Abstract
Background/Objectives: Temporary artery ligation or compression is commonly used to reduce intraoperative blood loss in various surgeries, including uterine procedures. In head and neck surgery, the external carotid artery (ECA) typically branches into eight vessels, supplying most of the head and neck except for the brain. Severe and uncontrolled bleeding can occur if these branches are inadvertently damaged during surgery. However, limited research exists on temporary arterial ligation during head and neck surgeries. This study aimed to evaluate the effects of temporary ECA restriction and internal maxillary artery (IMA) ligation on minimizing intraoperative blood loss during head and neck surgery. Methods: This study involved 25 patients with terminal-stage maxillary tumors who underwent total maxillectomy. The effectiveness of IMA ligation and ECA restriction using a Rummel tourniquet in controlling intraoperative bleeding was compared. Results: The average blood loss was significantly lower in the ECA restriction (467 mL) and IMA ligation (461 mL) groups than in the control group (794 mL). However, no significant difference was observed between the IMA ligation and ECA restriction methods. Conclusions: Overall, our results suggest that either method is effective; however, ECA restriction is preferred for tumors involving the infratemporal fossa.
1. Introduction
Maxillectomy is performed to treat tumors invading the sinonasal area. Partial maxillectomy is relatively uncomplicated and typically involves no significant intraoperative bleeding. However, the terminal stage of oral cancer often necessitates total maxillectomy, which carries significant risks owing to potential intraoperative bleeding that can lead to severe surgical morbidity [1,2]. The internal maxillary artery (IMA), the primary arterial supply to the maxilla, represents the main source of rapid bleeding during total maxillectomy [3]. Ligating the IMA before initiating the bone cut can significantly reduce bleeding volume [4], thereby decreasing intraoperative bleeding and surgical complications while reducing stress for the surgeon. Additionally, given that the main feeder to the IMA is the external carotid artery (ECA), temporarily applying a tourniquet to ligate the ECA can theoretically control bleeding.
Previous studies have explored bleeding control during cesarean sections through temporary uterine ligation or cross-clamping of the infrarenal abdominal aorta during cesarean hysterectomy to control operative blood loss [5,6]. Nevertheless, similar surgical techniques have not been investigated for bleeding control in head and neck surgery. Therefore, we aimed to determine the potential of temporarily restricting the ECA to reduce bleeding. Specifically, we sought to compare the effects of IMA ligation and ECA restriction before performing a total maxillectomy, assessing outcomes among the IMA ligation, ECA restriction, and control groups.
2. Materials and Methods
2.1. Patients
This study is a retrospective cohort study that includes patients registered at Hualien Tzu Chi Hospital in Eastern Taiwan between 2009 and 2022. The inclusion criteria are tumors that invade the upper gum, maxilla, or sinus and require total maxillectomy. The exclusion criteria are patients with coagulopathy, such as liver cirrhosis, chronic kidney disease, or those using anticoagulants. Additionally, uncontrolled hypertension was also excluded.
After CT scanning, if the tumor is less than 1 cm from the IMA or involves the IMA, proceeding with IMA ligation would be unsuitable due to margins of safety. ECA restriction would be more appropriate in such cases.
2.2. Surgical Techniques
2.2.1. IMA Ligation
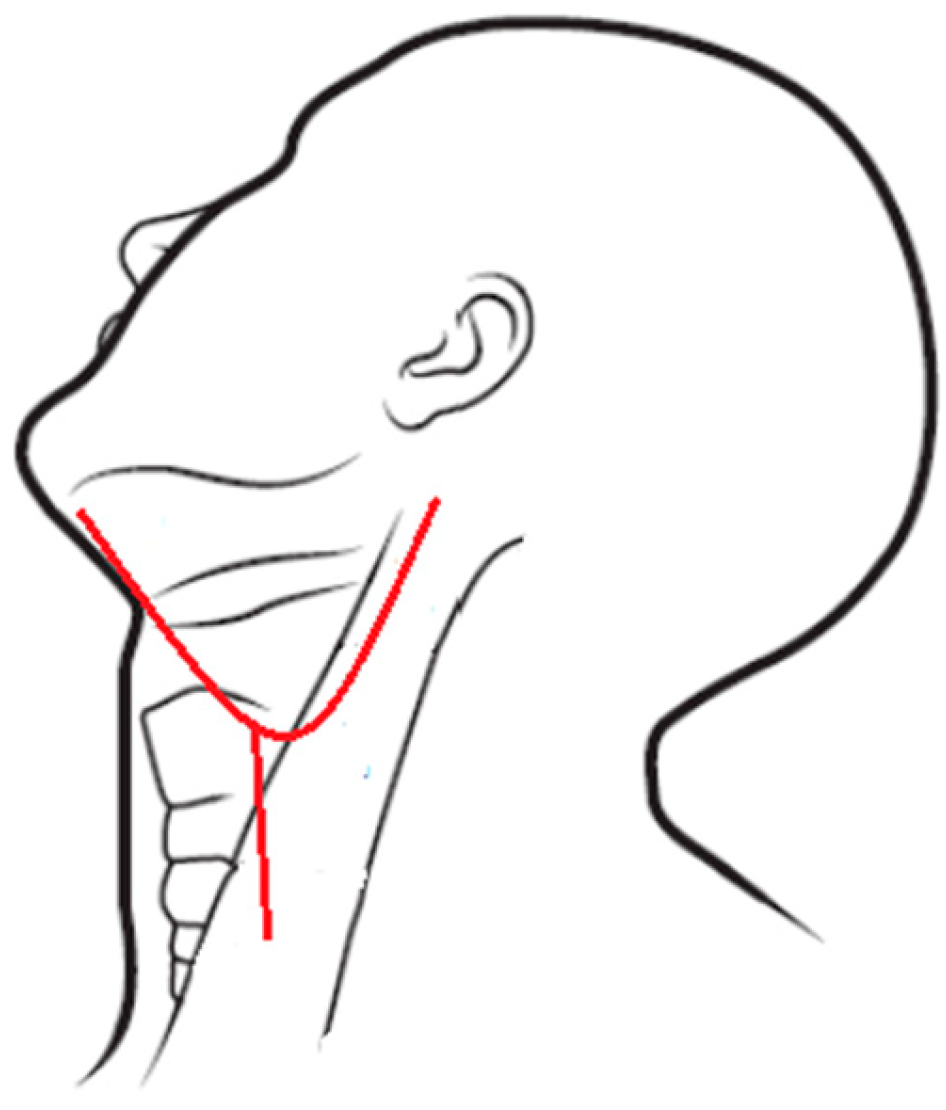
IMA ligation was conducted under general anesthesia, using either oral intubation or a tracheotomy. A Weber–Ferguson incision (Figure 1) was made, and the cheek flap was elevated. The plane was easily separated along the subperiosteal surface of the maxilla (Figure 2). Subsequently, the infraorbital nerve was divided and ligated. The infraorbital foramen is located just medial to the midpupillary line, approximately 0.7–1 cm below the infraorbital rim [7]. Access to the postmaxillary space was achieved by separating the masseter muscle from the zygomatic arch. The intraoral incision followed the buccogingival sulcus, extending to the third upper molar or, if necessary, the ascending ramus of the mandible.
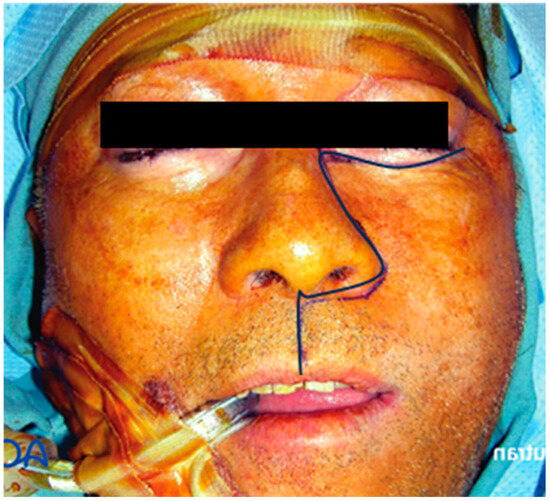
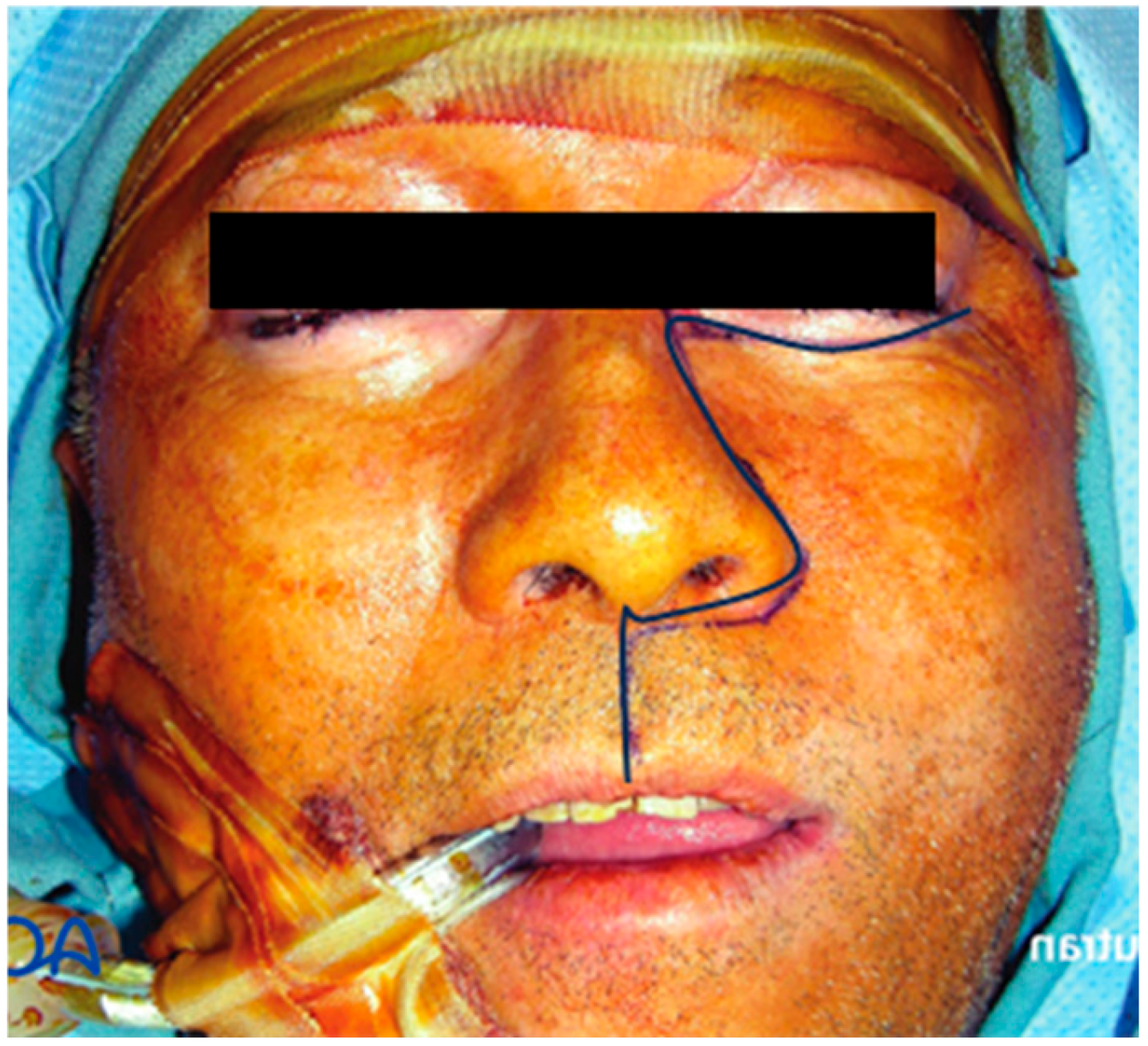
Figure 1.
Weber–Ferguson incision.
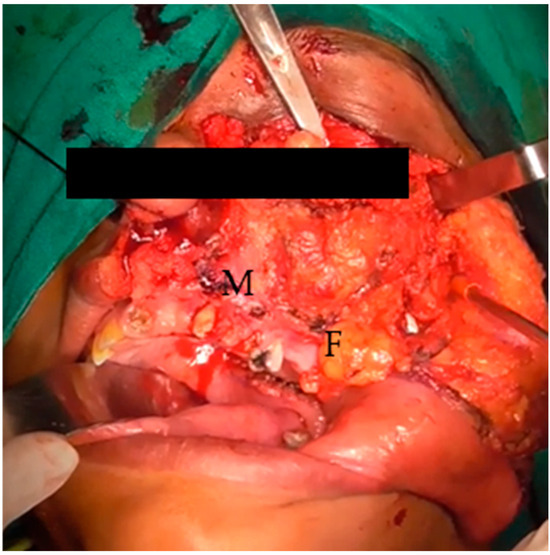
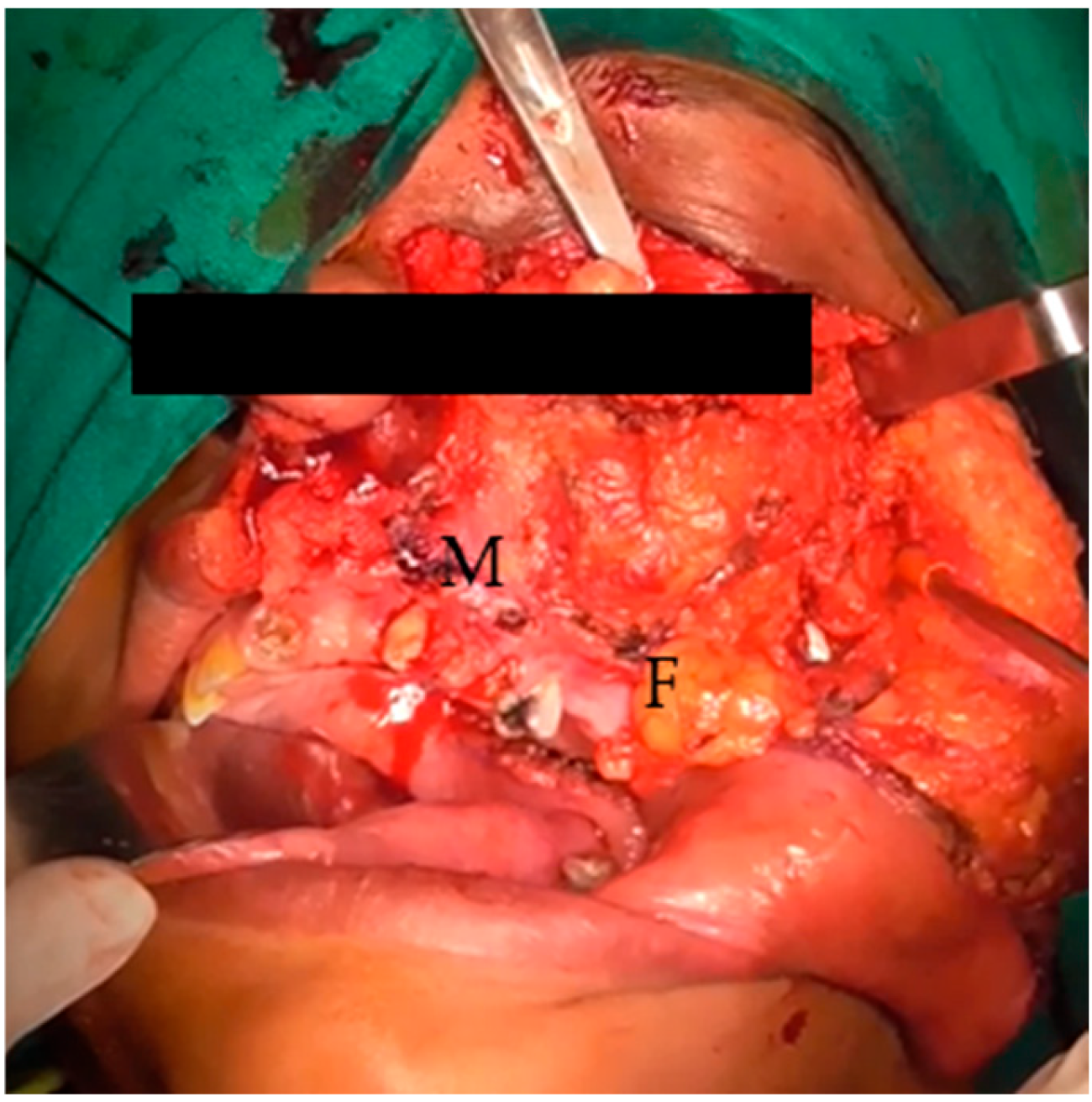
Figure 2.
Elevated cheek flap, exposed maxilla (M), and buccal fat (F).
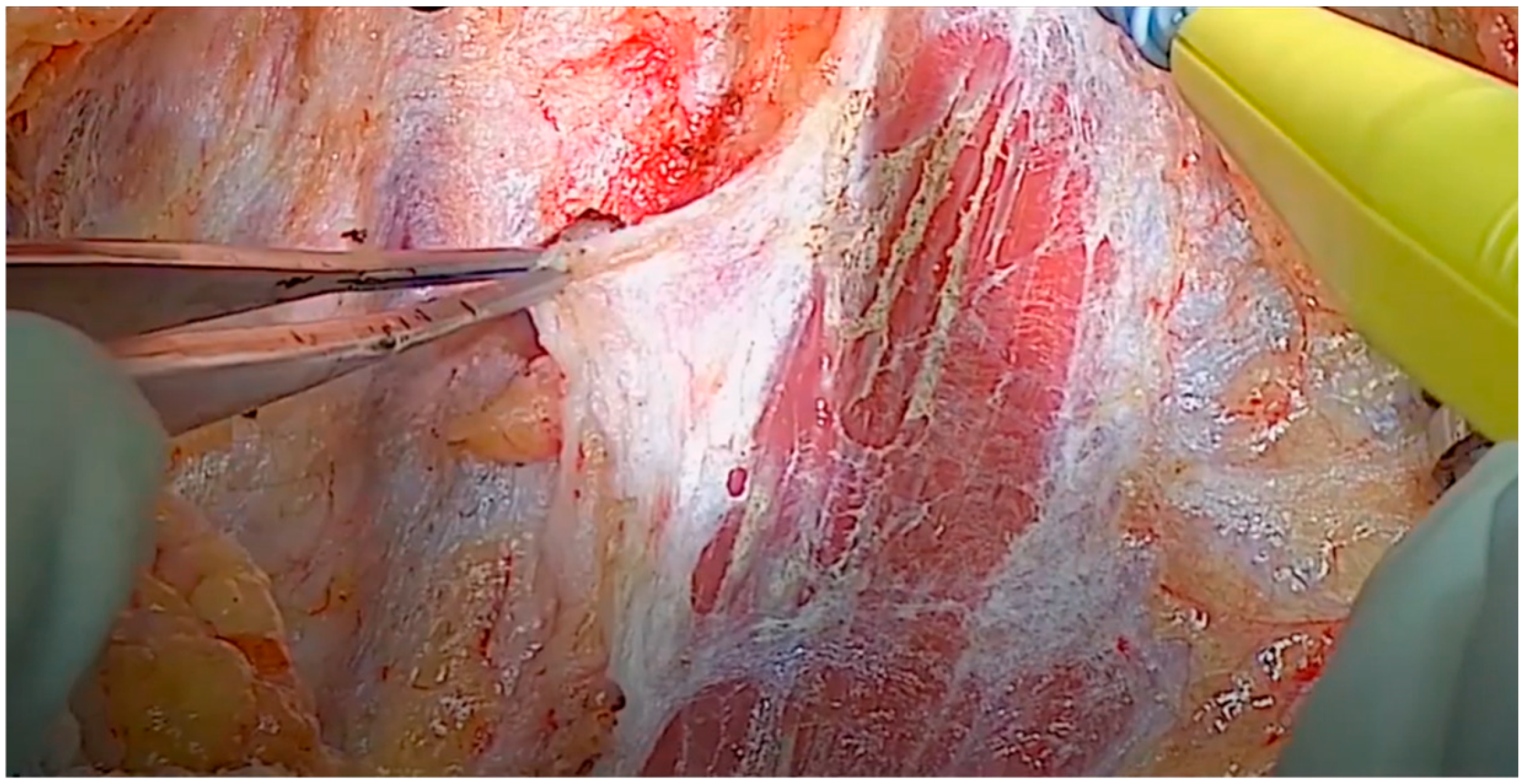
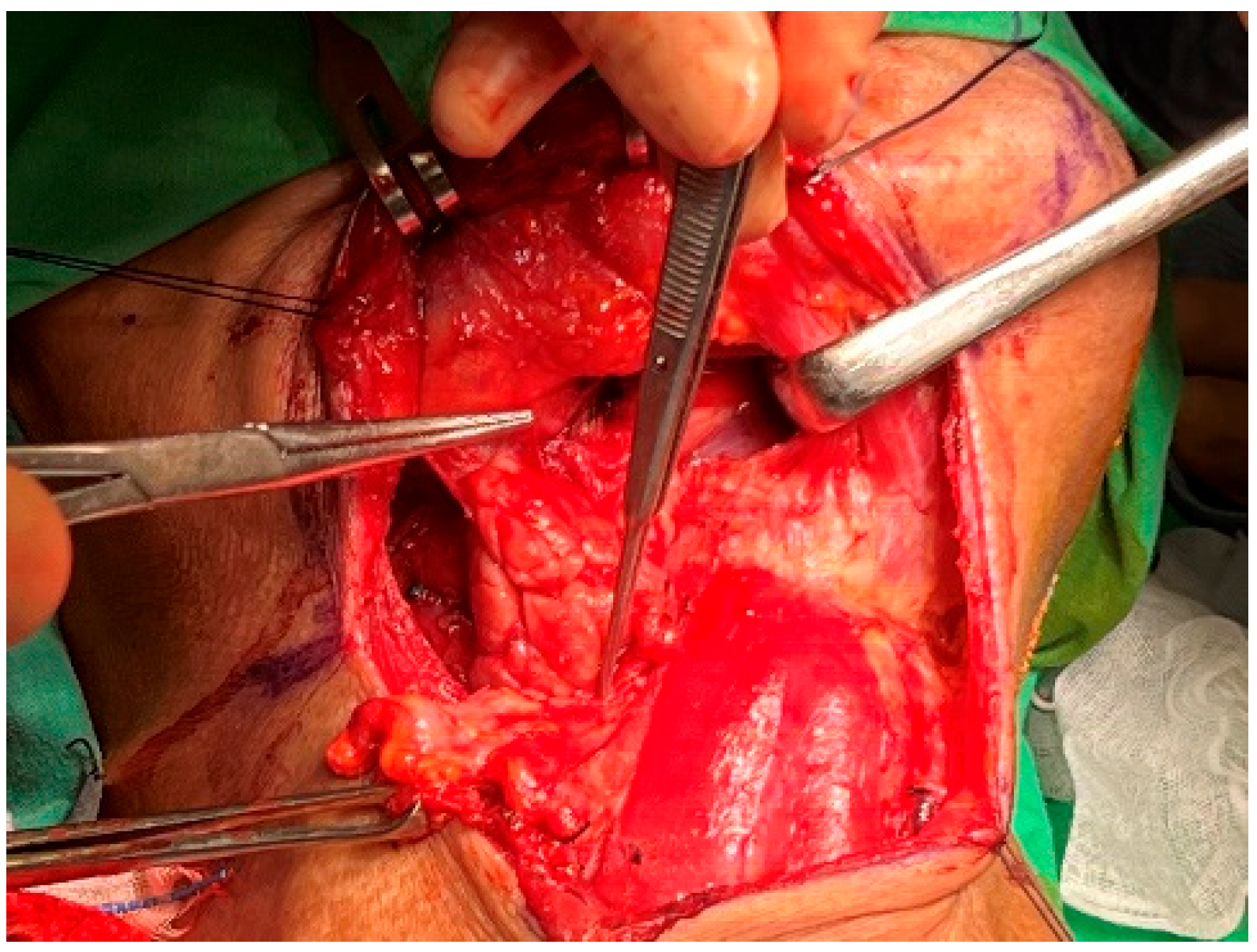
The IMA was accessed from the lateral pterygoid muscle by navigating through the space between the lateral and posterior walls of the maxilla and masseter muscle [8]. Buccal fat was removed either via direct electrocauterization or via ligation using hemostatic forceps and scissors. The lateral pterygoid muscle, located behind the temporalis muscle, was used as a landmark to locate the IMA, which was just above the lateral pterygoid muscle or below the inferior margin of the muscle (Figure 3) [9,10].
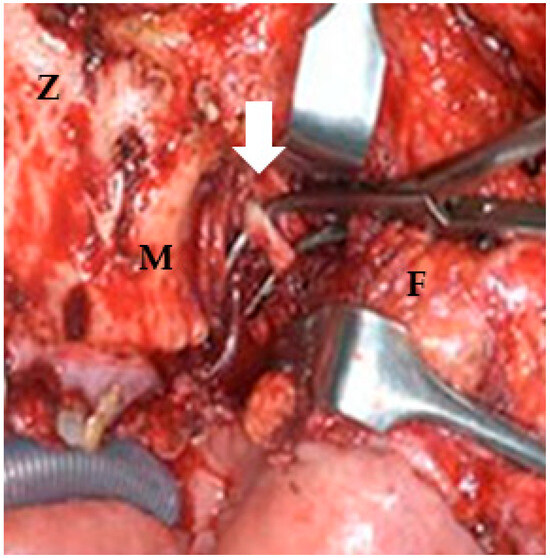
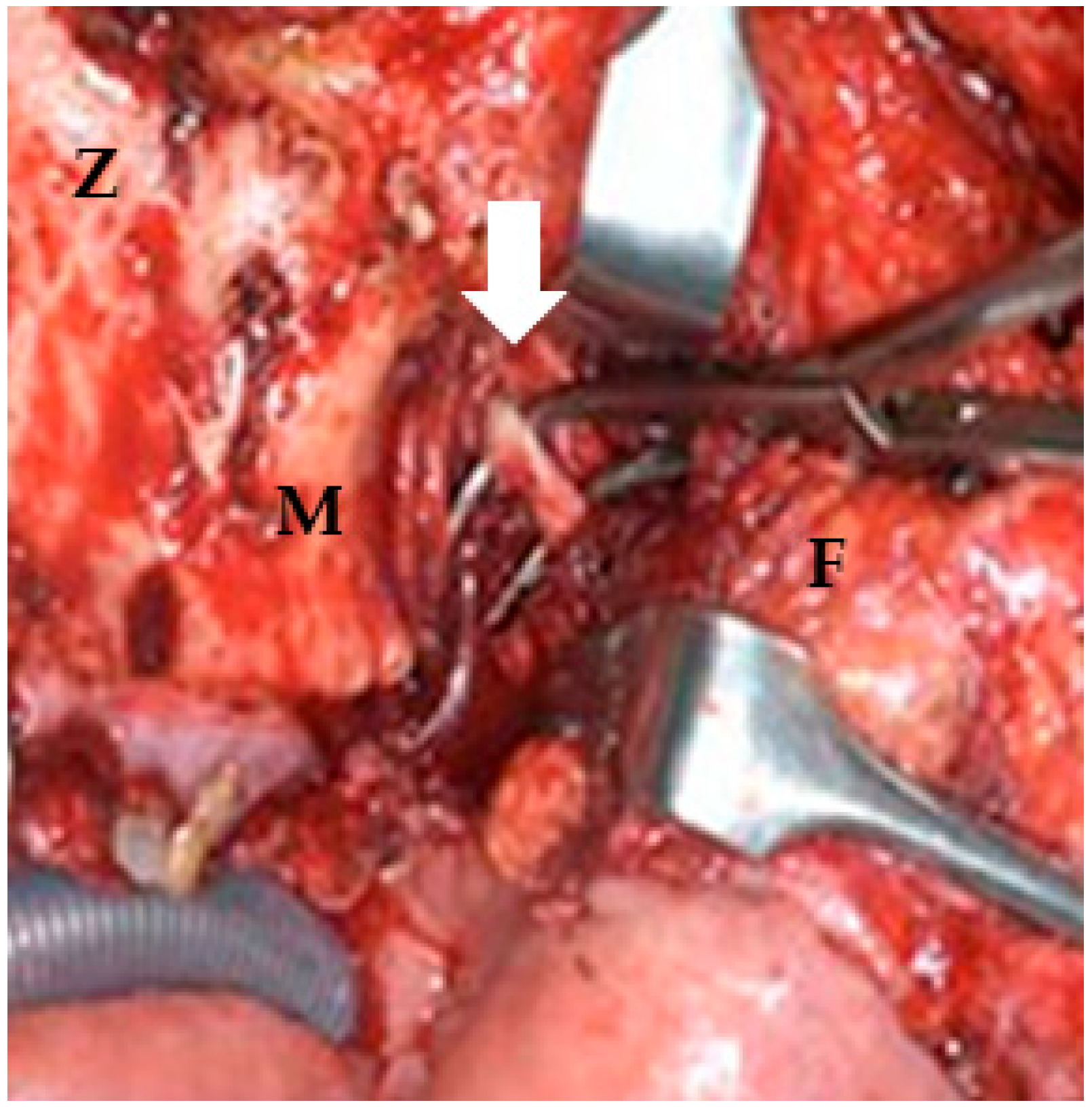
Figure 3.
Postmaxillary space. The white arrow shows the internal maxillary artery in the postmaxillary space. Z: zygoma; M: maxilla; F: buccal fat.
2.2.2. Rummel Tourniquet Restriction of the ECA
If the IMA was difficult to approach, ECA restriction was performed through a neck dissection wound. Neck dissection was performed using either a modified apron incision (Figure 4) or a single-Y incision (Figure 5) if radical neck dissection was required [11,12,13]. Both incisions provided easy access to the areas of interest.
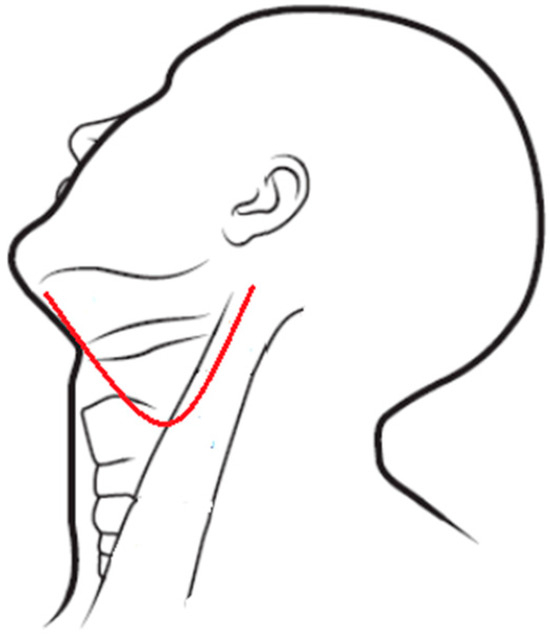
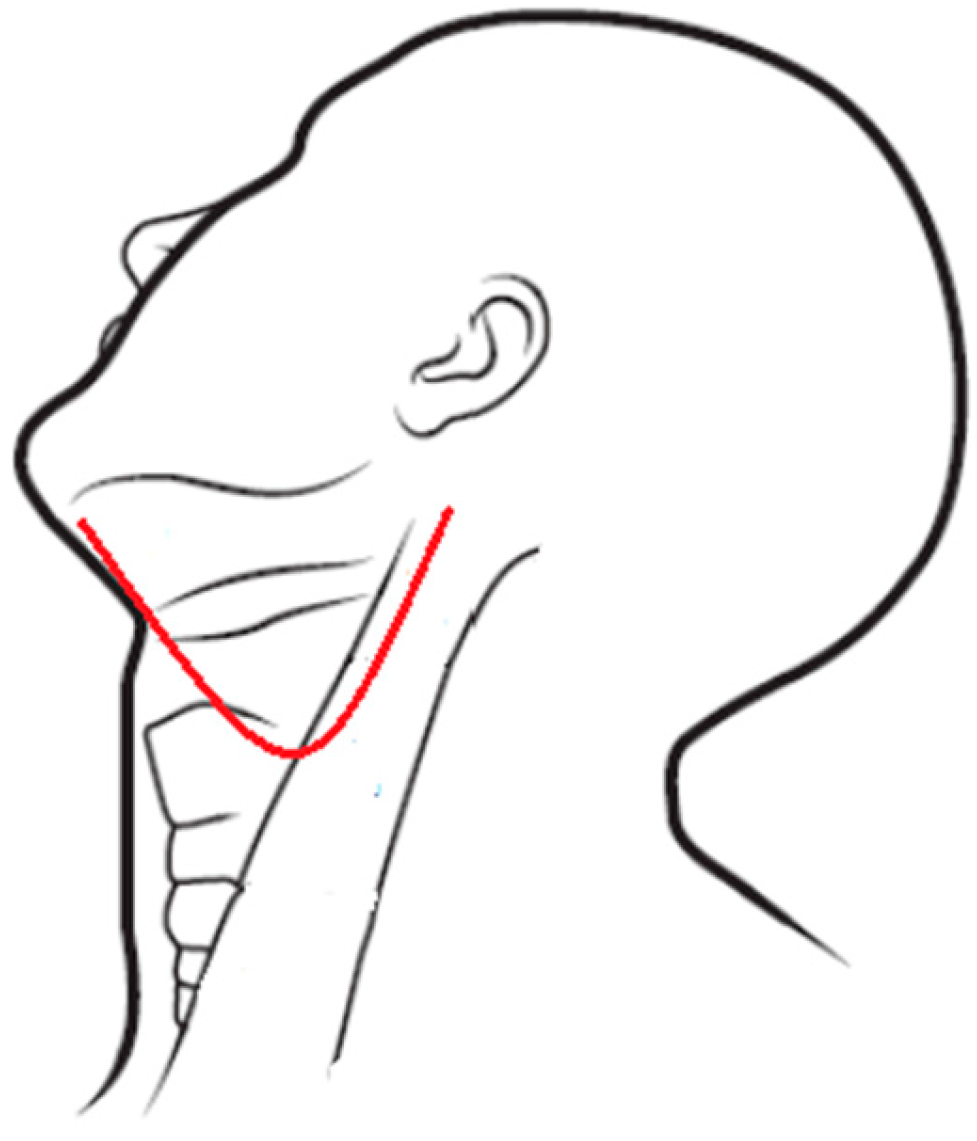
Figure 4.
Modified apron incision.
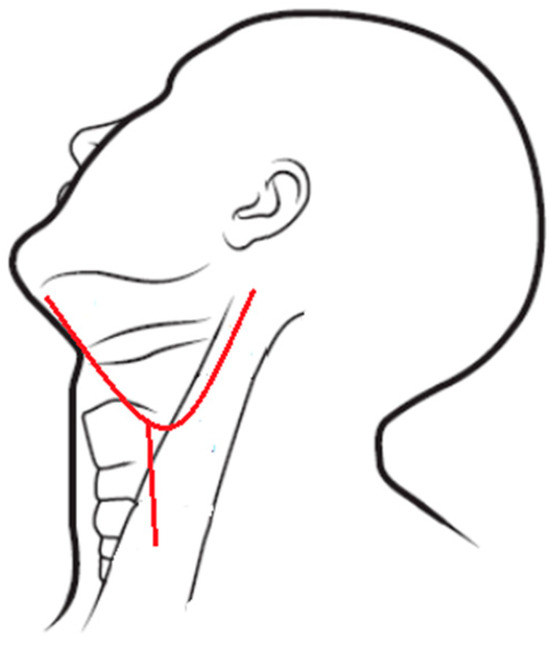
Figure 5.
Single-Y incision.
Anteriorly, the incision was curved upward or extended across the midline to reach the submental triangle. The incision extended posteriorly to the upper part of the sternocleidomastoid (SCM) muscle, exposing the tail of the parotid gland, and extended two finger widths below the mandible to avoid injuring the marginal mandibular branch of the facial nerve. The strap muscles were exposed medially, and the posterior border of the SCM was exposed in a selective neck dissection, or the anterior border of the trapezius muscle was exposed in a radical neck dissection [12,13,14]. The incision was made up to the platysma layer, and the flap was elevated. At this stage, the external jugular vein and great auricular nerve were carefully divided. When the flap reached the lower border of the submandibular gland (SMG), it was extended along the surface of the submandibular gland to avoid injuring the marginal mandibular branch of the facial nerve, located approximately 2 cm below the inferior border of the mandible and near the platysma layer [15,16]. Additionally, the Hayes Martin Maneuver, another method to protect the facial nerve, was employed. This method involves ligating the posterior facial vein and reflecting the investing fascia superiorly below the mandible to preserve the marginal mandibular nerve [17].
After sufficient exposure, the SCM, SMG, and strap muscles were in view. A small incision was made on the medial side of the upper SCM muscle, between the lymphoareolar tissue and the SCM muscle (Figure 6). The SCM muscle was retracted using a retractor, and the lymphoareolar tissue was clamped using Allis forceps. The internal jugular vein (IJV) was carefully and slowly freed from the SCM muscle medially and beneath [18,19].
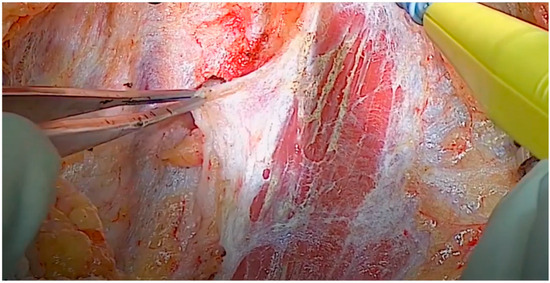
Figure 6.
Division of the SCM muscle and lymphoareolar tissue.
Care was taken regarding the surface of the SCM muscle to prevent injury to the external jugular vein while separating the upper part of the SCM muscle. In the depth of the SCM muscle, the spinal accessory nerve (SAN) is located medial to the digastric and stylohyoid muscles and lateral or immediately posterior to the IJV [20]. The SAN was palpated and felt with the tip of the finger, feeling similar to a rubber band in the lymphoareolar tissue.
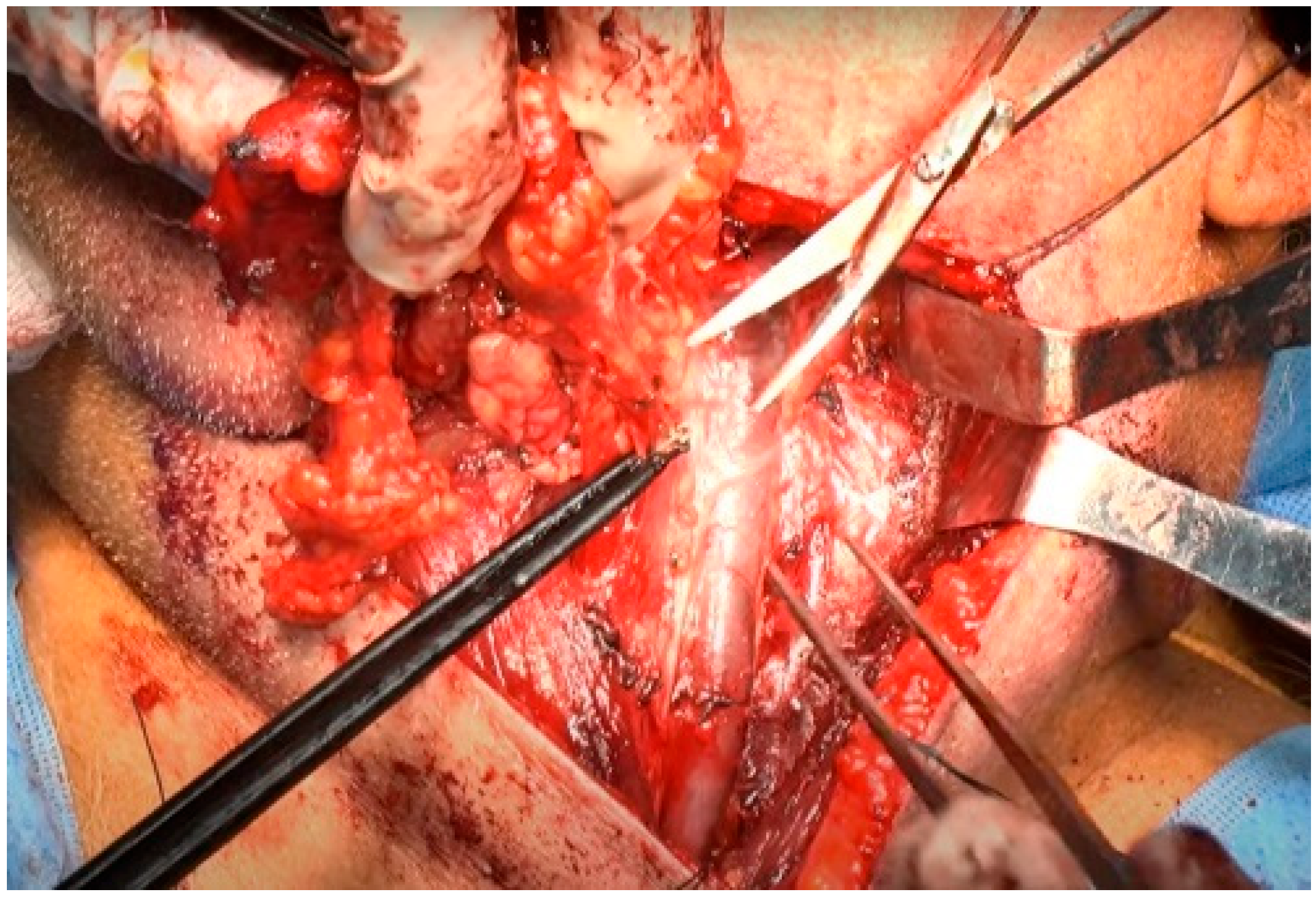
The dissection proceeded along the inner aspect of the SCM muscle in a cephalad-to-caudal direction. The main objective at this stage was to identify and dissect the cervical rootlets while preserving their contributions to the trapezius branch of the SAN. The specimen, involving levels II and III (and subsequently IV), was dissected superficially to each cervical rootlet [21]. As each rootlet was dissected, the specimen was progressively released. The plane of the rootlets established the posterior boundary of levels III and IV, circumventing the need to go deeper at this stage. In level IIb of the neck, the lymphatic packet was dissected from the deep layer of the deep cervical fascia and passed beneath the SAN [22]. Subsequently, the lymphoareolar tissue was reflected upward and removed along the anterior surface of the IJV using Allis forceps. Numerous branches of the IJV were encountered and carefully ligated at this stage [22,23]. Given that the IJV is located superficial and lateral to the carotid artery [22], care was taken when handling the lymphoareolar tissue on the medial side of the IJV (Figure 7). The tissue was carefully dissected free from the carotid sheath, with caution taken to avoid injuring the vagus nerve. The nodal tissue was elevated until it reached the level of the hyoid bone.
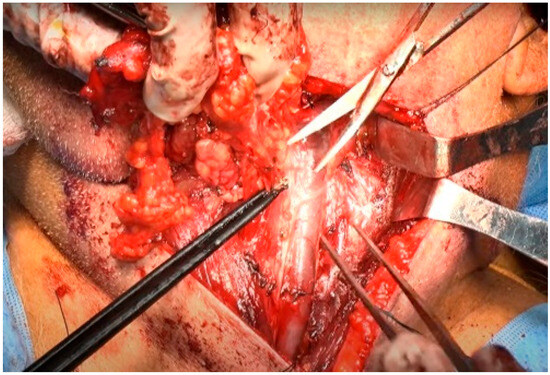
Figure 7.
Dissection of the lymphoareolar tissue from the medial side of the IJV.
The dissection proceeded to the submental area (level IA). The contralateral anterior belly of the digastric muscle was identified, and the superficial fat over the anterior bellies of both digastric muscles (submental fat) was dissected laterally (Figure 8). The dissection proceeded laterally over the ipsilateral mylohyoid muscle, which was retracted anterosuperiorly using an Army–Navy retractor [24,25]. In this region, an abundant venous plexus was present, which could bleed easily. To reduce the risk of bleeding, dissection was continued in the correct plane, avoiding injury to the submental muscle. After completely freeing the specimen from the submental area, the submandibular gland was sacrificed, as during neck dissection in patients undergoing curative surgery for oral squamous cell carcinoma [26].
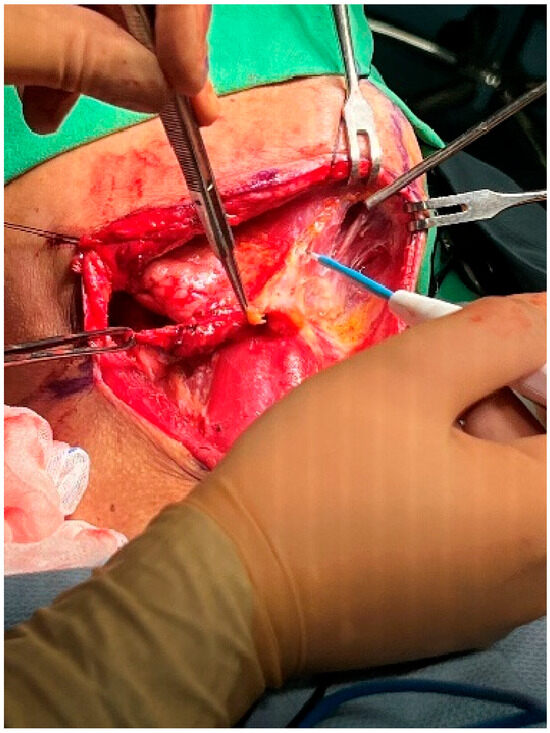
Figure 8.
Dissection of the submental area.
The fascia of the submandibular gland, adhered to the mandible, was first dissected from the anterior to the lateral direction. This process was conducted carefully and slowly to encounter and ligate the distal facial artery and vein. After freeing the submandibular gland from the mandible, it was retracted inferiorly. The submandibular fat and lymph nodes were gently moved inferiorly with a sponge, revealing the lingual nerve and submandibular ganglion (Figure 9). The submandibular ganglion and duct were tied off and cut. Dissection of the submandibular triangle proceeded from the medial to lateral, and the proximal facial artery was severed for a second time at the gland’s posterior [24,25,26,27].
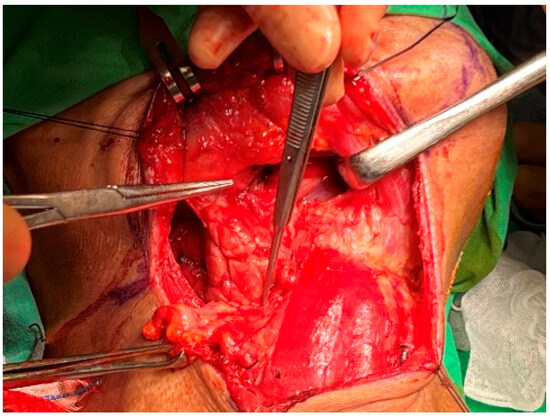
Figure 9.
Submandibular space. Dissection of the submandibular gland.
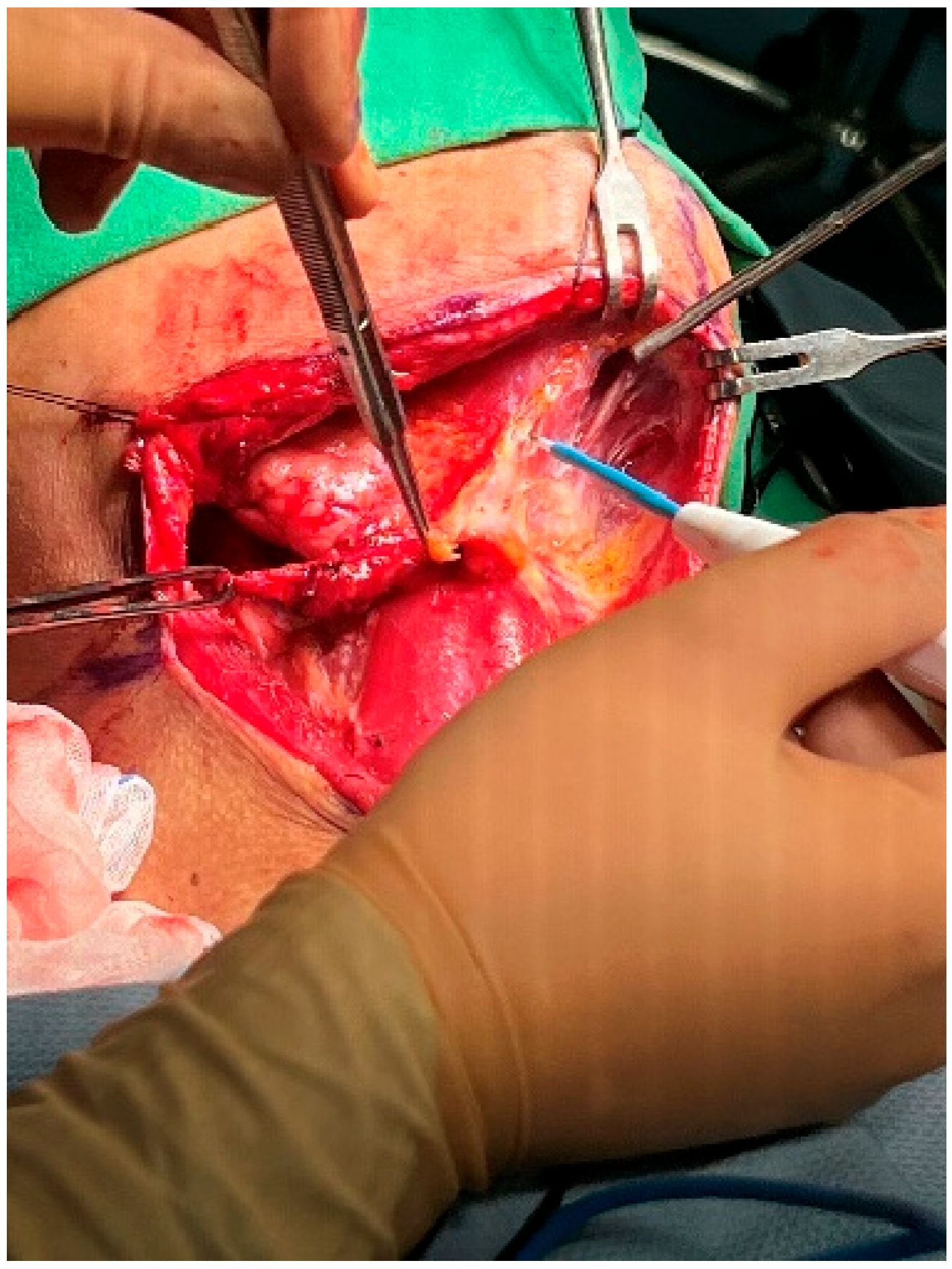
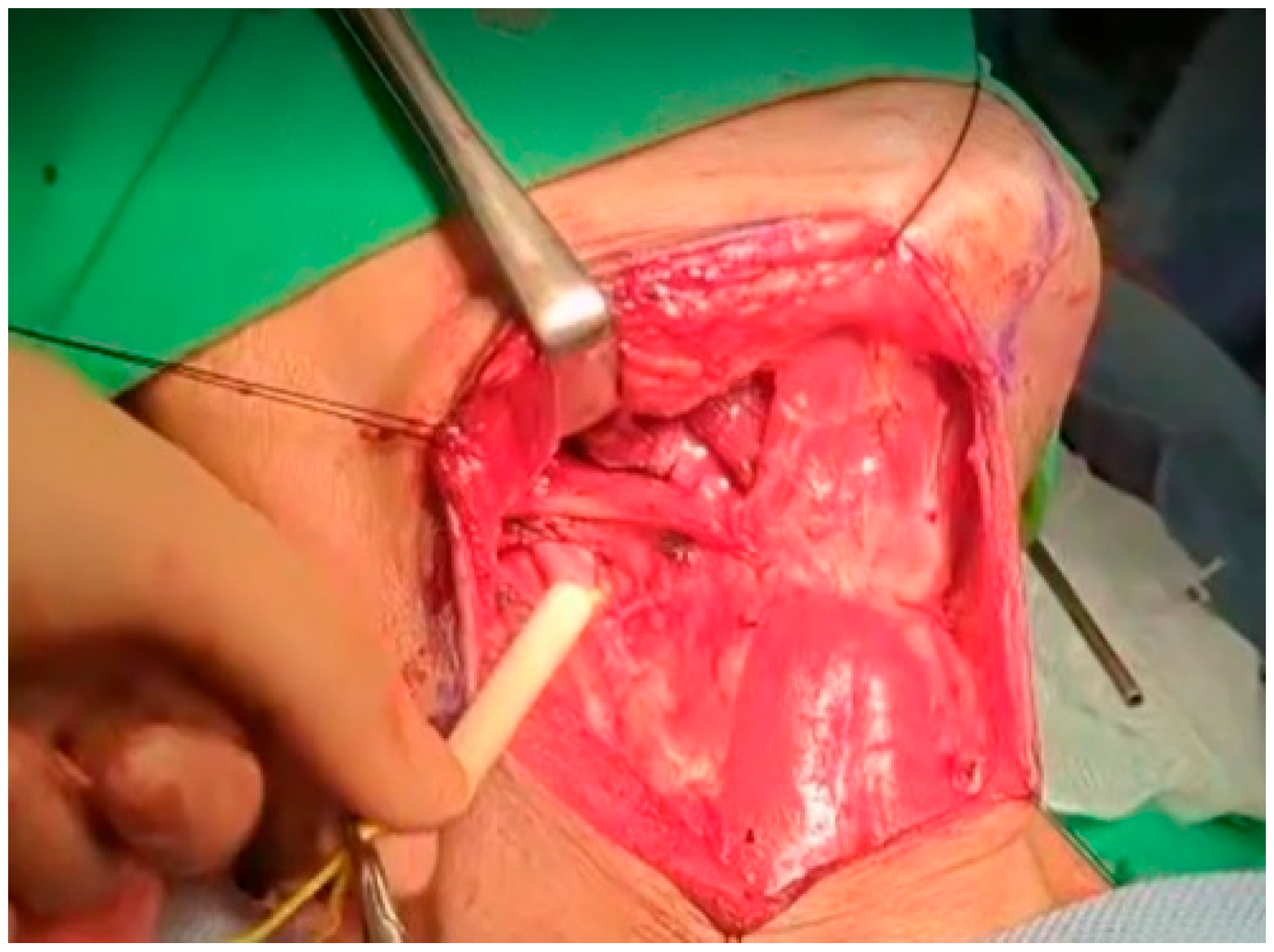
At this point, the neck dissection was complete, and the facial artery was identified, which helped differentiate between the internal and external carotid arteries. By opening the carotid sheath using curved pean forceps, the common carotid artery was made visible. Tracing upward led to the bifurcation of the carotid artery. The presence of branches serves as a characteristic feature of the external carotid artery. The superior thyroid artery, the first branch of the external carotid artery, originates below the level of the greater horn of the hyoid bone. The lingual artery, closely associated with the hyoid bone, originates from the external carotid artery above the level of the greater horn [28]. The root of the facial artery is located obliquely beneath the digastric and stylohyoid muscles [29,30]. These branches were used to identify the external carotid artery. After separating the external carotid artery from the internal carotid artery and vagus nerve, a Rummel tourniquet was used to restrict the external carotid artery and control bleeding (Figure 10).
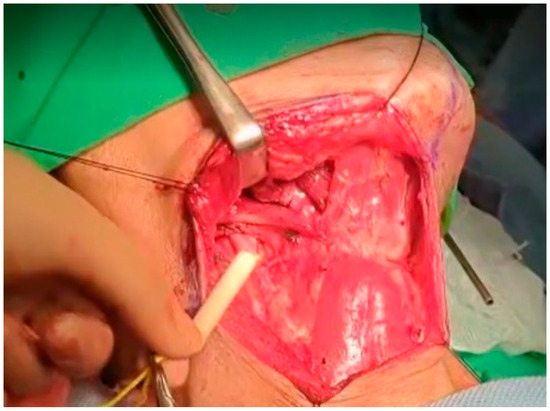
Figure 10.
Rummel tourniquet restriction of the left ECA.
2.3. Statistical Analysis
The Kruskal–Wallis test was used to compare the effect of the different surgical methods on blood loss. If significant differences were observed, Dunn’s test was conducted for post hoc analysis. All statistical results were based on two-tailed tests, and a p-value < 0.05 was considered statistically significant. All statistical analyses were performed using the IBM SPSS Statistics v29.0 (SPSS, Inc., Chicago, IL, USA).
3. Results
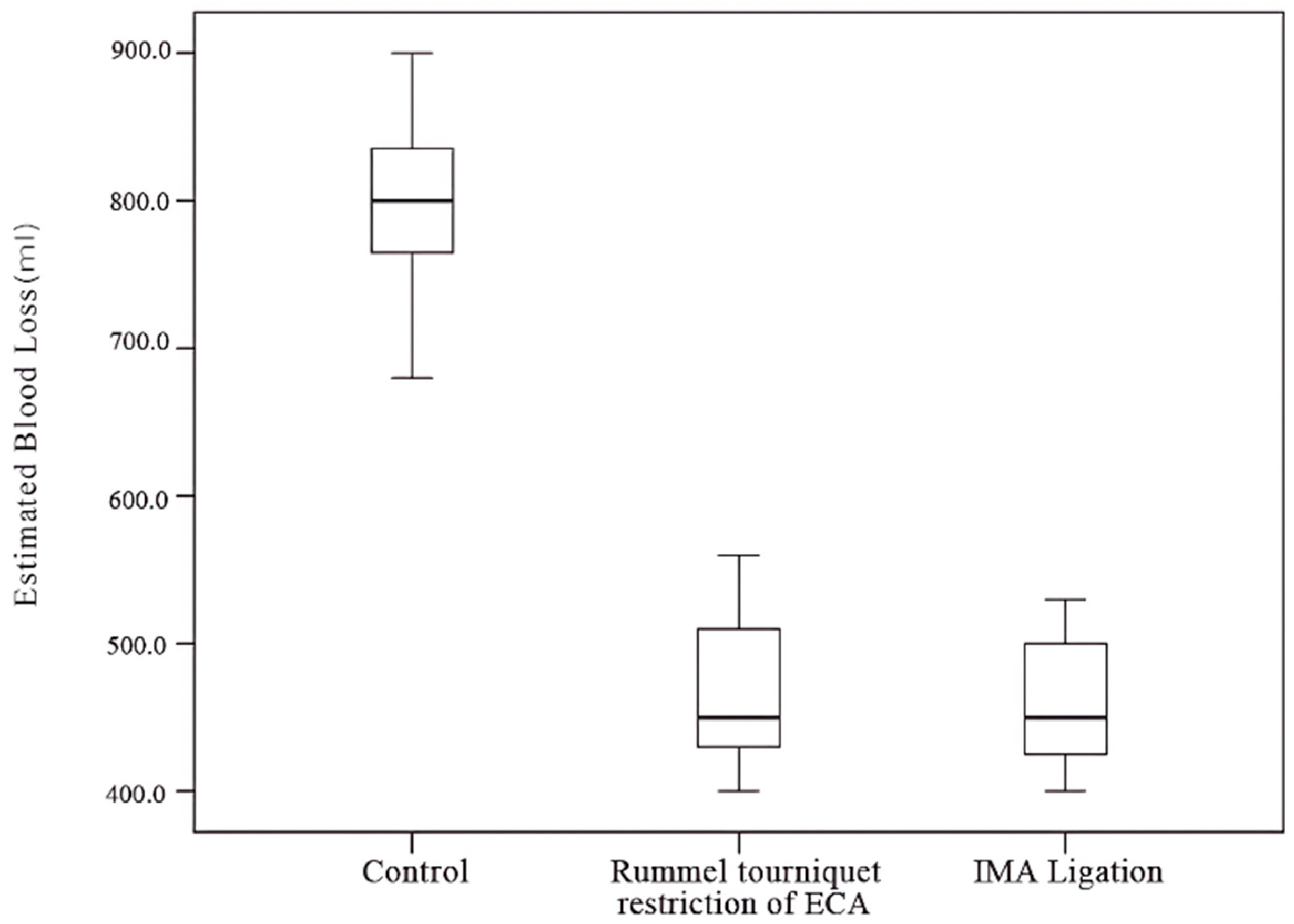
Twenty-five patients were enrolled in this study (eighteen males and seven females) (Table 1). All patients were in the terminal stage with maxilla involvement. If the IMA could be approached intraoperatively, IMA ligation was performed. If the tumor involved the IMA, a Rummel tourniquet restriction of the ECA was applied to reduce IMA flow, achieving a similar effect to IMA ligation. This study included twelve patients (48%) in the control group (group A), six patients (24%) who underwent Rummel tourniquet restriction of the ECA (group B), and seven patients (28%) who underwent IMA ligation (group C). All patients had malignant tumors involving the maxillary sinus and underwent total maxillectomy with a Weber–Ferguson incision by the same surgeon. The average intraoperative blood loss was 467 mL and 461 mL for groups B and C, respectively (range: 400–600 mL), significantly lower than the mean of the control group, at 794 mL (range: 600–900 mL).

Table 1.
Patient data profile.
The median age was 57 years, with 18 males (72%) and 7 females (28%). The only stage III cancer case (4%) was palatal mucosal melanoma, whereas the remaining 24 cases (96%) were all stage IV (Table 2). None of the patients had distant metastasis and were operable with tumor eradication via total maxillectomy.

Table 2.
Descriptive statistics of sample demographics.
The Kruskal–Wallis test and post hoc Dunn’s test revealed a significant difference between the control group and both the IMA ligation and ECA restriction groups (Table 3). No significant difference in blood loss was observed between the IMA ligation and ECA restriction groups (Table 4, Figure 11).

Table 3.
Descriptive statistics of blood loss among different groups.

Table 4.
Comparison of blood loss among different groups.
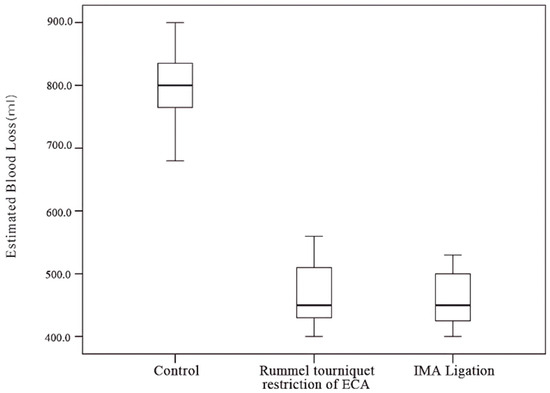
Figure 11.
Amount of blood loss among different groups.
4. Discussion
Maxillary surgery is among the head and neck cancer procedures most prone to significant bleeding and also stands as one of the most challenging. Managing intraoperative bleeding during maxillectomy is difficult due to the deep positioning of the IMA and pterygoid plexus [2], which can be easily injured or inevitably sacrificed before the specimen is completely removed [1,2,3]. Rapid bleeding often leads surgeons to prioritize tumor removal before attempting hemostasis. However, this fast bleeding causes anxiety for the surgeon and obstructs their view, complicating adherence to standard surgical steps and making it challenging to perform a total maxillectomy systematically. In the past, the Reverse Trendelenburg position and hypotensive anesthesia have been used to minimize blood loss in head and neck surgery [31,32]. However, both techniques have limited effectiveness in controlling blood loss. Hypotensive anesthesia is only applicable under strict physiological criteria [32]. Embolization is another effective method for controlling intraoperative bleeding [33]. Preoperative embolization, which involves coordination with the radiology department, requires time, incurs additional costs, and introduces additional risks and burdens for the patient [34,35]. These factors may decrease the patient’s willingness to undergo the treatment.
Currently, it has been confirmed that performing IMA ligation before surgery can effectively reduce intraoperative bleeding during maxillectomy, similar to how preoperative uterine artery ligation can reduce postoperative bleeding in cesarean sections [4,5]. However, the concept of temporary uterine ligation or temporary clamping of the infrarenal abdominal aorta has been less commonly applied in head and neck surgeries [5,6]. Therefore, this study uses a Rummel tourniquet to temporarily restrict the external carotid artery to limit IMA blood flow and reduce bleeding during maxillectomy. This method can be applied when neck dissection is performed, as in oral cancer surgeries, where it is customary to perform neck dissection before addressing the primary tumor, making the steps feasible.
The IMA is located in the pterygoid segment, just lateral to the lateral pterygoid muscle. Therefore, ligating the IMA before accessing the posterior side of the maxilla can significantly reduce intraoperative bleeding [4]. Nevertheless, advanced maxillary cancers involving the infratemporal fossa may disrupt the IMA and pterygoid plexus, complicating bleeding control. In such cases, using a Rummel tourniquet to restrict the ECA can help control bleeding. In our experience, we use the facial artery to identify the external carotid artery, as it is commonly found and frequently used for flap reconstruction during combined surgeries. The facial artery is typically located between the upper edge of the posterior digastric muscle and the submandibular gland. Notably, the lingual and hypoglossal nerves are also present in this space; therefore, careful identification and ligation are required [36]. The facial artery can be differentiated from nerves by its pulsation when palpated. Additionally, after opening the carotid sheath and identifying the external carotid artery, caution should be taken to avoid stimulating or compressing the carotid sinus at the common carotid artery bifurcation, as this can lead to bradycardia and hypotension [37].
In this study, no significant difference was observed between the efficacy of groups B and C in terms of reducing intraoperative bleeding. However, bleeding was significantly reduced in both groups compared to that seen in group A.
The minimal difference in effectiveness between groups B and C could be attributed to the vascular supply to the maxilla, which includes not only the IMA but also the ascending palatine artery from the facial artery and branches of the ascending pharyngeal artery [38,39]. Therefore, ligating only the IMA may counterbalance the effect of using a Rummel tourniquet to restrict the ECA in group B. To minimize potential complications, artery ligation should be performed as close to the bleeding site as possible. Permanent ligation of the external carotid artery (ECA) can lead to tissue ischemia, necrosis, neurological deficits, facial swelling, and infection [40]. Nevertheless, temporary ligation of the ECA rarely causes significant side effects [41]. IMA ligation and temporary restriction of the ECA can reduce intraoperative bleeding, provide a clearer surgical field, enhance patient safety, and reduce anxiety and stress for the surgeon.
This method still has limitations. For cancers that do not require routine neck dissection and when the wound cannot be exposed in advance, a small incision is needed to access the external carotid artery. Additionally, exposing the carotid sheath introduces risks not only to the external carotid artery but also to the internal carotid artery. Mistaking the internal carotid artery for the external and inadvertently restricting it can lead to irreversible complications [42]. Furthermore, this study faces several limitations, including a small sample size and the potential for varying bleeding levels due to different tumor types. The duration of the surgery also significantly influences outcomes; while IMA ligation or ECA restriction may reduce blood loss, longer surgical times are still associated with increased bleeding. Consequently, the experience of the surgeon is also a crucial factor.
5. Conclusions
IMA ligation and Rummel tourniquet restriction of the ECA effectively reduced bleeding during maxillary surgery before bone cuts, with no significant differences in their effectiveness. For tumors involving the infratemporal fossa that affect the IMA and pterygoid plexus, using a Rummel tourniquet to restrict the ECA is recommended. Conversely, IMA ligation is recommended if the tumor does not invade the infratemporal fossa.
Author Contributions
Conceptualization, P.-R.C.; methodology, P.-R.C.; formal analysis, Y.-C.L.; data curation, P.-R.C.; writing—original draft preparation, Y.-C.L.; writing—review and editing, P.-R.C.; supervision, P.-R.C.; project administration, P.-R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Research Ethics Committee, Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation (IRB113-161-B) on 10 September 2024.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
We would like to thank Wan-Ting Huang for assisting with the statistical analysis and providing guidance on statistical methods.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Choi, J.; Park, H.S. The clinical anatomy of the maxillary artery in the pterygopalatine fossa. J. Oral Maxillofac. Surg. 2003, 61, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Lanigan, D.T.; Hey, J.H.; West, R.A. Major vascular complications of orthognathic surgery: False aneurysms and arteriovenous fistulas following orthognathic surgery. J. Oral Maxillofac Surg. 1991, 49, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Lanigan, D.T.; Hey, J.H.; West, R.A. Major vascular complications of orthognathic surgery: Hemorrhage associated with Le Fort I osteotomies. J. Oral Maxillofac. Surg. 1990, 48, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.P.; Yang, T.L.; Ko, J.Y.; Lou, P.J. Ligation of the internal maxillary artery to reduce intraoperative bleeding during total maxillectomy. Laryngoscope 2007, 117, 1978–1981. [Google Scholar] [CrossRef]
- Erin, R.; İssak, A.; Baki Erin, K.; Kulaksiz, D.; Bayoğlu Tekin, Y. The efficiency of temporary uterine artery ligation on prevention of the bleeding in cesarean section. Gynecol. Obstet. Investig. 2021, 86, 486–493. [Google Scholar] [CrossRef]
- Hashiguchi, M. Temporary cross-clamping of the infrarenal abdominal aorta during cesarean hysterectomy to control operative blood loss. Surg. J. 2021, 7, S7–S10. [Google Scholar] [CrossRef] [PubMed]
- Votto, S.S.; Read-Fuller, A.; Reddy, L. Lip augmentation. Oral Maxillofac. Surg. Clin. North Am. 2021, 33, 185–195. [Google Scholar] [CrossRef]
- Sashi, R.; Tomura, N.; Hashimoto, M.; Kobayashi, M.; Watarai, J. Angiographic anatomy of the first and second segments of the maxillary artery. Radiat. Med. 1996, 14, 133–138. [Google Scholar]
- Pretterklieber, M.L.; Skopakoff, C.; Mayr, R. The human maxillary artery reinvestigated: I. Topographical relations in the infratemporal fossa. Acta. Anat. 1991, 142, 281–287. [Google Scholar] [CrossRef]
- Krizan, Z. Contribution to the descriptive and topographic anatomy of the maxillary artery. Acta. Anat. 1960, 41, 319–333. [Google Scholar]
- Crile, G. Landmark article Dec 1, 1906: Excision of cancer of the head and neck. With special reference to the plan of dissection based on one hundred and thirty-two operations. By George Crile. JAMA 1987, 258, 3286–3293. [Google Scholar] [CrossRef] [PubMed]
- Bocca, E.; Pignataro, O. A conservation technique in radical neck dissection. Ann. Otol. Rhinol. Laryngol. 1967, 76, 975–987. [Google Scholar] [CrossRef] [PubMed]
- Roger, V.; Hitier, M.; Robard, L.; Babin, E. Morbidity of neck dissection submuscular recess (sublevel IIb) in head and neck cancer. Rev. Laryngol. Otol. Rhinol. 2014, 135, 135–140. [Google Scholar]
- Byers, R.M.; Clayman, G.L.; McGill, D.; Andrews, T.; Kare, R.P.; Roberts, D.B.; Goepfert, H. Selective neck dissections for squamous carcinoma of the upper aerodigestive tract: Patterns of regional failure. Head Neck 1999, 21, 499–505. [Google Scholar] [CrossRef]
- Hwang, K.; Kim, J.Y.; Lim, J.H. Anatomy of the platysma muscle. J. Craniofac. Surg. 2017, 28, 539–542. [Google Scholar] [CrossRef]
- Hoerter, J.E.; Patel, B.C. Anatomy, Head and Neck, Platysma. StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545294/ (accessed on 13 August 2024). [PubMed]
- Riffat, F.; Buchanan, M.A.; Mahrous, A.K.; Fish, B.M.; Jani, P. Oncological safety of the Hayes-Martin manoeuvre in neck dissections for node-positive oropharyngeal squamous cell carcinoma. J. Laryngol. Otol. 2012, 126, 1045–1048. [Google Scholar] [CrossRef]
- Rodrigo, J.P.; Grilli, G.; Shah, J.P.; Medina, J.E.; Robbins, K.T.; Takes, R.P.; Hamoir, M.; Kowalski, L.P.; Suárez, C.; López, F.; et al. Selective neck dissection in surgically treated head and neck squamous cell carcinoma patients with a clinically positive neck: Systematic review. Eur. J. Surg. Oncol. 2018, 44, 395–403. [Google Scholar] [CrossRef]
- Lanišnik, B. Different branching patterns of the spinal accessory nerve: Impact on neck dissection technique and postoperative shoulder function. Curr. Opin. Otolaryngol. Head Neck Surg. 2017, 25, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.B.; Boone, J.L.; Schmalbach, C.E.; Miller, F.R. Intraoperative relationship of the spinal accessory nerve to the internal jugular vein: Variation from cadaver studies. Am. J. Otolaryngol. 2013, 34, 527–529. [Google Scholar] [CrossRef]
- David, M.; Mayaud, A.; Timochenko, A.; Asanau, A.; Prades, J.M. Topographical and functional anatomy of trapezius muscle innervation by spinal accessory nerve and C2 to C4 nerves of cervical plexus. Surg. Radiol. Anat. 2016, 38, 917–922. [Google Scholar] [CrossRef]
- Wistermayer, P.; Anderson, K.G. Radical Neck Dissection; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK563186/ (accessed on 13 August 2024). [PubMed]
- Hemmat, S.M.; Wang, S.J.; Ryan, W.R. Neck dissection technique commonality and variance: A survey on neck dissection technique preferences among head and neck oncologic surgeons in the American Head and Neck Society. Int. Arch. Otorhinolaryngol. 2017, 21, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Myers, E.N. Chapter 78, Neck Dissection. In Operative Otolaryngology: Head and Neck Surgery, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 1, pp. 679–708. [Google Scholar]
- Medina, J.E. Neck Dissection. In Head & Neck Surgery-Otolaryngology, 4th ed.; Bailey, B.J., Johnson, J.T., Eds.; Lippincoott Williams & Wilkins: Philadelphia, PA, USA, 2006; pp. 1585–1609. [Google Scholar]
- Yang, S.; Wang, X.; Su, J.Z.; Yu, G.Y. Rate of submandibular gland involvement in oral squamous cell carcinoma. J. Oral Maxillofac. Surg. 2019, 77, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Robbins, K.T.; Clayman, G.; Levine, P.A.; Medina, J.; Sessions, R.; Shaha, A.; Som, P.; Wolf, G.T.; American Head and Neck Society; American Academy of Otolaryngology—Head and Neck Surgery. Neck dissection classification update: Revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology—Head and Neck Surgery. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 751–758. [Google Scholar] [CrossRef]
- Lemaire, V.; Jacquemin, G.; Nelissen, X.; Heymans, O. Tip of the greater horn of the hyoid bone: A landmark for cervical surgery. Surg. Radiol. Anat. 2005, 27, 33–36. [Google Scholar] [CrossRef]
- Parson, S.H. Clinically Oriented Anatomy. J. Anat. 2009, 215, 474. [Google Scholar] [CrossRef] [PubMed Central]
- Pessa, J.E.; Rohrich, R.J. Facial Topography: Clinical Anatomy of the Face, 1st ed.; Thieme: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- Nooraei, N.; Dabbagh, A.; Niazi, F.; Mohammadi, S.; Mohajerani, S.A.; Radmand, G.; Hashemian, S.M. The Impact of Reverse Trendelenburg Versus Head-up Position on Intraoperative Bleeding of Elective Rhinoplasty. Int. J. Prev. Med. 2013, 4, 1438–1441. [Google Scholar] [PubMed]
- Conley, J.; Hicks, R.G.; Jasaitis, J.E. Hypotensive Anesthesia in Surgery of the Head and Neck. Arch Otolaryngol. 1965, 81, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Taketomi, T.; Sanui, T.; Fukuda, T.; Takeshita, G.; Nojiri, J. Preoperative endovascular arterial embolization to avoid maxillary artery injury in maxillary gingival cancer surgery. Radiol. Case Rep. 2024, 19, 3561–3568. [Google Scholar] [CrossRef]
- Christensen, N.P.; Smith, D.S.; Barnwell, S.L.; Wax, M.K. Arterial embolization in the management of posterior epistaxis. Otolaryngol. Head Neck Surg. 2005, 133, 748–753. [Google Scholar] [CrossRef]
- Strong, E.B.; Bell, D.A.; Johnson, L.P.; Jacobs, J.M. Intractable epistaxis: Transantral ligation vs. embolization: Efficacy review and cost analysis. Otolaryngol. Head. Neck. Surg. 1995, 113, 674–678. [Google Scholar] [CrossRef]
- Schrank, T.P.; Mikhaylov, Y.; Zanation, A.M. Surgical excision of the submandibular gland. Oper. Tech. Otolaryngol. Head Neck Surg. 2018, 29, 162–167. [Google Scholar] [CrossRef]
- Kikuta, S.; Iwanaga, J.; Kusukawa, J.; Tubbs, R.S. Carotid sinus nerve: A comprehensive review of its anatomy, variations, pathology, and clinical applications. World Neurosurg. 2019, 127, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, A.; Lézy, J.P.; Vacher, C. Vascularization of the palate in maxillary osteotomies: Anatomical study. Surg Radiol Anat. 2002, 24, 13–17. [Google Scholar] [CrossRef]
- Siebert, J.W.; Angrigiani, C.; McCarthy, J.G.; Longaker, M.T. Blood supply of the Le Fort I maxillary segment: An anatomic study. Plast. Reconstr. Surg. 1997, 100, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Cooke, E.T. An evaluation and clinical study of severe epistaxis treated by arterial ligation. J. Laryngol. Otol. 1985, 99, 745–749. [Google Scholar] [CrossRef]
- Waldron, J.; Stafford, N. Ligation of the external carotid artery for severe epistaxis. J. Otolaryngol. 1992, 21, 249–251. [Google Scholar]
- Voris, H.C. Complications of ligation of the internal carotid artery. J. Neurosurg. 1951, 8, 119–131. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).