | 1/15 (6.7%) | |
| Type 3 | 9/49 (18%) | 3/15 (20%) | |
| Type 4 | 1/49 (2.0%) | 0/15 (0%) |
| Variable | Cluster 1, N = 71 1 | Cluster 2, N = 83 1 | p-Value 2 |
|---|---|---|---|
| Age, years | 50.9 ± 13.3 | 48.5 ± 14.2 | 0.27 |
| BMI, kg/m2 | 26.4 (22.5, 31.0) | 27.0 (22.8, 30.5) | 0.93 |
| Sex (Female) | 44/71 (62%) | 50/83 (60%) | 0.83 |
| Race | 0.017 | ||
| White | 61/71 (86%) | 77/83 (93%) | |
| Black | 4/71 (5.6%) | 4/83 (4.8%) | |
| DTA | 0/71 (0%) | 2/83 (2.4%) | |
| Other | 6/71 (8.5%) | 0/83 (0%) | |
| CA Funnel width (mm) | 4.37 ± 1.12 | 5.90 ± 1.34 | † |
| CA Funnel length (mm) | 3.98 ± 0.73 | 5.37 ± 0.92 | † |
| CA Midpoint diameter (mm) | 0.29 (0.22, 0.37) | 0.41 (0.31, 0.51) | † |
| CA Length (mm) | 11.94 ± 1.63 | 13.65 ± 2.06 | † |
| Migirov-Kronenberg Classification | 0.10 | ||
| Type 1 | 43/71 (61%) | 53/83 (64%) | |
| Type 2 | 10/71 (14%) | 19/83 (23%) | |
| Type 3 | 18/71 (25%) | 11/83 (13%) | |
| Superior canal pathology group | 0.017 | ||
| Control | 21/71 (30%) | 42/83 (51%) | |
| SCD | 13/71 (18%) | 15/83 (18%) | |
| SCDS | 37/71 (52%) | 26/83 (31%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagururu, N.V.; Jung, D.; Hui, F.; Pearl, M.S.; Carey, J.P.; Ward, B.K. Cochlear Aqueduct Morphology in Superior Canal Dehiscence Syndrome. Audiol. Res. 2023, 13, 367-377. https://doi.org/10.3390/audiolres13030032
Nagururu NV, Jung D, Hui F, Pearl MS, Carey JP, Ward BK. Cochlear Aqueduct Morphology in Superior Canal Dehiscence Syndrome. Audiology Research. 2023; 13(3):367-377. https://doi.org/10.3390/audiolres13030032
Chicago/Turabian StyleNagururu, Nimesh V., Diane Jung, Ferdinand Hui, Monica S. Pearl, John P. Carey, and Bryan K. Ward. 2023. "Cochlear Aqueduct Morphology in Superior Canal Dehiscence Syndrome" Audiology Research 13, no. 3: 367-377. https://doi.org/10.3390/audiolres13030032
APA StyleNagururu, N. V., Jung, D., Hui, F., Pearl, M. S., Carey, J. P., & Ward, B. K. (2023). Cochlear Aqueduct Morphology in Superior Canal Dehiscence Syndrome. Audiology Research, 13(3), 367-377. https://doi.org/10.3390/audiolres13030032







