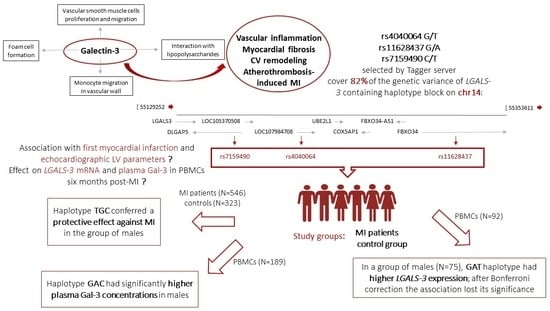
Variants Tagging LGALS-3 Haplotype Block in Association with First Myocardial Infarction and Plasma Galectin-3 Six Months after the Acute Event
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Doppler Echocardiography
2.3. Selection of Tag Variants
2.4. Genetic Analysis
2.5. Reverse Transcription Quantitative Real-Time PCR (RT-qPCR)
2.6. Quantification of pGal-3 Levels
2.7. Statistical Methods
3. Results
3.1. Association of rs4040064, rs11628437, and rs7159490 Haplotypes with MI
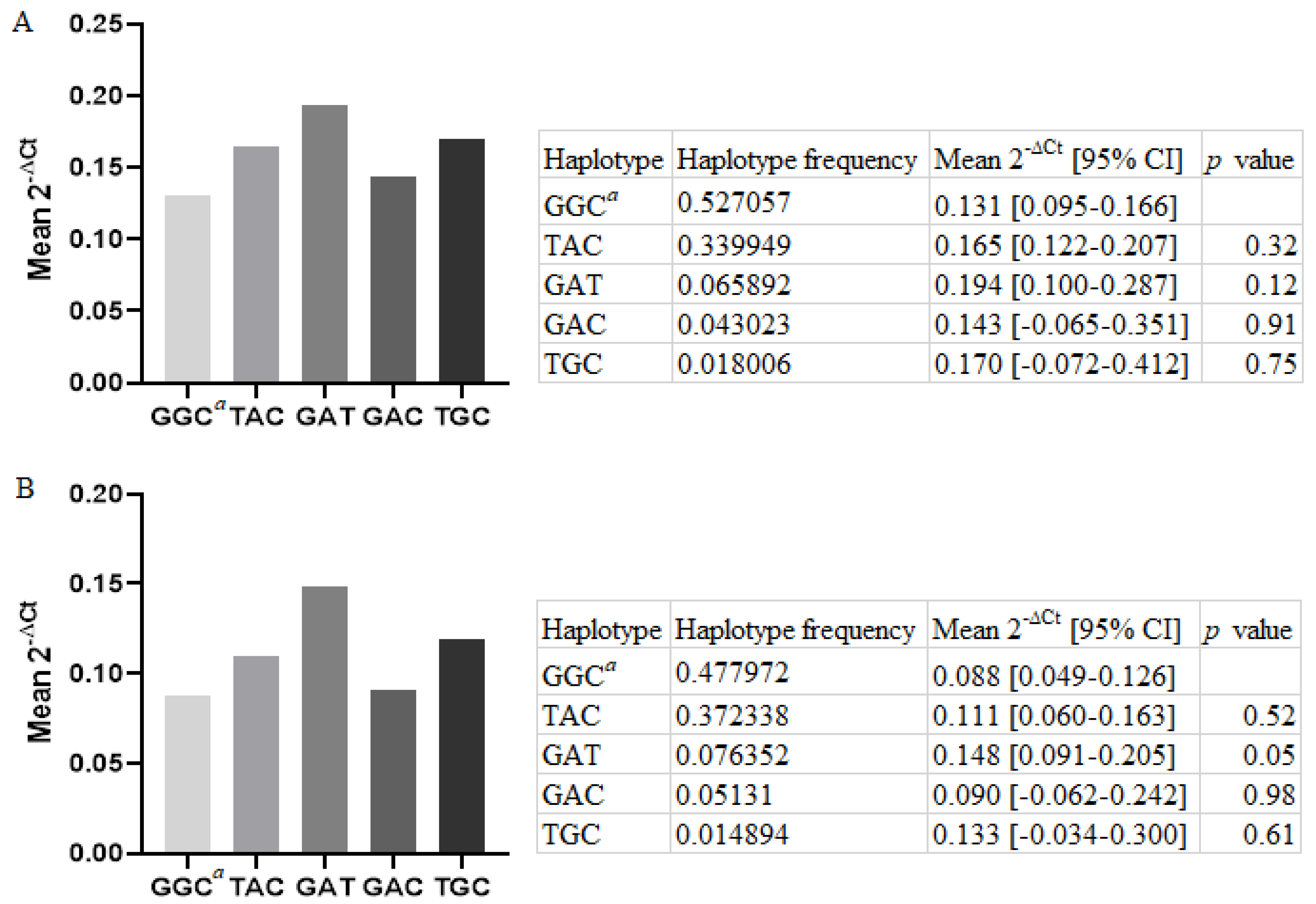
3.2. Relative LGALS-3 mRNA Expression in PBMCs Six Months Post-MI, in Regard to the Haplotypes Inferred from rs4040064, rs11628437, and rs7159490
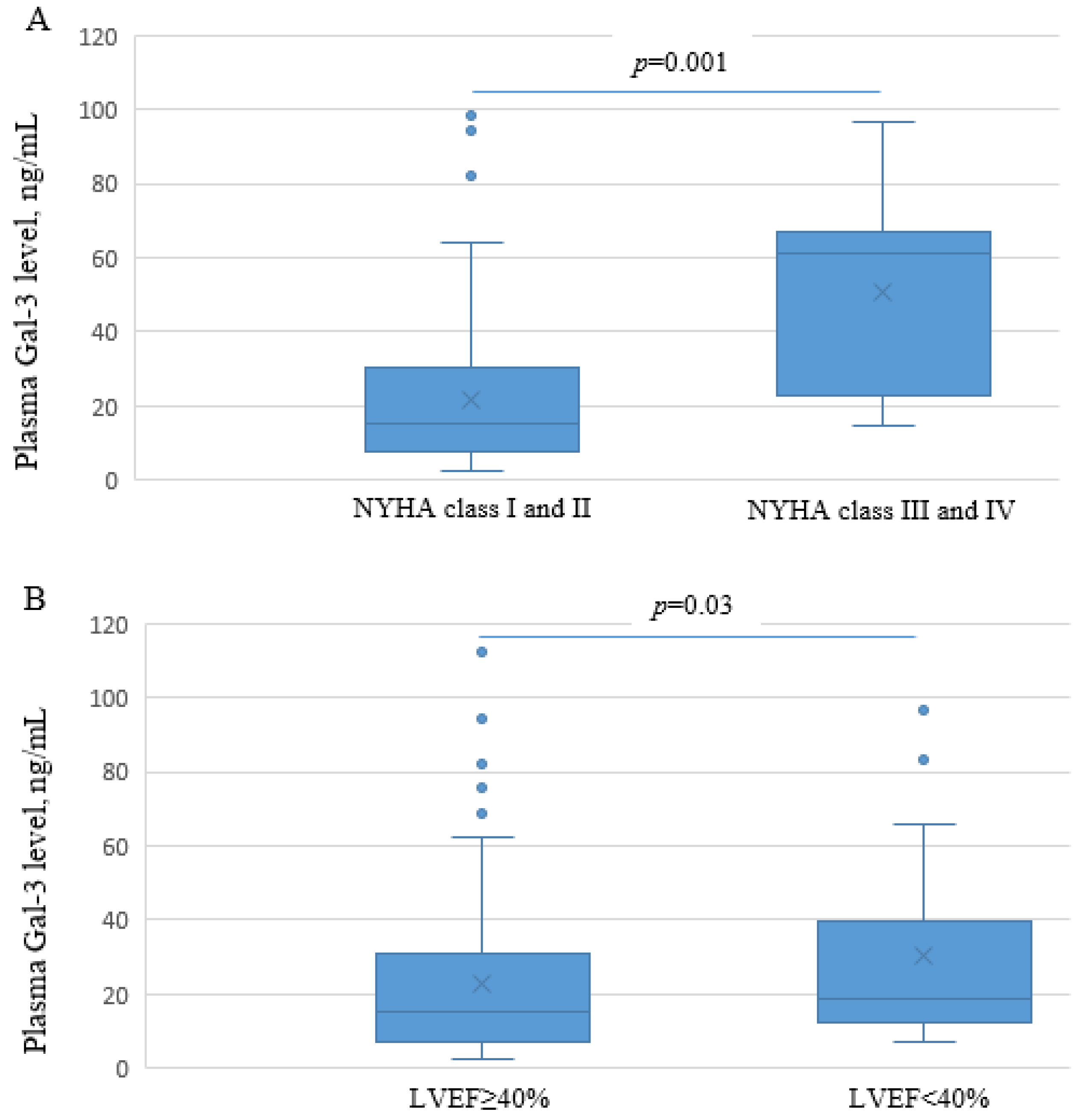
3.3. Plasma Gal-3 in Patients Six Months Post-MI, in Regard to the Haplotypes Inferred from rs4040064, rs11628437, and rs7159490 and in Association with Echocardiographic Parameters Serving for Assessment of LV Function and Structure
3.4. Association of rs4040064, rs11628437, and rs7159490 Haplotypes with a Change in Echocardiographic Parameters Serving for Assessment of LV Function and Structure within Six Months Post-MI
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 9 December 2020).
- Kessler, T.; Vilne, B.; Schunkert, H. The impact of genome-wide association studies on the pathophysiology and therapy of cardiovascular disease. EMBO Mol. Med. 2016, 8, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Zaliaduonyte-Peksiene, D.; Simonyte, S.; Lesauskaite, V.; Vaskelyte, J.; Gustiene, O.; Mizariene, V.; Jurkevicius, R.; Jariene, G.; Tamosiunas, A.; Zaliunas, R. Left ventricular remodelling after acute myocardial infarction: Impact of clinical, echocardiographic parameters and polymorphism of angiotensinogen gene. J. Renin. Angiotensin Aldosterone Syst. 2014, 15, 286–293. [Google Scholar] [CrossRef] [PubMed]
- McLean, R.C.; Hirsch, G.A.; Becker, L.C.; Kasch-Semenza, L.; Gerstenblith, G.; Schulman, S.P. Polymorphisms of the beta adrenergic receptor predict left ventricular remodeling following acute myocardial infarction. Cardiovasc. Drugs Ther. 2011, 25, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Agnello, L.; Bivona, G.; Lo Sasso, B.; Scazzone, C.; Bazan, V.; Bellia, C.; Ciaccio, M. Galectin-3 in acute coronary syndrome. Clin. Biochem. 2017, 50, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.Q.; Yu, X.; Leng, P. Research progress on the role of gal-3 in cardio/cerebrovascular diseases. Biomed. Pharmacother. 2021, 133, 111066. [Google Scholar] [CrossRef]
- Suthahar, N.; Meijers, W.C.; Silljé, H.H.W.; Ho, J.E.; Liu, F.T.; de Boer, R.A. Galectin-3 Activation and Inhibition in Heart Failure and Cardiovascular Disease: An Update. Theranostics 2018, 8, 593–609. [Google Scholar] [CrossRef]
- Meijers, W.C.; van der Velde, A.R.; Pascual-Figal, D.A.; de Boer, R.A. Galectin-3 and post-myocardial infarction cardiac remodeling. Eur. J. Pharmacol. 2015, 763, 115–121. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef]
- De Boer, R.A.; Verweij, N.; van Veldhuisen, D.J.; Westra, H.J.; Bakker, S.J.; Gansevoort, R.T.; Muller Kobold, A.C.; van Gilst, W.H.; Franke, L.; Leach, I.M.; et al. A genome-wide association study of circulating galectin-3. PLoS ONE 2012, 7, e47385. [Google Scholar] [CrossRef]
- Folkersen, L.; Fauman, E.; Sabater-Lleal, M.; Strawbridge, R.J.; Frånberg, M.; Sennblad, B.; Baldassarre, D.; Veglia, F.; Humphries, S.E.; Rauramaa, R.; et al. Mapping of 79 loci for 83 plasma protein biomarkers in cardiovascular disease. PLoS Genet. 2017, 13, e1006706. [Google Scholar] [CrossRef]
- Folkersen, L.; Gustafsson, S.; Wang, Q.; Hansen, D.H.; Hedman, Å.K.; Schork, A.; Page, K.; Zhernakova, D.V.; Wu, Y.; Peters, J.; et al. Genomic and drug target evaluation of 90 cardiovascular proteins in 30,931 individuals. Nat. Metab. 2020, 2, 1135–1148. [Google Scholar] [CrossRef]
- De Bakker, P.I.; Yelensky, R.; Pe’er, I.; Gabriel, S.B.; Daly, M.J.; Altshuler, D. Efficiency and power in genetic association studies. Nat. Genet. 2005, 37, 1217–1223. [Google Scholar] [CrossRef]
- GTEx Consortium. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef]
- Kerimov, N.; Hayhurst, J.D.; Peikova, K.; Manning, J.R.; Walter, P.; Kolberg, L.; Samoviča, M.; Sakthivel, M.P.; Kuzmin, I.; Trevanion, S.J.; et al. A compendium of uniformly processed human gene expression and splicing quantitative trait loci. Nat. Genet. 2021, 53, 1290–1299. [Google Scholar] [CrossRef]
- Boyle, A.P.; Hong, E.L.; Hariharan, M.; Cheng, Y.; Schaub, M.A.; Kasowski, M.; Karczewski, K.J.; Park, J.; Hitz, B.C.; Weng, S.; et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 2012, 22, 1790–1797. [Google Scholar] [CrossRef]
- Djordjevic, A.; Dekleva, M.; Zivkovic, M.; Stankovic, A.; Markovic Nikolic, N.; Alavantic, D.; Djuric, T. Left ventricular remodeling after the first myocardial infarction in association with LGALS-3 neighbouring variants rs2274273 and rs17128183 and its relative mRNA expression: A prospective study. Mol. Biol. Rep. 2018, 45, 2227–2236. [Google Scholar] [CrossRef]
- Djordjevic, A.; Zivkovic, M.; Koncar, I.; Stankovic, A.; Kuveljic, J.; Djuric, T. Tag Variants of LGALS-3 Containing Haplotype Block in Advanced Carotid Atherosclerosis. J. Stroke Cerebrovasc. Dis. 2022, 31, 106212. [Google Scholar] [CrossRef]
- Bruyninckx, R.; Aertgeerts, B.; Bruyninckx, P.; Buntinx, F. Signs and symptoms in diagnosing acute myocardial infarction and acute coronary syndrome: A diagnostic meta-analysis. Br. J. Gen. Pract. 2008, 58, 105–111. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Schiller, N.B.; Shah, P.M.; Crawford, M.; DeMaria, A.; Devereux, R.; Feigenbaum, H.; Gutgesell, H.; Reichek, N.; Sahn, D.; Schnittger, I.; et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J. Am. Soc. Echocardiogr. 1989, 2, 358–367. [Google Scholar] [CrossRef]
- McMurray, J.J.; Adamopoulos, S.; Anker, S.D.; Auricchio, A.; Böhm, M.; Dickstein, K.; Falk, V.; Filippatos, G.; Fonseca, C.; Gomez-Sanchez, M.A.; et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2012, 14, 803–869. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, M.; New York Heart Association, Criteria Committee. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels; Little, Brown: Boston, MA, USA, 1994; pp. 253–256. [Google Scholar]
- Tsai, T.H.; Sung, P.H.; Chang, L.T.; Sun, C.K.; Yeh, K.H.; Chung, S.Y.; Chua, S.; Chen, Y.L.; Wu, C.J.; Chang, H.W.; et al. Value and level of galectin-3 in acute myocardial infarction patients undergoing primary percutaneous coronary intervention. J. Atheroscler. Thromb. 2012, 19, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, L.M.; Smith, K.D.; Boyer, S.H.; Borgaonkar, D.S.; Wachtel, S.S.; Miller, O.J.; Breg, W.R.; Jones, H.W., Jr.; Rary, J.M. Analysis of human Y-chromosome-specific reiterated DNA in chromosome variants. Proc. Natl. Acad. Sci. USA 1977, 74, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, A.; Zivkovic, M.; Stankovic, A.; Zivotic, I.; Koncar, I.; Davidovic, L.; Alavantic, D.; Djuric, T. Genetic Variants in the Vicinity of LGALS-3 Gene and LGALS-3 mRNA Expression in Advanced Carotid Atherosclerosis: An Exploratory Study. J. Clin. Lab. Anal. 2016, 30, 1150–1157. [Google Scholar] [CrossRef]
- Tregouet, D.A.; Escolano, S.; Tiret, L.; Mallet, A.; Golmard, J.L. A new algorithm for haplotype-based association analysis: The Stochastic-EM algorithm. Ann. Hum. Genet. 2004, 68, 165–177. [Google Scholar] [CrossRef]
- Tregouet, D.A.; Garelle, V. A new JAVA interface implementation of THESIAS: Testing haplotype effects in association studies. Bioinformatics 2007, 23, 1038–1039. [Google Scholar] [CrossRef]
- Menashe, I.; Rosenberg, P.S.; Chen, B.E. PGA: Power calculator for case-control genetic association analyses. BMC Genet. 2008, 9, 36. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alföldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef]
- Ibrahim, B.A.; Mohamed, S.H.; Hassaan, M.M.M.; Sabbah, N.A. Associations of galectin-3 expression and LGALS-3 (rs4652) gene variant with coronary artery disease risk in diabetics. J. Med. Biochem. 2021, 40, 395–406. [Google Scholar] [CrossRef]
- Cunha, E.G.C.; de Lima, C.A.D.; Vilar, K.M.; Nóbrega, M.F.; Almeida, A.R.; Pereira, M.C.; Dantas, A.T.; Gonçalves, R.S.G.; Rêgo, M.J.B.M.; Duarte, A.L.B.P.; et al. Genetic variants in LGALS3 are related to lower galectin-3 serum levels and clinical outcomes in systemic sclerosis patients: A case-control study. Autoimmunity 2021, 54, 187–194. [Google Scholar] [CrossRef]
- Howe, K.L.; Achuthan, P.; Allen, J.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Azov, A.G.; Bennett, R.; Bhai, J.; et al. Ensembl 2021. Nucleic Acids Res. 2021, 49, D884–D891. [Google Scholar] [CrossRef]
- Mitchell, B.D.; Fornage, M.; McArdle, P.F.; Cheng, Y.C.; Pulit, S.L.; Wong, Q.; Dave, T.; Williams, S.R.; Corriveau, R.; Gwinn, K.; et al. Using previously genotyped controls in genome-wide association studies (GWAS): Application to the Stroke Genetics Network (SiGN). Front. Genet. 2014, 5, 95. [Google Scholar] [CrossRef][Green Version]
- Carty, C.L.; Keene, K.L.; Cheng, Y.C.; Meschia, J.F.; Chen, W.M.; Nalls, M.; Bis, J.C.; Kittner, S.J.; Rich, S.S.; Tajuddin, S.; et al. Meta-Analysis of Genome-Wide Association Studies Identifies Genetic Risk Factors for Stroke in African Americans. Stroke 2015, 46, 2063–2068. [Google Scholar] [CrossRef]
- Behr, E.R.; Ritchie, M.D.; Tanaka, T.; Kääb, S.; Crawford, D.C.; Nicoletti, P.; Floratos, A.; Sinner, M.F.; Kannankeril, P.J.; Wilde, A.A.; et al. Genome wide analysis of drug-induced torsades de pointes: Lack of common variants with large effect sizes. PLoS ONE 2013, 8, e78511. [Google Scholar] [CrossRef]
- Kotnik, P.; Knapič, E.; Kokošar, J.; Kovač, J.; Jerala, R.; Battelino, T.; Horvat, S. Identification of novel alleles associated with insulin resistance in childhood obesity using pooled-DNA genome-wide association study approach. Int. J. Obes. 2018, 42, 686–695. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Daniels, L.B.; Clopton, P.; Laughlin, G.A.; Maisel, A.S.; Barrett-Connor, E. Galectin-3 is independently associated with cardiovascular mortality in community-dwelling older adults without known cardiovascular disease: The Rancho Bernardo Study. Am. Heart J. 2014, 167, 674–682.e1. [Google Scholar] [CrossRef]
- De Boer, R.A.; van Veldhuisen, D.J.; Gansevoort, R.T.; Muller Kobold, A.C.; van Gilst, W.H.; Hillege, H.L.; Bakker, S.J.; van der Harst, P. The fibrosis marker galectin-3 and outcome in the general population. J. Intern. Med. 2012, 272, 55–64. [Google Scholar] [CrossRef]
- Florido, R.; Kwak, L.; Echouffo-Tcheugui, J.B.; Zhang, S.; Michos, E.D.; Nambi, V.; Goldberg, R.B.; Hoogeveen, R.C.; Lazo, M.; Gerstenblith, G.; et al. Obesity, Galectin-3, and Incident Heart Failure: The ARIC Study. J. Am. Heart Assoc. 2022, 11, e023238. [Google Scholar] [CrossRef]
- Liu, Y.; Beyer, A.; Aebersold, R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef]
- Fortelny, N.; Overall, C.M.; Pavlidis, P.; Freue, G.V.C. Can we predict protein from mRNA levels? Nature 2017, 547, E19–E20. [Google Scholar] [CrossRef] [PubMed]
- Al-Salam, S.; Hashmi, S. Myocardial Ischemia Reperfusion Injury: Apoptotic, Inflammatory and Oxidative Stress Role of Galectin-3. Cell Physiol. Biochem. 2018, 50, 1123–1139. [Google Scholar] [CrossRef] [PubMed]
- Cassaglia, P.; Penas, F.; Betazza, C.; Estevez, F.F.; Miksztowicz, V.; Naya, N.M.; Llamosas, M.C.; Truant, S.N.; Wilensky, L.; Volberg, V.; et al. Genetic Deletion of Galectin-3 Alters the Temporal Evolution of Macrophage Infiltration and Healing Affecting the Cardiac Remodeling and Function after Myocardial Infarction in Mice. Am. J. Pathol. 2020, 190, 1789–1800. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.C.; Mosleh, W.; Chaudhari, M.R.; Katkar, R.; Weil, B.; Evelo, C.; Cimato, T.R.; Pokharel, S.; Blankesteijn, W.M.; Suzuki, G. Myocardial and Serum Galectin-3 Expression Dynamics Marks Post-Myocardial Infarction Cardiac Remodelling. Heart Lung Circ. 2017, 26, 736–745. [Google Scholar] [CrossRef]
- Van der Velde, A.R.; Gullestad, L.; Ueland, T.; Aukrust, P.; Guo, Y.; Adourian, A.; Muntendam, P.; van Veldhuisen, D.J.; de Boer, R.A. Prognostic value of changes in galectin-3 levels over time in patients with heart failure: Data from CORONA and COACH. Circ. Heart Fail. 2013, 6, 219–226. [Google Scholar] [CrossRef]
- Felker, G.M.; Fiuzat, M.; Shaw, L.K.; Clare, R.; Whellan, D.J.; Bettari, L.; Shirolkar, S.C.; Donahue, M.; Kitzman, D.W.; Zannad, F.; et al. Galectin-3 in ambulatory patients with heart failure: Results from the HF-ACTION study. Circ. Heart Fail. 2012, 5, 72–78. [Google Scholar] [CrossRef]
- Pecherina, T.; Kutikhin, A.; Kashtalap, V.; Karetnikova, V.; Gruzdeva, O.; Hryachkova, O.; Barbarash, O. Serum and Echocardiographic Markers May Synergistically Predict Adverse Cardiac Remodeling after ST-Segment Elevation Myocardial Infarction in Patients with Preserved Ejection Fraction. Diagnostics 2020, 10, 301. [Google Scholar] [CrossRef]
- Mayr, A.; Klug, G.; Mair, J.; Streil, K.; Harrasser, B.; Feistritzer, H.J.; Jaschke, W.; Schocke, M.; Pachinger, O.; Metzler, B. Galectin-3: Relation to infarct scar and left ventricular function after myocardial infarction. Int. J. Cardiol. 2013, 163, 335–337. [Google Scholar] [CrossRef]
| Variable | Control Group, N = 323 | MI Group, N = 546 | p Value |
|---|---|---|---|
| Age, years | 54.1 ± 14.2 | 58.4 ± 11.4 | <0.01 § |
| Gender, f/m, % | 44.58/55.42 | 28.39/71.61 | <0.01 § |
| BMI, kg/m2 | 25.09 ± 3.68 | 27.26 ± 4.01 | <0.01 § |
| TC, mmol/L | 5.61 ± 1.30 | 5.61 ± 1.51 | ns |
| HDLC, mmol/L | 1.48 ± 0.86 | 1.12 ± 0.34 | <0.01 § |
| LDLC, mmol/L | 3.31 ± 1.23 | 3.67 ± 1.04 | <0.01 § |
| TG, mmol/L | 1.58 ± 1.09 | 1.86 ± 1.27 | <0.01 § |
| T2DM, % | 0.00 | 17.10 | N/A |
| Hypertension, % | 27.43 | 65.76 | <0.01 |
| Current smokers, % | 55.04 | 64.09 | 0.06 |
| Haplotype § | Haplotype Frequency | Haplotype Effect on Risk of MI | ||
|---|---|---|---|---|
| Control Group | MI Group | OR [95% CI] # | p Value | |
| Overall | N = 323 | N = 546 | ||
| GGC | 0.61625 | 0.6113 | referent haplotype | |
| TGC ¥ | 0.01667 | 0.00437 | N/A | N/A |
| TAC | 0.25505 | 0.25879 | 1.06 [0.83–1.37] | 0.62 |
| GAT | 0.07233 | 0.0886 | 1.30 [0.85–1.98] | 0.22 |
| GAC | 0.01799 | 0.02834 | 1.69 [0.77–3.71] | 0.19 |
| TGT ¥ | 0.00953 | 0.00146 | N/A | N/A |
| TAT ¥ | 0.0074 | 0.00223 | N/A | N/A |
| GGT ¥ | 0.00478 | 0.00492 | N/A | N/A |
| Males | N = 179 | N = 391 | ||
| GGC | 0.59517 | 0.58863 | referent haplotype | |
| TGC | 0.03082 | 0.00543 | 0.19 [0.05–0.72] | 0.015 |
| TAC | 0.2674 | 0.27426 | 1.12 [0.80–1.57] | 0.49 |
| GAT | 0.06479 | 0.09247 | 1.54 [0.87–2.72] | 0.13 |
| GAC | 0.01739 | 0.02933 | 1.79 [0.64–4.50] | 0.27 |
| TGT ¥ | 0.01083 | 0.00193 | N/A | N/A |
| TAT ¥ | 0.00772 | 0.00278 | N/A | N/A |
| GGT | 0.00587 | 0.00517 | 0.99 [0.10–9.64] | 0.99 |
| Females | N = 144 | N = 155 | ||
| GGC | 0.63392 | 0.67037 | referent haplotype | |
| TGC ¥ | 0.00507 | 0.00022 | N/A | N/A |
| TAC | 0.2443 | 0.22015 | 0.88 [0.58–1.35] | 0.56 |
| GAT | 0.0856 | 0.07816 | 0.90 [0.46–1.76] | 0.75 |
| GAC | 0.01915 | 0.02566 | 1.25 [0.33–4.66] | 0.74 |
| TGT ¥ | 0.00448 | 0.0013 | N/A | N/A |
| TAT ǂ | 0.00 | 0.00 | N/A | N/A |
| GGT ¥ | 0.00749 | 0.00414 | N/A | N/A |
| Haplotype § | Haplotype Frequency | Mean pGal-3 [95% CI], ng/mL | p Value |
|---|---|---|---|
| Overall, N = 189 | |||
| GGC | 0.594489 | 41.2 [36.3–46.2] | referent haplotype |
| TAC | 0.285885 | 43.3 [35.1–51.5] | 0.68 |
| GAT | 0.06574 | 45.7 [31.4–59.9] | 0.59 |
| GAC | 0.03387 | 55.2 [34.5–75.9] | 0.19 |
| Males, N = 142 | |||
| GGC | 0.529661 | 18.9 [14.5–23.4] | referent haplotype |
| TAC | 0.327339 | 17.2 [10.7–23.7] | 0.69 |
| GAT | 0.074224 | 19.3 [3.8–34.8] | 0.96 |
| GAC | 0.041146 | 48.3 [37.3–59.4] | <0.0001 |
| Cardiac Parameter | Haplotype Frequency | Means [95% CI] | p Value |
|---|---|---|---|
| Δ LV End diastolic volume (mL) | |||
| GGC | 0.579682 | 5.78 [0.01–11.55] | ref. haplotype |
| TAC | 0.292608 | −2.72 [−13.81–8.37] | 0.23 |
| GAT | 0.070432 | 6.12 [−15.85–28.09] | 0.98 |
| GAC | 0.035608 | −11.09 [−42.46–20.28] | 0.3 |
| Δ LV End systolic volume (mL) | |||
| GGC | 0.579747 | 3.24 [−0.78–7.27] | ref. haplotype |
| TAC | 0.292733 | −2.98 [−11.12–5.16] | 0.22 |
| GAT | 0.070111 | 17.10 [4.18–30.02] | 0.04 |
| GAC | 0.035804 | −11.49 [−43.27–20.29] | 0.37 |
| Δ LV Ejection fraction (%) | |||
| GGC | 0.582037 | 1.13 [−0.54–2.80] | ref. haplotype |
| TAC | 0.28883 | 3.22 [0.07–6.36] | 0.31 |
| GAT | 0.072803 | −6.31 [−11.15–−1.47] | 0.005 |
| GAC | 0.035033 | 8.83 [−1.99–19.65] | 0.17 |
| Δ Left atrial dimension (mm) | |||
| GGC | 0.582591 | 1.90 [0.97–2.83] | ref. haplotype |
| TAC | 0.287416 | 0.32 [−1.73–2.37] | 0.21 |
| GAT | 0.073188 | −0.86 [−6.65–4.94] | 0.36 |
| GAC | 0.035369 | −1.01 [−16.96–14.93] | 0.72 |
| Δ Global radial strain (%) | |||
| GGC | 0.59579 | 4.12 [1.76–6.48] | ref. haplotype |
| TAC | 0.281092 | −1.01 [−5.60–3.59] | 0.09 |
| GAT | 0.069458 | −3.30 [−10.43–3.83] | 0.06 |
| GAC | 0.040206 | 4.61 [−7.89–17.11] | 0.94 |
| Δ Stroke volume (mL) | |||
| GGC | 0.584989 | 8.03 [3.38–12.68] | ref. haplotype |
| TAC | 0.290219 | 0.78 [−8.12–9.67] | 0.2 |
| GAT | 0.067594 | −13.50 [−35.46–8.46] | 0.06 |
| GAC | 0.034273 | −7.99 [−32.82–16.83] | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djordjevic, A.; Zivkovic, M.; Boskovic, M.; Dekleva, M.; Stankovic, G.; Stankovic, A.; Djuric, T. Variants Tagging LGALS-3 Haplotype Block in Association with First Myocardial Infarction and Plasma Galectin-3 Six Months after the Acute Event. Genes 2023, 14, 109. https://doi.org/10.3390/genes14010109
Djordjevic A, Zivkovic M, Boskovic M, Dekleva M, Stankovic G, Stankovic A, Djuric T. Variants Tagging LGALS-3 Haplotype Block in Association with First Myocardial Infarction and Plasma Galectin-3 Six Months after the Acute Event. Genes. 2023; 14(1):109. https://doi.org/10.3390/genes14010109
Chicago/Turabian StyleDjordjevic, Ana, Maja Zivkovic, Maja Boskovic, Milica Dekleva, Goran Stankovic, Aleksandra Stankovic, and Tamara Djuric. 2023. "Variants Tagging LGALS-3 Haplotype Block in Association with First Myocardial Infarction and Plasma Galectin-3 Six Months after the Acute Event" Genes 14, no. 1: 109. https://doi.org/10.3390/genes14010109
APA StyleDjordjevic, A., Zivkovic, M., Boskovic, M., Dekleva, M., Stankovic, G., Stankovic, A., & Djuric, T. (2023). Variants Tagging LGALS-3 Haplotype Block in Association with First Myocardial Infarction and Plasma Galectin-3 Six Months after the Acute Event. Genes, 14(1), 109. https://doi.org/10.3390/genes14010109