Adverse Audio-Vestibular Effects of Drugs and Vaccines Used in the Treatment and Prevention of COVID-19: A Review
Abstract
:1. Introduction
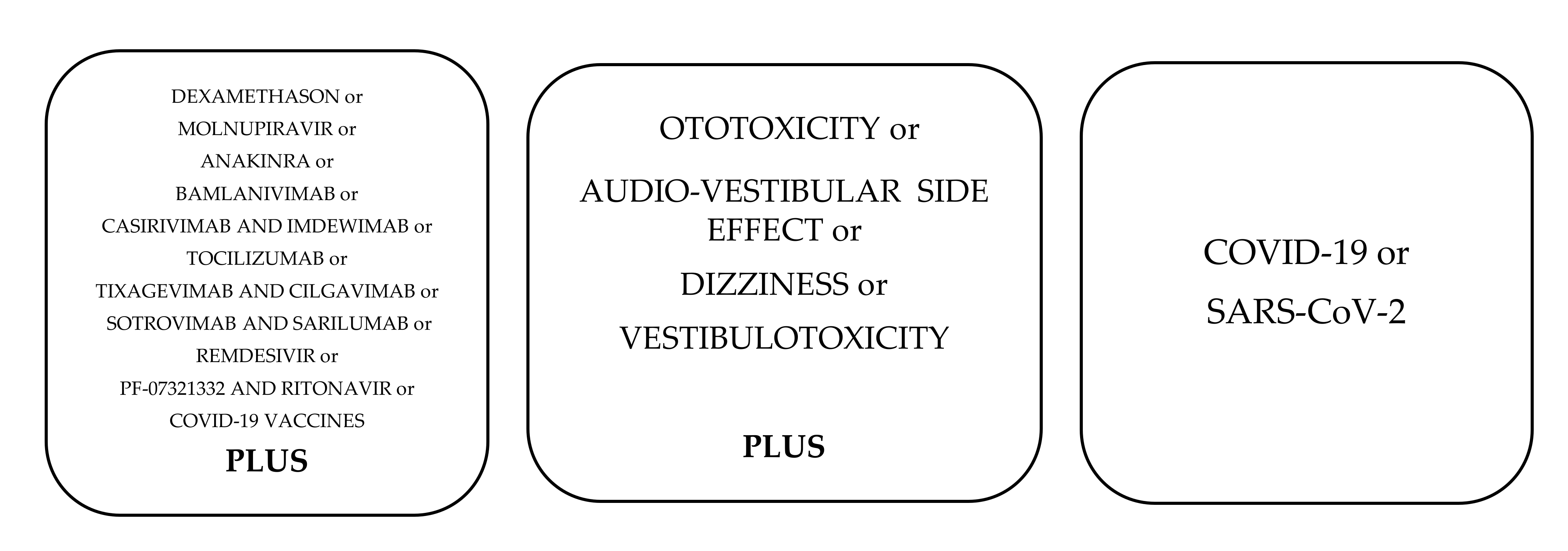
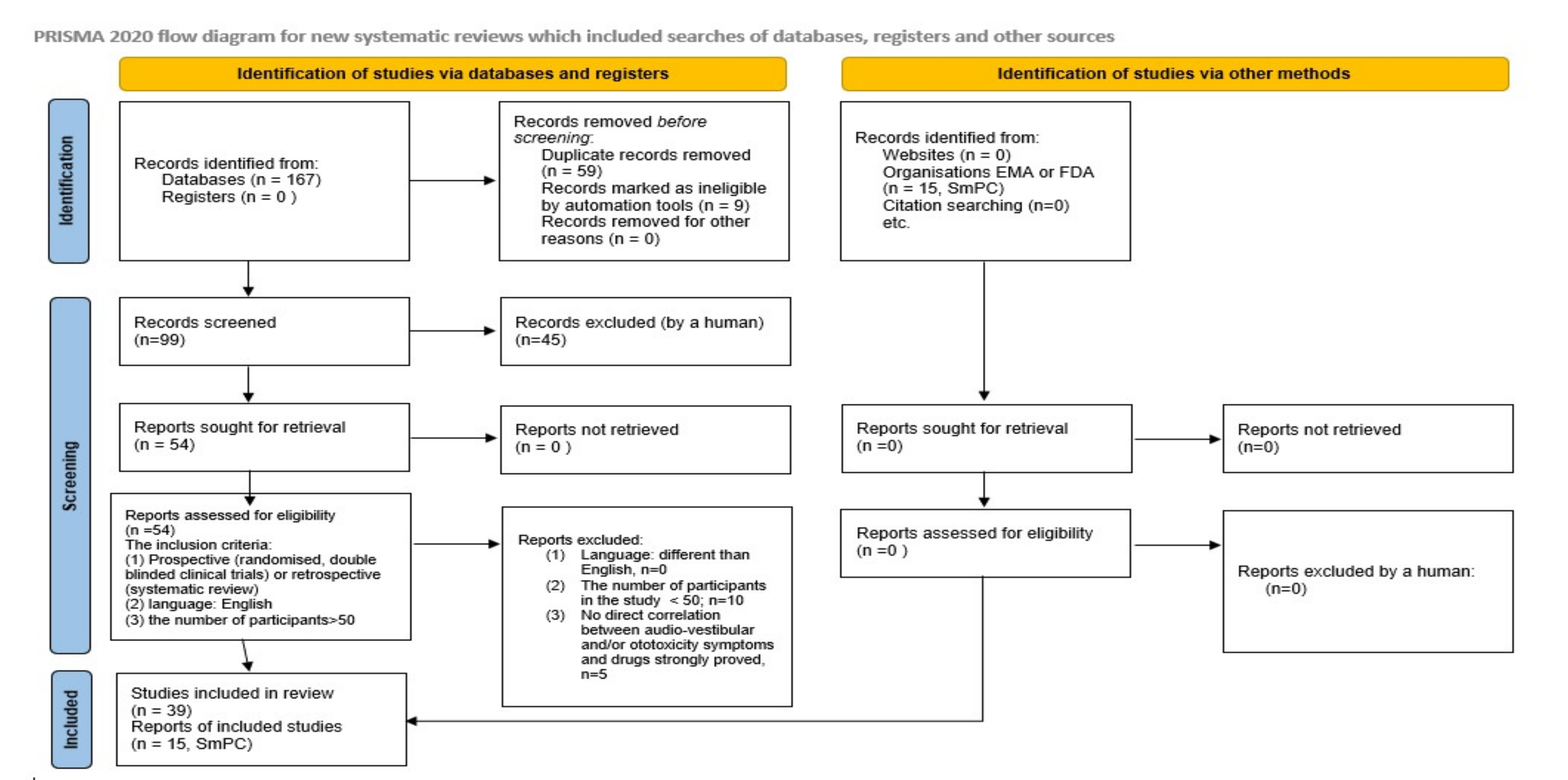
2. Materials and Methods
3. Results
3.1. Anti-COVID-19 Drugs
3.1.1. Dexamethasone
3.1.2. Anakinra
3.2. Monoclonal Antibodies
3.2.1. Bamlanivimab
3.2.2. Casirivimab and Imdewimab
3.2.3. Tocilizumab and Sarilumab
3.2.4. Tixagevimab/Cilgavimab
3.2.5. Sotrovimab
3.3. Oral Treatments against COVID-19
3.3.1. Molnupiravir
3.3.2. PF-07321332/Ritonavir (Brand Name: Paxlovid)
3.4. COVID-19 Vaccines
3.4.1. Pfizer + BioNTechVaccine: Comirnaty
3.4.2. COVID-19 Vaccine: Moderna
3.4.3. COVID-19 Vaccine: Astra Zeneca
3.4.4. COVID-19 Vaccine: Janssen
3.4.5. COVID-19 Vaccine: Novovax (Nuvaxovid)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hospitalized Adults: Therapeutic Management. Available online: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/hospitalized-adults--therapeutic-management/ (accessed on 19 January 2022).
- Cennimo, D. Coronavirus Disease 2019 (COVID-19) Treatment & Management: Approach Considerations, Prevention, Antiviral Agents. 2022. Available online: https://emedicine.medscape.com/article/2500114-treatment (accessed on 20 February 2022).
- An EUA for Bamlanivimab—A Monoclonal Antibody for COVID-19. JAMA 2021, 325, 880–881. [CrossRef] [PubMed]
- Chen, P.; Nirula, A.; Heller, B.; Gottlieb, R.L.; Boscia, J.; Morris, J.; Huhn, G.; Cardona, J.; Mocherla, B.; Stosor, V.; et al. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with COVID-19. N. Engl. J. Med. 2021, 384, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Patterson, T.F.; Mehta, A.K.; Tomashek, K.M.; Wolfe, C.R.; Ghazaryan, V.; Marconi, V.C.; Ruiz-Palacios, G.M.; Hsieh, L.; Kline, S.; et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N. Engl. J. Med. 2021, 384, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Regeneron Pharmaceuticals Inc. Regeneron’s COVID-19 Outpatient Trial Prospectively Demonstrates That REGN-COV2 Antibody Cocktail Significantly Reduced Virus Levels and Need for Further Medical Attention. Available online: https://investor.regeneron.com/news-releases/news-release-details/regenerons-covid-19-outpatient-trial-prospectively-demonstrates/ (accessed on 19 January 2022).
- Casirivimab and Imdevimab. Available online: https://www.regeneron.com/medicines/casirivimab-imdevimab (accessed on 20 February 2022).
- US Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19 (accessed on 19 January 2022).
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19–Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Goldman, J.D.; Lye, D.C.B.; Hui, D.S.; Marks, K.M.; Bruno, R.; Montejano, R.; Spinner, C.D.; Galli, M.; Ahn, M.-Y.; Nahass, R.G.; et al. Remdesivir for 5 or 10 Days in Patients with Severe COVID-19. N. Engl. J. Med. 2020, 383, 1827–1837. [Google Scholar] [CrossRef]
- Spinner, C.D.; Gottlieb, R.L.; Criner, G.J.; Arribas López, J.R.; Cattelan, A.M.; Soriano Viladomiu, A.; Ogbuagu, O.; Malhotra, P.; Mullane, K.M.; Castagna, A.; et al. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 1048–1057. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in Adults with Severe COVID-19: A Randomised, Double-Blind, Placebo-Controlled, Multicentre Trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef]
- Matthay, M.A.; Thompson, B.T. Dexamethasone in Hospitalised Patients with COVID-19: Addressing Uncertainties. Lancet. Respir. Med. 2020, 8, 1170–1172. [Google Scholar] [CrossRef]
- Ganesan, P.; Schmiedge, J.; Manchaiah, V.; Swapna, S.; Dhandayutham, S.; Kothandaraman, P.P. Ototoxicity: A Challenge in Diagnosis and Treatment. J. Audiol. Otol. 2018, 22, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Lord, S.G. Monitoring Protocols for Cochlear Toxicity. Semin. Hear. 2019, 40, 122–143. [Google Scholar] [CrossRef]
- Fligor, B.J. Pediatric Ototoxicity: Current Trends and Management. Semin. Hear. 2019, 40, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Durrant, J. American Academy of Audiology Position Statement and Clinical Practice Guidelines: Ototoxicity Monitoring. 2011. Available online: https://audiology-web.s3.amazonaws.com/migrated/OtoMonGuidelines.pdf_539974c40999c1.58842217.pdf (accessed on 16 February 2022).
- Rizk, H.G.; Lee, J.A.; Liu, Y.F.; Endriukaitis, L.; Isaac, J.L.; Bullington, W.M. Drug-Induced Ototoxicity: A Comprehensive Review and Reference Guide. Pharmacotherapy 2020, 40, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Altissimi, G.; Colizza, A.; Cianfrone, G.; Vincentiis, M.D.; Greco, A.; Taurone, S.; Musacchio, A.; Ciofalo, A.; Turchetta, R.; Angeletti, D.; et al. Drugs Inducing Hearing Loss, Tinnitus, Dizziness and Vertigo: An Updated Guide. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7946–7952. [Google Scholar] [PubMed]
- Dusan, M.; Milan, S.; Nikola, D. COVID-19 Caused Hearing Loss. Eur. Arch. Otorhinolaryngol. 2021, 1–10. [Google Scholar] [CrossRef]
- McIntyre, K.M.; Favre, N.M.; Kuo, C.C.; Carr, M.M. Systematic Review of Sensorineural Hearing Loss Associated With COVID-19 Infection. Cureus 2021, 13. [Google Scholar] [CrossRef]
- Jeong, M.; Ocwieja, K.E.; Han, D.; Wackym, P.A.; Zhang, Y.; Brown, A.; Moncada, C.; Vambutas, A.; Kanne, T.; Crain, R.; et al. Direct SARS-CoV-2 Infection of the Human Inner Ear May Underlie COVID-19-Associated Audiovestibular Dysfunction. Commun. Med. 2021, 1, 1–14. [Google Scholar] [CrossRef]
- Thrane, J.F.; Britze, A.; Fjaeldstad, A.W. Incidence and Duration of Self-Reported Hearing Loss and Tinnitus in a Cohort of COVID-19 Patients with Sudden Chemosensory Loss: A STROBE Observational Study–ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S1879729621002246 (accessed on 20 February 2022).
- Ciorba, A.; Skarżyński, P.H.; Pelucchi, S.; Hatzopoulos, S. Ototoxicity Prevention during the SARS-CoV-2 (COVID-19) Emergency. J. Glob. Antimicrob. Resist. 2020, 23, 263–264. [Google Scholar] [CrossRef]
- Fancello, V.; Hatzopoulos, S.; Corazzi, V.; Bianchini, C.; Skarżyńska, M.B.; Pelucchi, S.; Skarżyński, P.H.; Ciorba, A. SARS-CoV-2 (COVID-19) and Audio-Vestibular Disorders. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211027372. [Google Scholar] [CrossRef]
- Ciorba, A.; Corazzi, V.; Skarżyński, P.H.; Skarżyńska, M.B.; Bianchini, C.; Pelucchi, S.; Hatzopoulos, S. Don’t Forget Ototoxicity during the SARS-CoV-2 (Covid-19) Pandemic! Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420941754. [Google Scholar] [CrossRef]
- Formeister, E.J.; Chien, W.; Agrawal, Y.; Carey, J.P.; Stewart, C.M.; Sun, D.Q. Preliminary Analysis of Association Between COVID-19 Vaccination and Sudden Hearing Loss Using US Centers for Disease Control and Prevention Vaccine Adverse Events Reporting System Data. JAMA Otolaryngol. Head Neck Surg. 2021, 147, 674–676. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, S.; Cidlowski, J.A. Corticosteroids: Mechanisms of Action in Health and Disease. Rheum. Dis. Clin. North Am. 2016, 42, 15–31, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, M.H.; Hassan, A. Dexamethasone for the Treatment of Coronavirus Disease (COVID-19): A Review. SN Compr. Clin. Med. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.E. Basic and Clinical Pharmacology of Glucocorticosteroids. Anesth. Prog. 2013, 60, 25–31; [Google Scholar] [CrossRef]
- Zoorob, R.J.; Cender, D. A Different Look at Corticosteroids. Am. Fam. Physician 1998, 58, 443–450. [Google Scholar]
- Saraya, M.; Amal, A. Dexamethasone as Adjunctive Therapy for Treatment of Varicella Pneumonia. Egypt. J. Chest Dis. Tuberc. 2012, 61, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Topete, D.; Cidlowski, J.A. One Hormone, Two Actions: Anti- and pro-Inflammatory Effects of Glucocorticoids. Neuroimmunomodulation 2015, 22, 20–32. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Li, W.; Farzan, M.; Harrison, S.C. Structure of SARS Coronavirus Spike Receptor-Binding Domain Complexed with Receptor. Science 2005, 309, 1864–1868. [Google Scholar] [CrossRef]
- Turski, W.A.; Wnorowski, A.; Turski, G.N.; Turski, C.A.; Turski, L. AhR and IDO1 in Pathogenesis of Covid-19 and the “Systemic AhR Activation Syndrome:” A Translational Review and Therapeutic Perspectives. Restor. Neurol. Neurosci. 2020, 38, 343–354. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Czarska-Thorley, D. Ema Endorses Use of Dexamethasone in COVID-19 Patients on Oxygen or Mechanical Ventilation. Available online: https://www.ema.europa.eu/en/news/ema-endorses-use-dexamethasone-covid-19-patients-oxygen-mechanical-ventilation (accessed on 19 January 2022).
- Boehringer Ingelheim RCV GmbH & Co KG. Pfizer Health AB Kineret–Summary of Product Characteristics; Boehringer Ingelheim RCV GmbH & Co KG: Wien, Austria, 2021. [Google Scholar]
- Kyriazopoulou, E.; Poulakou, G.; Milionis, H.; Metallidis, S.; Adamis, G.; Tsiakos, K.; Fragkou, A.; Rapti, A.; Damoulari, C.; Fantoni, M.; et al. Early Treatment of COVID-19 with Anakinra Guided by Soluble Urokinase Plasminogen Receptor Plasma Levels: A Double-Blind, Randomized Controlled Phase 3 Trial. Nat. Med. 2021, 27, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Convertino, I.; Tuccori, M.; Ferraro, S.; Valdiserra, G.; Cappello, E.; Focosi, D.; Blandizzi, C. Exploring Pharmacological Approaches for Managing Cytokine Storm Associated with Pneumonia and Acute Respiratory Distress Syndrome in COVID-19 Patients. Crit. Care 2020, 24, 331. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Anderson, A.O.; Tang, J.W.; Tuccori, M. Convalescent Plasma Therapy for COVID-19: State of the Art. Clin. Microbiol. Rev. 2020, 33, e00072-20. [Google Scholar] [CrossRef] [PubMed]
- Sajna, K.V.; Kamat, S. Antibodies at Work in the Time of Severe Acute Respiratory Syndrome Coronavirus 2. Cytotherapy 2021, 23, 101–110. [Google Scholar] [CrossRef] [PubMed]
- FDA Issues EUA for Bamlanivimab to Treat COVID-19. Available online: http://www.pharmacist.com/Pharmacy-News/fda-issues-eua-for-bamlanivimab-to-treat-covid-19 (accessed on 19 January 2022).
- Bamlanivimab and Etesevimab Emergency Use Authorization (EUA) for COVID-19. Available online: https://www.covid19.lilly.com/bam-ete/hcp (accessed on 19 January 2022).
- Dougan, M.; Nirula, A.; Azizad, M.; Mocherla, B.; Gottlieb, R.L.; Chen, P.; Hebert, C.; Perry, R.; Boscia, J.; Heller, B.; et al. Bamlanivimab plus Etesevimab in Mild or Moderate COVID-19. N. Engl. J. Med. 2021, 385, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Casirivimab. Available online: https://www.ncbi.nlm.nih.gov/books/NBK572124/ (accessed on 19 January 2022).
- Imdevimab. Available online: https://www.ncbi.nlm.nih.gov/books/NBK572065/ (accessed on 19 January 2022).
- REGEN-COV®. Casirivimab and Imdevimab. Available online: https://www.regencov.com/hcp (accessed on 19 January 2022).
- US Food and Drug Administration. Coronavirus (COVID-19) Update: Daily Roundup, March 24, 2020. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-daily-roundup-march-24-2020 (accessed on 19 January 2022).
- Weinreich, D.M.; Sivapalasingam, S.; Norton, T.; Ali, S.; Gao, H.; Bhore, R.; Xiao, J.; Hooper, A.T.; Hamilton, J.D.; Musser, B.J.; et al. REGEN-COV Antibody Combination and Outcomes in Outpatients with Covid-19. N. Engl. J. Med. 2021, 385, e81. [Google Scholar] [CrossRef]
- Gordon, A.C.; Mouncey, P.R.; Al-Beidh, F.; Rowan, K.M.; Nichol, A.D.; Arabi, Y.M.; Annane, D.; Beane, A.; van Bentum-Puijk, W.; Berry, L.R.; et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 385, 1491–1502. [Google Scholar]
- COVID Research. A Year of Scientific Milestones. Nature, 2021. Available online. (accessed on 19 January 2022). [CrossRef]
- Anonymous. RoActemra. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/roactemra (accessed on 19 January 2022).
- Roche Registration. GmbH Roactemra–Summary of Product Characteristics. 2022. Available online: https://www.rocheresources.co.uk/content/dam/hcp-portals/uk2/documents/roactemra/RoActemra_product_summary.pdf (accessed on 19 January 2022).
- Kevzara 150 Mg Solution for Injection in Pre-Filled Syringe–Summary of Product Characteristics (SmPC)–(Emc). Available online: https://www.medicines.org.uk/emc/product/762/smpc#gref (accessed on 19 January 2022).
- Stone, J.H.; Frigault, M.J.; Serling-Boyd, N.J.; Fernandes, A.D.; Harvey, L.; Foulkes, A.S.; Horick, N.K.; Healy, B.C.; Shah, R.; Bensaci, A.M.; et al. Efficacy of Tocilizumab in Patients Hospitalized with COVID-19. N. Engl. J. Med. 2020, 383, 2333–2344. [Google Scholar] [CrossRef]
- AstraZeneca. Tixagevimab Co-Packaged with Cilgavimab–Fact Sheet For Healthcare Providers: Emergency Use Authorization For Evusheld. 2021. Available online: https://www.fda.gov/media/154701/download (accessed on 19 January 2022).
- Levin, M.J.; Ustianowski, A.; De Wit, S.; Launay, O.; Avila, M.; Seegobin, S.; Templeton, A.; Yuan, Y.; Ambery, P.; Arends, R.H.; et al. LB5. PROVENT: Phase 3 Study of Efficacy and Safety of AZD7442 (Tixagevimab/Cilgavimab) for Pre-Exposure Prophylaxis of COVID-19 in Adults. Open Forum Infect. Dis. 2021, 8, S810. [Google Scholar] [CrossRef]
- GlaxoSmithKline Manufacturing S.p.A. Sotrovimab–Summary of Product Characteristics. 2021. Available online: https://www.ema.europa.eu/en/documents/product-information/xevudy-epar-product-information_en.pdf (accessed on 19 January 2022).
- Gupta, A.; Gonzalez-Rojas, Y.; Juarez, E.; Crespo Casal, M.; Moya, J.; Falci, D.R.; Sarkis, E.; Solis, J.; Zheng, H.; Scott, N.; et al. Early Treatment for COVID-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N. Engl. J. Med. 2021, 385, 1941–1950. [Google Scholar] [CrossRef]
- Merck & Co., Inc. Molnupiravir—Fact Sheet for Healthcare Providers: Emergency Use Authorization; Merck & Co., Inc.: New York, NY, USA, 2022. [Google Scholar]
- Covid-19: Molnupiravir Reduces Risk of Hospital Admission or Death by 50% in Patients at Risk, MSD Reports. BMJ. Available online: https://www.bmj.com/content/375/bmj.n2422 (accessed on 17 February 2022).
- Pfizer Labs Paxlovid: Fact Sheet For Healthcare Providers: Emergency Use Authorization. 2022. Available online: https://www.fda.gov/media/155050/download (accessed on 19 January 2022).
- Awadasseid, A.; Wu, Y.; Tanaka, Y.; Zhang, W. Current Advances in the Development of SARS-CoV-2 Vaccines. Int. J. Biol. Sci. 2021, 17, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.P.; Gupta, V. COVID-19 Vaccine: A Comprehensive Status Report. Virus Res. 2020, 288, 198114. [Google Scholar] [CrossRef] [PubMed]
- AstraZeneca’s COVID-19 Vaccine Authorised for Emergency Supply in the UK. Available online: https://www.astrazeneca.com/media-centre/press-releases/2020/astrazenecas-covid-19-vaccine-authorised-in-uk.html (accessed on 19 January 2022).
- CDC Ensuring the Safety of COVID-19 Vaccines in the United States. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety.html (accessed on 19 January 2022).
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Tseng, P.-T.; Chen, T.-Y.; Sun, Y.-S.; Chen, Y.-W.; Chen, J.-J. The Reversible Tinnitus and Cochleopathy Followed First-Dose AstraZeneca COVID-19 Vaccination. QJM 2021, 114, 663–664. [Google Scholar] [CrossRef]
- Jeong, J.; Choi, H.S. Sudden Sensorineural Hearing Loss after COVID-19 Vaccination. Int. J. Infect. Dis. 2021, 113, 341–343. [Google Scholar] [CrossRef]
- Pfizer. COMIRNATY®|Pfizer UK. Available online: https://www.pfizer.co.uk/products/prescription-medicines/comirnaty (accessed on 19 January 2022).
- Summary of Product Characteristics for Spikevax. Available online: https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-moderna/information-for-healthcare-professionals-on-covid-19-vaccine-moderna (accessed on 19 January 2022).
- Regulatory Approval of Vaxzevria (Previously COVID-19 Vaccine AstraZeneca). Available online: https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-astrazeneca (accessed on 19 January 2022).
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 NCoV-19) COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef]
- Novavax CZ a.s. Nuvaxovid-Summary of Product Characteristics. 2022. Available online: https://www.ema.europa.eu/en/documents/product-information/nuvaxovid-epar-product-information_en.pdf?_x_tr_sl=en&_x_tr_tl=fi&_x_tr_hl=fi&_x_tr_pto=sc (accessed on 19 January 2022).
- Dexamethasone in Hospitalized Patients with Covid-19. Available online: https://www.nejm.org/doi/full/10.1056/nejmoa2021436 (accessed on 19 January 2022).
- Tharaux, P.-L.; Pialoux, G.; Pavot, A.; Mariette, X.; Hermine, O.; Resche-Rigon, M.; Porcher, R.; Ravaud, P.; Bureau, S.; Dougados, M.; et al. Effect of Anakinra versus Usual Care in Adults in Hospital with COVID-19 and Mild-to-Moderate Pneumonia (CORIMUNO-ANA-1): A Randomised Controlled Trial. Lancet Resp. Med. 2021, 9, 295–304. [Google Scholar] [CrossRef]
- Abani, O.; Abbas, A.; Abbas, F. Casirivimab and Imdevimab in Patients Admitted to Hospital with COVID-19 (RECOVERY): A Randomised, Controlled, Open-Label, Platform Trial. Lancet 2022, 399, 665–676. [Google Scholar] [CrossRef]
- Subcutaneous REGEN-COV Antibody Combination to Prevent Covid-19. Available online: https://www.nejm.org/doi/full/10.1056/nejmoa2109682 (accessed on 19 January 2022).
- Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N. Engl. J. Med. 2021, 384, 1491–1502. [CrossRef]
- Tocilizumab and Remdesivir in Hospitalized Patients with Severe COVID-19 Pneumonia: A Randomized Clinical Trial. Available online: https://pubmed.ncbi.nlm.nih.gov/34609549/ (accessed on 19 January 2022).
- Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia. Available online: https://www.nejm.org/doi/full/10.1056/NEJMoa2030340 (accessed on 19 January 2022).
- Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. Available online: https://www.nejm.org/doi/full/10.1056/nejmoa2028836 (accessed on 19 January 2022).
- Kadali, R.A.K.; Janagama, R.; Peruru, S.; Malayala, S.V. Side Effects of BNT162b2 MRNA COVID-19 Vaccine: A Randomized, Cross-Sectional Study with Detailed Self-Reported Symptoms from Healthcare Workers. Int. J. Infect. Dis. 2021, 106, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 MRNA Covid-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef] [PubMed]
- Safety and Efficacy of the BNT162b2 MRNA Covid-19 Vaccine. Available online: https://www.nejm.org/doi/full/10.1056/nejmoa2034577 (accessed on 19 January 2022).
- Final Analysis of Efficacy and Safety of Single-Dose Ad26.COV2.S. Available online: https://www.nejm.org/doi/full/10.1056/NEJMoa2117608 (accessed on 19 January 2022).
- Safety and Efficacy of the ChAdOx1 NCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Available online: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)32661-1/fulltext (accessed on 27 April 2022).
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simón-Campos, A.; et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N. Engl. J. Med. 2022, 386, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martín-Quirós, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022, 386, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J. Everything You Need to Know about the COVID-19 Therapy Trials. Available online: https://pharmaceutical-journal.com/article/feature/everything-you-need-to-know-about-the-covid-19-therapy-trials (accessed on 19 January 2022).
- Di Mauro, P.; La Mantia, I.; Cocuzza, S.; Sciancalepore, P.I.; Rasà, D.; Maniaci, A.; Ferlito, S.; Tundo, I.; Anzivino, R. Acute Vertigo After COVID-19 Vaccination: Case Series and Literature Review. Front. Med. 2022, 8, 2766–2771. [Google Scholar] [CrossRef] [PubMed]
- Cianfrone, G.; Pentangelo, D.; Cianfrone, F.; Mazzei, F.; Turchetta, R.; Orlando, M.P.; Altissimi, G. Pharmacological Drugs Inducing Ototoxicity, Vestibular Symptoms and Tinnitus: A Reasoned and Updated Guide. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 601–636. [Google Scholar]
- Duggal, P.; Sarkar, M. Audiologic Monitoring of Multi-Drug Resistant Tuberculosis Patients on Aminoglycoside Treatment with Long Term Follow-Up. BMC Ear. Nose Throat. Disord. 2007, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Campbell, K.C.; Durrant, J. Audiologic Monitoring for Ototoxicity. Otolaryngol. Clin. North Am. 1993, 26, 903–914. [Google Scholar] [CrossRef]
- Campbell, K.C. Pharmacology and Ototoxicity for Audiologist, 1st ed.; Thomson/Delmar Learning: New York, NY, USA, 2007; ISBN 1-4180-1130-4. [Google Scholar]
- Bortoli, R.; Santiago, M. Chloroquine Ototoxicity. Clin. Rheumatol. 2007, 26, 1809–1810. [Google Scholar] [CrossRef]
- Fee, W.E. Aminoglycoside Ototoxicity in the Human. Laryngoscope 1980, 90, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.G.; Schaefer, S.D. Inner Ear Histopathology in Patients Treated with Cis-Platinum. Laryngoscope 1982, 92, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Schuknecht, H. Pathology of the Ear, 2nd ed.; Lea & Febiger: Malvern, UK, 1993; ISBN 0-8121-1562-7. [Google Scholar]
- Dreschler, W.A.; van der Hulst, R.J.; Tange, R.A.; Urbanus, N.A. Role of High-Frequency Audiometry in the Early Detection of Ototoxicity. II. Clinical Aspects. Audiol. Off. Organ. Int. Soc. Audiol. 1989, 28, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Dreschler, W.A.; vd Hulst, R.J.; Tange, R.A.; Urbanus, N.A. The Role of High-Frequency Audiometry in Early Detection of Ototoxicity. Audiol. Off. Organ. Int. Soc. Audiol. 1985, 24, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Fausti, S.A.; Frey, R.H.; Henry, J.A.; Olson, D.J.; Schaffer, H.I. Early Detection of Ototoxicity Using High-Frequency, Tone-Burst-Evoked Auditory Brainstem Responses. J. Am. Acad. Audiol. 1992, 3, 397–404. [Google Scholar] [PubMed]
- Frank, T. High-Frequency Hearing Thresholds in Young Adults Using a Commercially Available Audiometer. Ear Hear. 1990, 11, 450–454. [Google Scholar] [CrossRef]
- Osterhammel, D. High Frequency Audiometry. Clinical Aspects. Scand. Audiol. 1980, 9, 249–256. [Google Scholar] [CrossRef]
- Kujansuu, E.; Rahko, T.; Punnonen, R.; Karma, P. Evaluation of the Hearing Loss Associated with Cis-Platinum Treatment by High-Frequency Audiometry. Gynecol. Oncol. 1989, 33, 321–322. [Google Scholar] [CrossRef]
- Wiley, T.L.; Cruickshanks, K.J.; Nondahl, D.M.; Tweed, T.S.; Klein, R.; Klein, R.; Klein, B.E. Aging and High-Frequency Hearing Sensitivity. J. Speech Lang. Hear. Res. 1998, 41, 1061–1072. [Google Scholar] [CrossRef]
- Beck, A.; Maurer, J.; Welkoborsky, H.J.; Mann, W. [Changes in transitory evoked otoacoustic emissions in chemotherapy with cisplatin and 5FU]. HNO 1992, 40, 123–127. [Google Scholar]
- Ress, B.D.; Sridhar, K.S.; Balkany, T.J.; Waxman, G.M.; Stagner, B.B.; Lonsbury-Martin, B.L. Effects of Cis-Platinum Chemotherapy on Otoacoustic Emissions: The Development of an Objective Screening Protocol. Third Place--Resident Clinical Science Award 1998. Otolaryngol. Head Neck Surg. 1999, 121, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Zorowka, P.G.; Schmitt, H.J.; Gutjahr, P. Evoked Otoacoustic Emissions and Pure Tone Threshold Audiometry in Patients Receiving Cisplatinum Therapy. Int. J. Pediatric Otorhinolaryngol. 1993, 25, 73–80. [Google Scholar] [CrossRef]
- Norton, S.J. Cochlear Function and Otoacoustic Emissions. Semin. Hear. 1992, 13, 1–14. [Google Scholar] [CrossRef]
- Probst, R.; Lonsbury-Martin, B.L.; Martin, G.K. A Review of Otoacoustic Emissions. J. Acoust. Soc. Am. 1991, 89, 2027–2067. [Google Scholar] [CrossRef]
- Rybak, L.P. Ototoxicity of Loop Diuretics. Otolaryngol Clin. North Am. 1993, 26, 829–844. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering and Medicine. Assessment of Long-Term Health Effects of Antimalarial Drugs When Used for Prophylaxis; Savitz, D.A., Styka, A.N., Eds.; The National Academies Press: Washington, DC, USA, 2020; ISBN 978-0-309-67210-8. [Google Scholar]
| Name of Medication: Anti-COVID-19 Treatment (Monoclonal Antibodies, Anti-Inflammatory Treatment) | Population | Route of Administration | Dose and Dosage | Ototoxicity as an Adverse Reaction and the Source of Information | Clinical Trial Information |
|---|---|---|---|---|---|
| Dexamethasone Phosphate | Adults and children from 12 years of age and weighing at least 40 kg, who received supplemental oxygen therapy | Orally Or Injection Or Infusion (drip) into a vein | 6 mg once a day for up to 10 days | Blurry Vision with Dizziness (SmPC) | |
| Publication: RECOVERY clinical study [78] | 2104 patients in dexamethasone group and 4321 to receive usual care | ||||
| Anakinra | The treatment of coronavirus disease 2019 (COVID-19) in adult patients with pneumonia requiring supplemental oxygen (low- or high-flow oxygen) who are at risk of progressing to severe respiratory failure | Subcutaneous injection | 100 mg administered once a day | No Adverse Reactions Identified as Audio-Vestibular Disorders (SmPC) | |
| and publications: | 594 adult patients with COVID-19 at risk of progressing to respiratory failure (189 patients were allocated to the placebo arm; and 405 patients were allocated to the anakinra arm) [40]. | ||||
| -publication: | 116 patients: 59 were assigned to the anakinra group, and 57 were assigned to the usual care group [79]. | ||||
| Bamlanivimab | Adults and pediatric patients > 12 years old, weight > 40 kg, and at high risk for progressing to severe disease and/or hospitalization | Intravenously | Single 700-mg IV infusion over at least 60 min | Dizziness (frequency: common) (SmPC) | BLAZE-1 clinical trial |
| Publication: | 1035 patients (the dose: 2800 mg of bamlanivimab and 2800 mg of etesevimab, administered together) or placebo within 3 days after a laboratory diagnosis of SARS-CoV-2 [46]. | ||||
| Casirivimab and Imdevimab | Adults and pediatric patients (12 years of age or older weighing at least 40 kg) | Administered together by intravenous (IV) infusion | 1200 mg of casirivimab and 1200 mg of imdevimab administered as a single intravenous infusion over at least 60 min as soon as possible after positive Vidal test for SARS-CoV-2 and within 10 days of symptom onset | Limited Data about Ototoxicity (SMPC) | Randomized, double-blind, placebo-controlled clinical trial in 799 non-hospitalized adults with mild to moderate COVID-19 symptoms. |
| and publications: | 5197 adult participants; adverse events occurred in 35% and 34% of participants administered casirivimab and imdevimab) and placebo, respectively, and injection site reactions occurred in 2.4% and 2.1% of participants, respectively [59]. | ||||
| Publication: | 1355 patients in the regen-cov group and 1341 patients in placebo group [80]. | ||||
| publication: | 753 participants in the regen-cov group and 752 participants in the placebo group [51]. | ||||
| publication: | 275 adult participants [81]. | ||||
| Tocilizumab | Critically ill patients, aged> 18 years | IV infusion via central or peripheral line over a 1-h period | Single dose of 8 mg/kg estimated or measured body weight, with a maximum total dose of 800 mg | NASOPHARYNGITIS, DIZZINESS (SmPC) | Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community Acquired Pneumonia (REMAP-CAP). |
| Publication: | no adverse reaction in the area of ENT was reported. serious adverse events occurred in 128 (29.8%) tocilizumab plus remdesivir and 72 (33.8%) placebo plus remdesivir patients; 78 (18.2%) and 42 (19.7%) patients, respectively, died by day 28 [82]. | ||||
| -publication: | 389 patients, (249 patients in the tocilizumab group and 128 patients in the placebo group) [83]. | ||||
| -publication: | 243 adult patients [84]. | ||||
| publication: | 2046 patients who had severe disease had undergone randomization in at least one remap-cap domain and 895 had undergone randomization in the immune modulation therapy domain [85]. | ||||
| Tixagevimab and Cilgavimab | The pre-exposure prophylaxis of coronavirus disease 2019 (COVID-19) in adults and pediatric individuals (12 years of age and older weighing at least 40 kg | Intramuscular injections | Single injection of 150 mg tixagevimab and 150 mg cilgavimab | No adverse reactions identified as audio-vestibular disorders (SmPC) | 4220 subjects in two clinical trials, phase III, PROVENT and STORM CHASER |
| Sotrovimab | The treatment of adults and adolescents of age above 12 years and weighing minimum 40 kg, suffering from COVID-19 disease and not requiring supplemental oxygen and at the same time are at risk of progressing to severe phase of COVID-19 | Infusion | 500 mg of sotrovimab in 8 mL (62.5 mg/mL) in 50 mL or 100 mL of sodium chloride (0.9%) or 5% glucose | No adverse reactions identified as audio-vestibular disorders (SmPC) | clinical trial (COMET-ICE) of 1057 subjects |
| publication: | 583 patients (291 in the sotrovimab group and 292 in the placebo group [61] | ||||
| Sarilumab | Critically ill patients, aged> 18 years Adults and pediatric patients >16 years of age | IV infusion through peripheral or central line over a 1-h period | Single dose of 400 mg | NASOPHARYNGITIS (SmPC) | Sarilumab COVID-19 clinical trial |
| Name of Medication and Source of Information Vaccines | Population | Route of Administration | Dose and Dosage | Ototoxicity as an Adverse Reaction | Clinical Trial Information |
| Pfizer + Biontech Vaccine: Comirnaty | Adults and children above 5 years old | One dose (0.3 mL) contains 30 µg of COVID-19 mRNA embedded in lipid nanoparticles for adults and 10 µg/dose for children | Intramuscularly—twice with an interval of 21 days between doses | Dizziness may occur only in hypersensitive people as apart of anaphylactic shock and as adverse reaction (SmPC) | Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community Acquired Pneumonia (REMAP-CAP) 2 clinical studies that include 21,744 participants, who received at least one dose of vaccine |
| Dizziness and nasal stuffiness | 1245 participants; the aim of the study was to assess the safety and side-effects of the bnt162b2 mRNA vaccine for coronavirus disease 2019 (COVID-19) [86]. | ||||
| -publication: | 890,828 persons; 42-day follow-up; aim: evaluation of the safety of the bnt162b2 mRNA vaccine [87]. | ||||
| Publication: | 43,448 patients (above 16 years old): 21,720 with bnt162b2 and 21,728 with placebo. -adverse reactions: the safety profile of bnt162b2 was characterized by short-term, mild-to-moderate pain at the injection site, fatigue, and headache. the incidence of serious adverse events was low and was similar in the vaccine and placebo groups [88]. | ||||
| COVID-19 Vaccine Moderna | Adults and children above 5 years old | One dose (0.5 mL) contains 100 µg of COVID-19 messenger RNA embedded in lipid nanoparticles for adults and 50 µg/dose for children | Intramuscularly—twice with an interval of 28 days between the doses | Dizziness may occur only in hypersensitive people as apart of anaphylactic shock and as adverse reaction (SmPC) | Clinical Phase 3 trial (randomised, placebo controlled, observer blind) involving 30,351 participants who received at least one dose of vaccine |
| COVID-19 Vaccine AstraZeneca | Adults | One dose (0.5 mL) contains COVID-19 vaccine (ChAdOx 1-S* recombinant) 5 × 1010 viral particles | Intramuscularly—twice with an interval of 28 days between two doses (from 4 to 26 weeks) | Dizziness may occur only in hypersensitive people as apart of anaphylactic shock and as adverse reaction (SmPC) | 4 clinical trials involving; 23,745 participants; Adverse reaction: dizziness may occur only in hypersensitive people as apart of anaphylactic shock and as adverse reaction. |
| publication: | 43,788 participants underwent randomization and received vaccine or placebo, and 39,185 participants who were seronegative for SARS-CoV-2 at baseline were included in the per-protocol analysis population for the double-blind phase [89]. | ||||
| publication: | adverse reactions: unsolicited adverse events were recorded for all participants for 28 days after each dose of azd1222 or placebo, but no in the area of ENT [76] | ||||
| publication: | 175 severe adverse events occurred in 168 participants, 84 events in the chadox1 ncov-19 group, and 91 in the control group, but none in the area of ENT [90]. | ||||
| Publication: | the incidence of serious adverse events was balanced between the two groups. Three deaths occurred in the vaccine group (none were COVID-19-related), and 16 in the placebo group (5 were COVID-19-related) [91]. | ||||
| Covid-19 Vaccine Novovax (Nuvaxovid) | Adults | One dose (0.5 mL) contains 5 µg of SARS-CoV-2 spike protein and is adjuvanted with Matrix-M | Intramuscularly—twice with an interval of 28 days between the doses | dizziness may occur only in hypersensitive people as apart of anaphylactic shock and as adverse reaction (smpc) | 5 clinical trials. Total number of patients enrolled to this study was 49,950 adult participants (Nuvaxovid group N = 30,058 and placebo group N = 19,892) |
| Covid-19 Vaccine Janssen | Adults | Janssen is an adenovirus vaccine against COVID-19 diseases and one dose (0.5 mL) contains adenovirus type 26 encoding the SARS-CoV-2 spike glycoprotein (Ad26.COV2-S), not less than 8.92 log10 infectious units | Intramuscularly—at least 2 months after the primary vaccination | dizziness, tinnitus (smpc) | The safety of COVID-19 Vaccine Janssen was evaluated in an ongoing Phase 3 study (COV3001), with a total of 21,895 adults |
| Name of Medication and Source of Information Oral Treatment | Population | Route of Administration | Dose and Dosage | Ototoxicity as an Adverse Reaction | Clinical Trial Information |
| PF-07321332 and Ritonavir (Brand Name: Paxlovid) | Adults | 300 mg (two tablets of 150 mg each) of nirmatrelvir and 100 mg (one tablet) of ritonavir | Orally, twice a day for 5 days | dysgeusia, as a side effect in the area of ent (This side-effect was reported by 6% of subjects from Paxlovid group in comparison with the 1% in the placebo group) (SmPC) | The safety and adverse reactions were assessed in a clinical trial (phase 2/3, randomized, placebo-controlled (C4671005 EPIC-HR)), 2224 adults |
| Publication: | -adverse events: that emerged during the treatment period was similar in the two groups (any adverse event, 22.6% with nirmatrelvir plus ritonavir vs. 23.9% with placebo; serious adverse events, 1.6% vs. 6.6%; and adverse events leading to discontinuation of the drugs or placebo, 2.1% vs. 4.2%), dysgeusia (5.6% vs. 0.3%) [92] | ||||
| Molnupiravir | adults | 800 mg | orally, twice a day for 5 days | dizziness (smpc) | Phase 3, double-blind, acronym MOVe-OUT), 1411 adults |
| publication: | adverse reactions were reported in 30.4% participants in the molnupiravir group and 33% of participants in the placebo group [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skarzynska, M.B.; Matusiak, M.; Skarzynski, P.H. Adverse Audio-Vestibular Effects of Drugs and Vaccines Used in the Treatment and Prevention of COVID-19: A Review. Audiol. Res. 2022, 12, 224-248. https://doi.org/10.3390/audiolres12030025
Skarzynska MB, Matusiak M, Skarzynski PH. Adverse Audio-Vestibular Effects of Drugs and Vaccines Used in the Treatment and Prevention of COVID-19: A Review. Audiology Research. 2022; 12(3):224-248. https://doi.org/10.3390/audiolres12030025
Chicago/Turabian StyleSkarzynska, Magdalena B., Monika Matusiak, and Piotr H. Skarzynski. 2022. "Adverse Audio-Vestibular Effects of Drugs and Vaccines Used in the Treatment and Prevention of COVID-19: A Review" Audiology Research 12, no. 3: 224-248. https://doi.org/10.3390/audiolres12030025
APA StyleSkarzynska, M. B., Matusiak, M., & Skarzynski, P. H. (2022). Adverse Audio-Vestibular Effects of Drugs and Vaccines Used in the Treatment and Prevention of COVID-19: A Review. Audiology Research, 12(3), 224-248. https://doi.org/10.3390/audiolres12030025








