Induction and Cancellation of Self-Motion Misperception by Asymmetric Rotation in the Light
Abstract
1. Introduction
2. Material and Methods
2.1. Participants
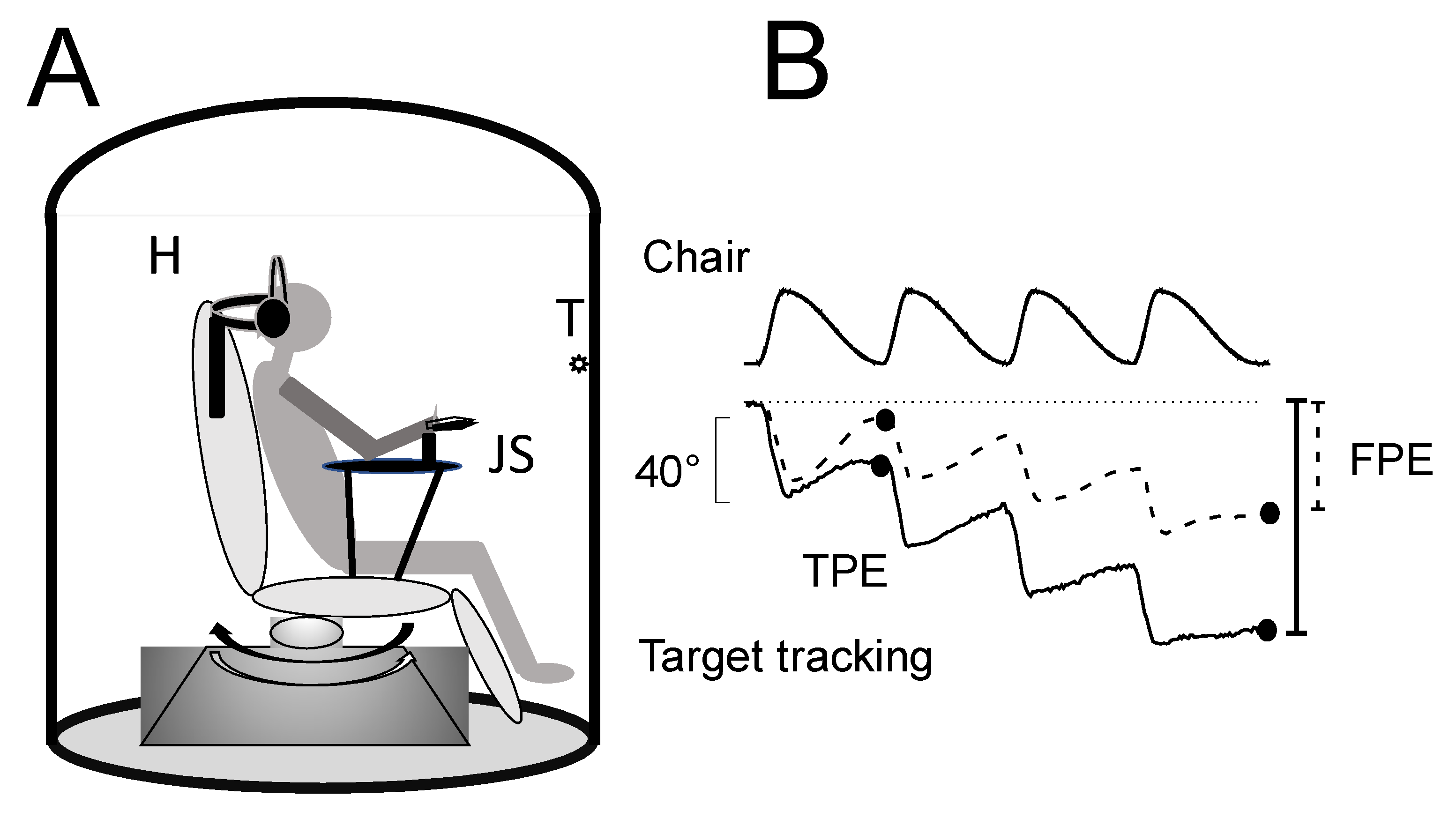
2.2. Test of Self-Motion Perception: Stimulation Apparatus and Recording
2.2.1. Stimulation Apparatus
2.2.2. Self-Motion Perception Conditioning
2.2.3. Self-Motion Perception Testing
2.2.4. Protocol for Conditioning and Testing Procedure
2.3. Data Evaluation and Statistical Analysis
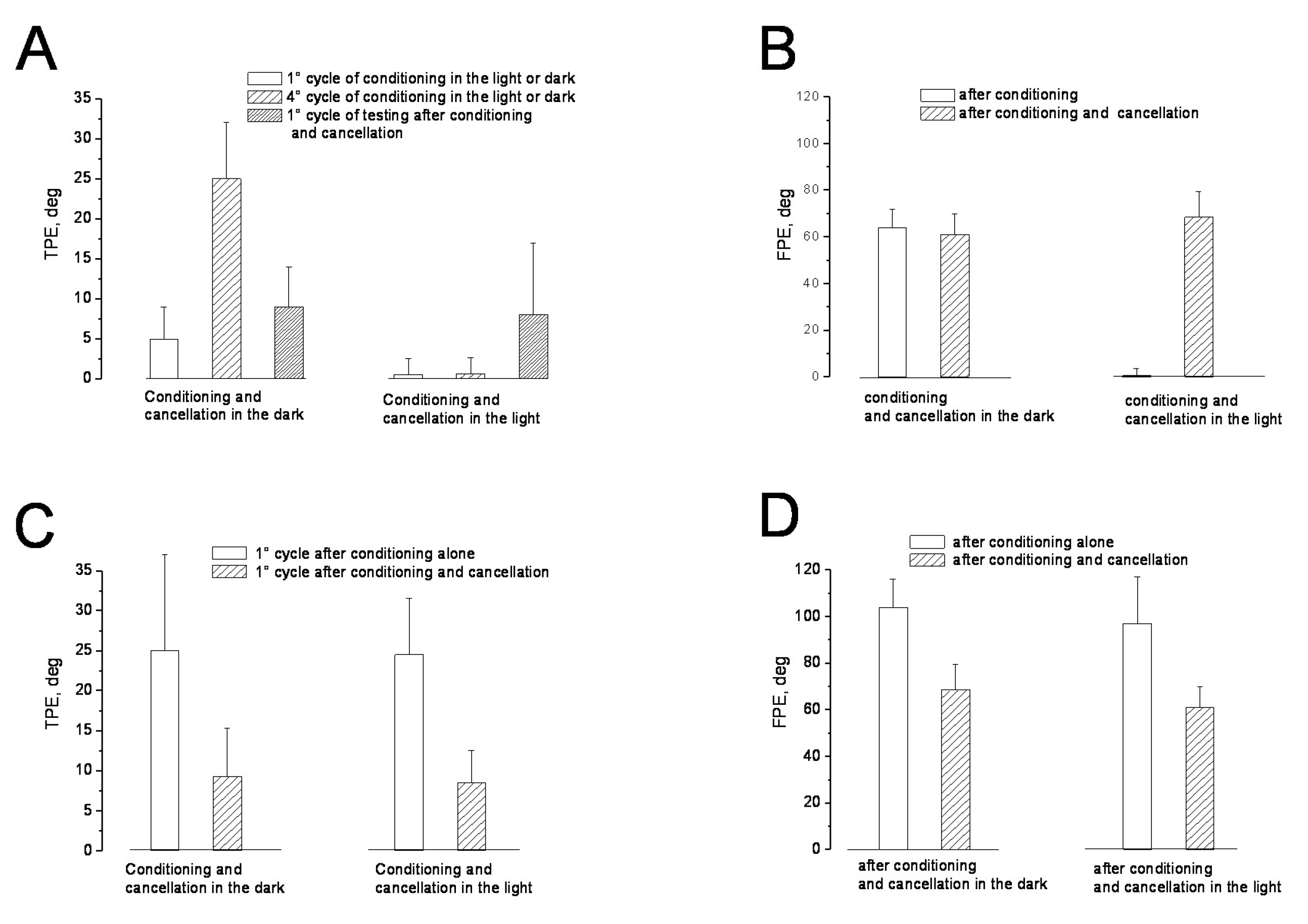
3. Results
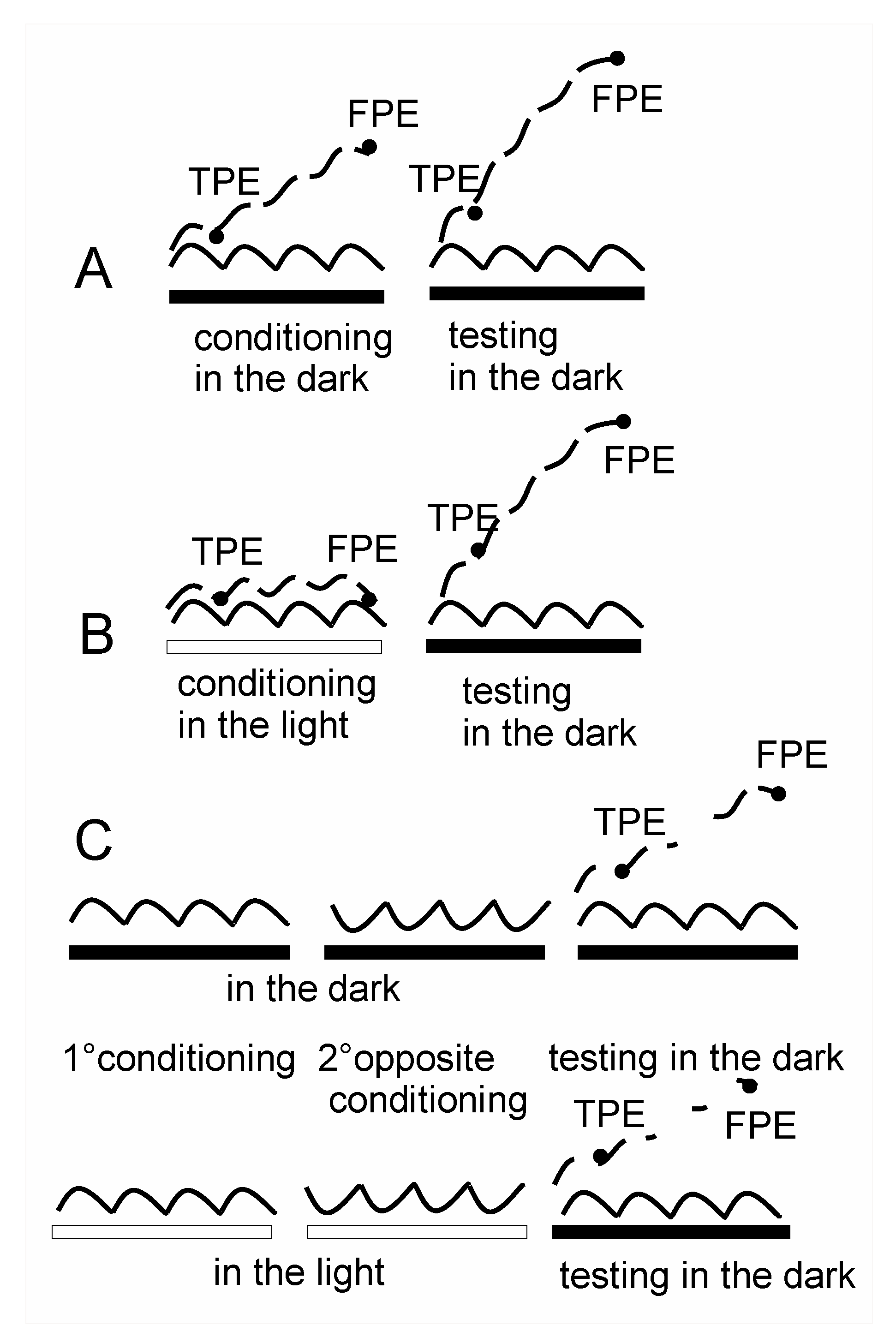
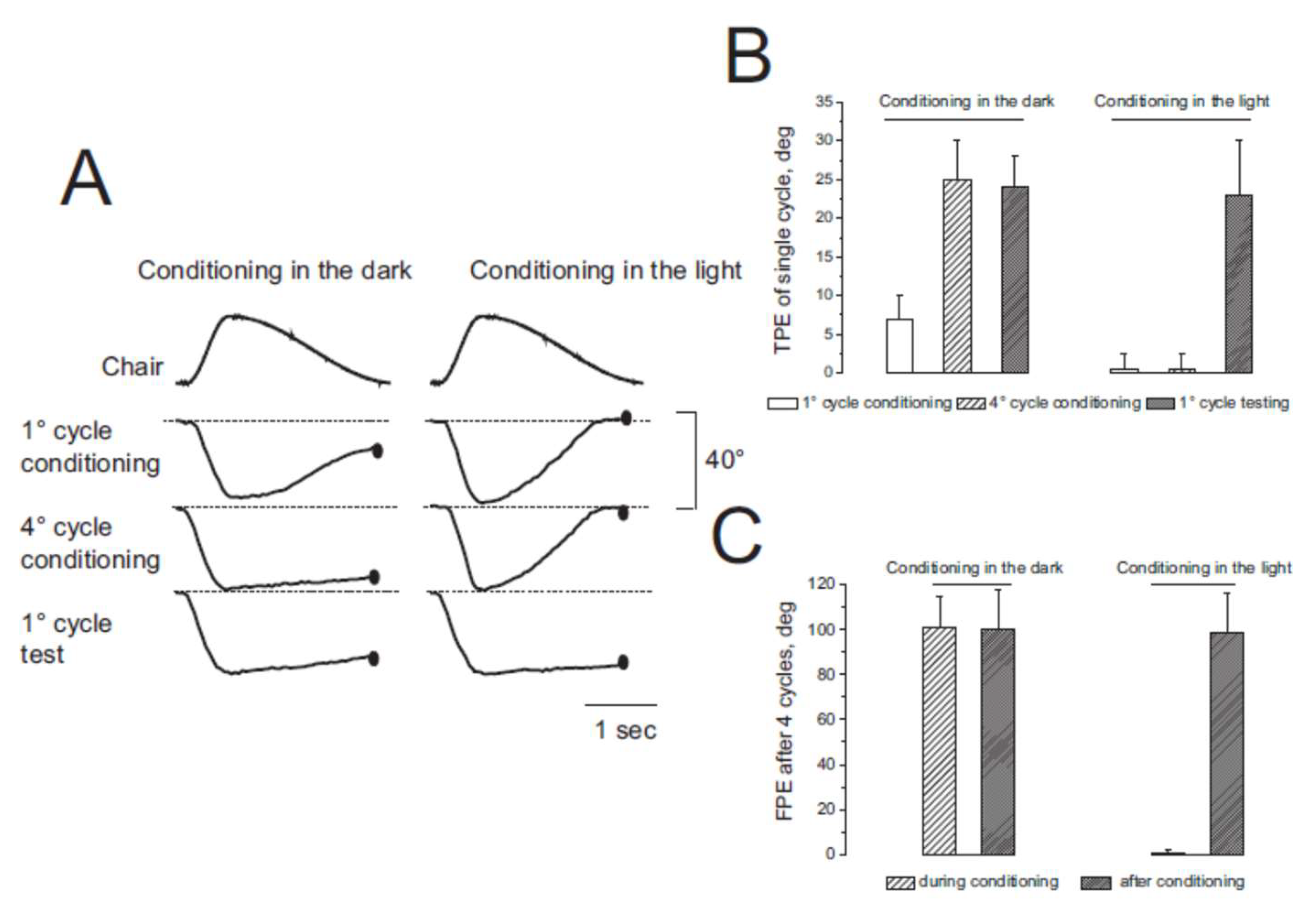
3.1. Misperception of Self-Movement Induced by Four Cycles of Asymmetric Rotation in the Dark
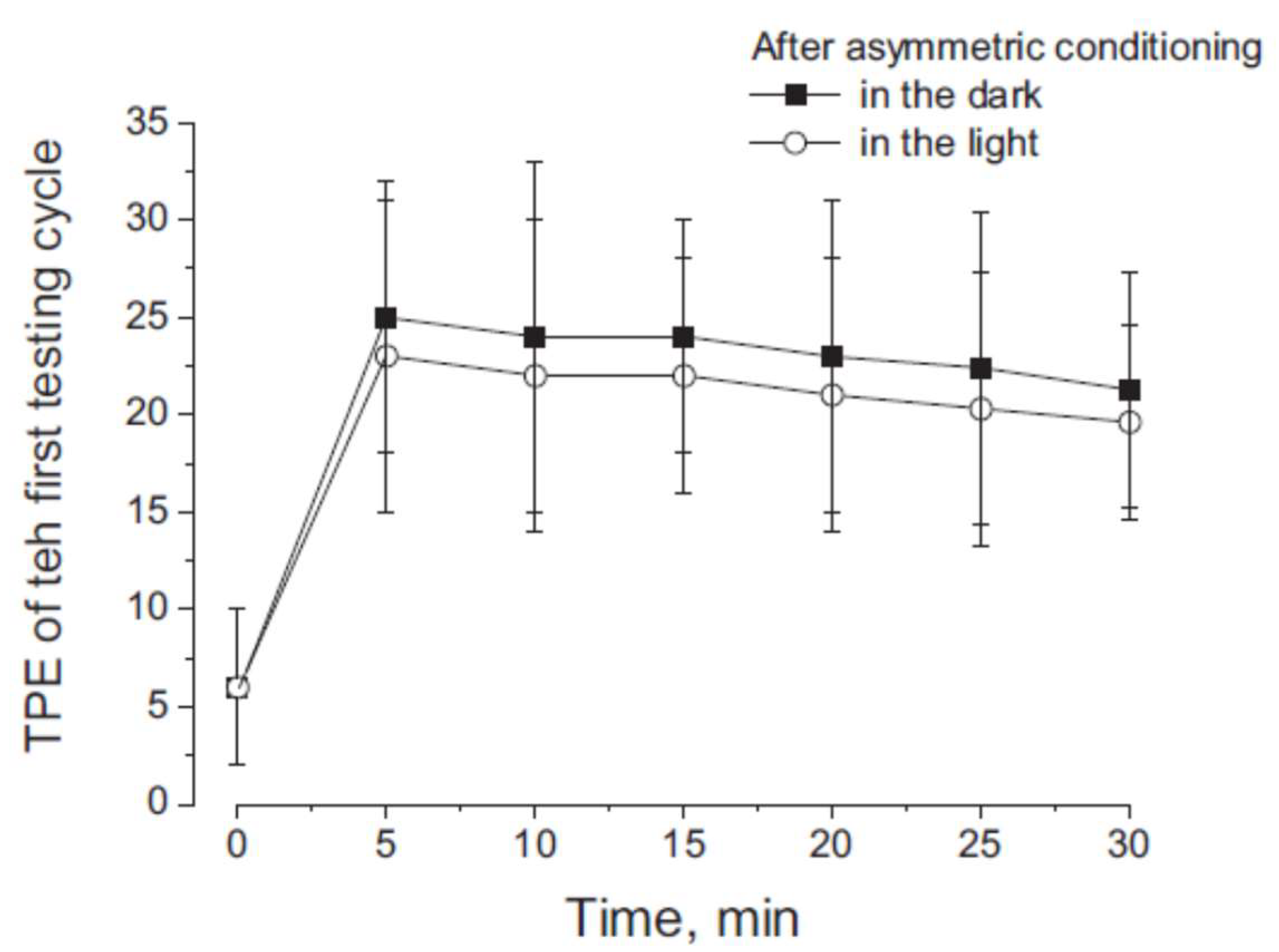
3.2. Misperception of Self-Movement Induced by Four Cycles of Asymmetrical Rotation in the Light
3.3. Cancellation of Perceptual Mismatch by Opposite Directed Asymmetric Rotation in the Dark and Light
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panichi, R.; Botti, F.M.; Ferraresi, A.; Faralli, M.; Kyriakareli, A.; Schieppati, M.; Pettorossi, V.E. Self-motion perception and vestibulo-ocular reflex during whole body yaw rotation in standing subjects: The role of head position and neck proprioception. Hum. Mov. Sci. 2011, 30, 314–332. [Google Scholar] [CrossRef] [PubMed]
- Pettorossi, V.E.; Panichi, R.; Botti, F.M.; Kyriakareli, A.; Ferraresi, A.; Faralli, M.; Schieppati, M.; Bronstein, A.M. Prolonged asymmetric vestibular stimulation induces opposite, long-term effects on self-motion perception and ocular responses. J. Physiol. 2013, 591, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Panichi, R.; Occhigrossi, C.; Ferraresi, A.; Faralli, M.; Lucertini, M.; Pettorossi, V.E. Adaptive changes in the perception of fast and slow movement at different head positions. Aerosp. Med. Hum. Perform. 2017, 88, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Faralli, M.; Pelliccia, C.; Occhigrossi, C.; Bruni, R.; Frati, F.; Ricci, G.; Pettorossi, V.E. Adaptive perceptual responses to asymmetric rotation for testing otolithic function. Exp. Brain. Res. 2022, 240, 2017–2025. [Google Scholar] [CrossRef]
- Sharp, P.E.; Blair, H.T.; Etkin, D.; Tzanetos, D.B. Influences of vestibular and visual motion information on the spatial firing patterns of hippocampal place cells. J. Neurosci. 1995, 15, 173–189. [Google Scholar] [CrossRef]
- Brandt, T.; Dieterich, M. The vestibular cortex. Its locations, functions, and disorders. Ann. N. Y. Acad. Sci. 1999, 871, 293–312. [Google Scholar] [CrossRef]
- Shaikh, A.G.; Meng, H.; Angelaki, D.E. Multiple reference frames for motion in the primate cerebellum. J. Neurosci. 2004, 24, 4491–4497. [Google Scholar] [CrossRef]
- Dieterich, M. Functional brain imaging: A window into the visuo-vestibular systems. Curr. Opin. Neurol. 2007, 20, 12–18. [Google Scholar] [CrossRef]
- Angelaki, D.E.; Cullen, K.E. Vestibular system: The many facets of a multimodal sense. Annu. Rev. Neurosci. 2008, 31, 125–150. [Google Scholar] [CrossRef]
- Bronstein, A.M.; Grunfeld, E.A.; Faldon, M.; Okada, T. Reduced self-motion perception in patients with midline cerebellar lesions. Neuroreport 2008, 19, 691–693. [Google Scholar] [CrossRef]
- Palla, A.; Straumann, D.; Bronstein, A.M. Vestibular neuritis: Vertigo and the high-acceleration vestibulo-ocular reflex. J. Neurol. 2008, 25, 1479–1482. [Google Scholar] [CrossRef]
- Seemungal, B.M.; Rizzo, V.; Gresty, M.A.; Rothwell, J.C.; Bronstein, A.M. Posterior parietal rTMS disrupts human Path Integration during a vestibular navigation task. Neurosci. Lett. 2008, 437, 88–92. [Google Scholar] [CrossRef]
- Helmchen, C.; Klinkenstein, J.; Machner, B.; Rambold, H.; Mohr, C.; Sander, T. Structural changes in the human brain following vestibular neuritis indicate central vestibular compensation. Ann. N. Y. Acad. Sci. 2009, 1164, 104–115. [Google Scholar] [CrossRef]
- Lopez, C.; Blanke, O. The thalamocortical vestibular system in animals and humans. Brain Res. Rev. 2011, 67, 119–146. [Google Scholar] [CrossRef] [PubMed]
- Cullen, K.E. The vestibular system: Multimodal integration and encoding of self-motion for motor control. Trends Neurosci. 2012, 35, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Cousins, S.; Kaski, D.; Cutfield, N.; Seemungal, B.M.; Golding, J.F.; Gresty, M.; Glasauer, S.; Bronstein, A.M. Vestibular perception following acute unilateral vestibular lesions. PLoS ONE 2013, 8, e61862. [Google Scholar] [CrossRef]
- Kaski, D.; Quadir, S.; Nigmatullina, Y.; Malhotra, P.A.; Bronstein, A.M.; Seemungal, B.M. Temporoparietal encoding of space and time during vestibular-guided orientation. Brain 2016, 139, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Panichi, R.; Faralli, M.; Bruni, R.; Kiriakarely, A.; Occhigrossi, C.; Ferraresi, A.; Bronstein, A.M.; Pettorossi, V.E. Asymmetric vestibular stimulation reveals persistent disruption of motion perception in unilateral vestibular lesions. J. Neurophysiol. 2017, 118, 2819–2832. [Google Scholar] [CrossRef]
- Faralli, M.; Ori, M.; Ricci, G.; Roscini, M.; Panichi, R.; Pettorossi, V.E. Disruption of self-motion perception without vestibular reflex alteration in Menieres disease. J. Vest Res. 2022, 32, 193–203. [Google Scholar] [CrossRef]
- Seemungal, B.M.; Gunaratne, I.A.; Fleming, I.O.; Gresty, M.A.; Bronstein, A.M. Perceptual and nystagmic thresholds of vestibular function in yaw. J. Vestib. Res. 2004, 14, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, L.; Nicoucar, K.; Mast, F.W.; Merfeld, D.M. Vestibular thresholds for yaw rotation about an earth-vertical axis as a function of frequency. Exp. Brain Res. 2008, 186, 677–681. [Google Scholar] [CrossRef]
- Valko, Y.; Lewis, R.F.; Priesol, A.J.; Merfeld, D.M. Vestibular labyrinth contributions to human whole-body motion discrimination. J. Neurosci. 2012, 32, 1353713542. [Google Scholar] [CrossRef]
- Pettorossi, V.E.; Panichi, R.; Bambagioni, D.; Grassi, S.; Botti, F.M. Contribution of eye position to movement perception. Acta Otolaryng. 2004, 124, 471–474. [Google Scholar] [CrossRef]
- Siegle, J.H.; Campos, J.L.; Mohler, B.J.; Loomis, J.M.; Bulthoff, H.H. Measurement of instantaneous perceived self-motion using continuous pointing. Exp. Brain Res. 2009, 195, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Lopez, C. Vestibular stimulation modifies the body schema. Neuropsychologia 2012, 50, 1830–1837. [Google Scholar] [CrossRef] [PubMed]
- Ferrè, E.R.; Haggard, P. The vestibular body: Vestibular contributions to bodily representations. Cogn. Neuropsychol. 2016, 33, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Britton, Z.; Arshad, Q. Vestibular and multi-sensory influences upon self-motion perception and the consequences for human behavior. Front. Neurol. 2019, 10, 63. [Google Scholar] [CrossRef]
- Occhigrossi, C.; Brosch, M.; Giommetti, G.; Panichi, R.; Ricci, G.; Ferraresi, A.; Roscini, M.; Pettorossi, V.E.; Faralli, M. Auditory perception is influenced by the orientation of the trunk relative to a sound source. Exp. Brain Res. 2021, 239, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Pettorossi, V.E.; Schieppati, M. Neck proprioception shapes body orientation and perception of motion. Front. Hum. Neurosci. 2014, 8, 895. [Google Scholar] [CrossRef]
- Pettorossi, V.E.; Panichi, R.; Botti, F.M.; Biscarini, A.; Filippi, G.M.; Schieppati, M. Long-lasting effects of neck muscle vibration and contraction on self-motion perception of vestibular origin. Clin. Neurophysiol. 2015, 126, 1886–1900. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pettorossi, V.E.; Occhigrossi, C.; Panichi, R.; Botti, F.M.; Ferraresi, A.; Ricci, G.; Faralli, M. Induction and Cancellation of Self-Motion Misperception by Asymmetric Rotation in the Light. Audiol. Res. 2023, 13, 196-206. https://doi.org/10.3390/audiolres13020019
Pettorossi VE, Occhigrossi C, Panichi R, Botti FM, Ferraresi A, Ricci G, Faralli M. Induction and Cancellation of Self-Motion Misperception by Asymmetric Rotation in the Light. Audiology Research. 2023; 13(2):196-206. https://doi.org/10.3390/audiolres13020019
Chicago/Turabian StylePettorossi, Vito Enrico, Chiara Occhigrossi, Roberto Panichi, Fabio Massimo Botti, Aldo Ferraresi, Giampietro Ricci, and Mario Faralli. 2023. "Induction and Cancellation of Self-Motion Misperception by Asymmetric Rotation in the Light" Audiology Research 13, no. 2: 196-206. https://doi.org/10.3390/audiolres13020019
APA StylePettorossi, V. E., Occhigrossi, C., Panichi, R., Botti, F. M., Ferraresi, A., Ricci, G., & Faralli, M. (2023). Induction and Cancellation of Self-Motion Misperception by Asymmetric Rotation in the Light. Audiology Research, 13(2), 196-206. https://doi.org/10.3390/audiolres13020019







