Serum Vitamin D in Children with Hemophilia A and Its Association with Joint Health and Quality of Life
Abstract
1. Introduction
2. Materials and Methods
2.1. Haemophilia Joint Health Score (HJHS)
2.2. Hemophilia-Specific Quality of Life Index (HaemoQoL and Haem-A-QoL Questionnaires) [11]
2.3. Determination of Serum Vitamin D
2.4. Assay Procedure
2.5. Ethical Considerations
2.6. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valentino, L.A.; Kaczmarek, R.; Pierce, G.F.; Noone, D.; O’Mahony, B.; Page, D.; Rotellini, D.; Skinner, M.W. Hemophilia gene therapy: First, do no harm. J. Thromb. Haemost. 2023, 21, 2354–2361. [Google Scholar] [CrossRef] [PubMed]
- Ragab, S.M.; Sharaf, A.E.; Bayomy, N.; Nour, B.A.; Mahmoud, A.A. Association between the levels of serum vitamin D and trace elements and joint health in children with hemophilia. Вoпрoсы Гематoлoгии/Онкoлoгии И Иммунoпатoлoгии В Педиатрии 2023, 22, 74–79. [Google Scholar]
- Hamed, E.M.; Ibrahim, A.R.; Meabed, M.H.; Khalaf, A.M.; El Demerdash, D.M.; Elgendy, M.O.; Saeed, H.; Salem, H.F.; Rabea, H. Therapeutic Outcomes of High Dose-Dexamethasone versus Prednisolone+ Azathioprine, Rituximab, Eltrombopag, and Romiplostim Strategies in Persistent, Chronic, Refractory, and Relapsed Immune Thrombocytopenia Patients. Pharmaceuticals 2023, 16, 1215. [Google Scholar] [CrossRef] [PubMed]
- Soares, B.M.D.; Imoto, A.M.; Ribeiro, A.J.T.; Simeoni, L.A.; de Almeida, K.J.Q.; Bezerra, L.B.; Braverman, M.S.; Fernandes, S.E.S.; Costa, A.M.; Amorim, F.F. Evaluation of Functional and Joint Health and Associated Factors in Adults with Hemophilia from a Developing Country with Government-Backed Efforts to Improve Hemophilia Care. Perm. J. 2023, 27, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Villalón-González, M.; Fernández de Luco-Santamaría, Í.; Cuesta-Barriuso, R.; López-Pina, J.A.; Pérez-Llanes, R. Hemophilic Arthropathy of the Knee and Its Association with Reduced Muscle Strength and Activation and the Pressure Pain Threshold: A Case-Control Study. J. Clin. Med. 2023, 12, 3275. [Google Scholar] [CrossRef] [PubMed]
- Ehsanbakhsh, A.; Azarkar, G.; Ziaee, M.; Taghavieh, A. Prevalence of bone density reduction and its related factors in hemophilia patients in South Khorasan Province in 2018. Galen Med. J. 2020, 9, e1711. [Google Scholar] [CrossRef] [PubMed]
- Abbasnezhad, A.; Habibi, M.; Abdolkarimi, B.; Zare, S.; Moghadam, E.F.; Choghakhori, R. Serum concentrations of vitamin D, calcium, phosphorus and trace minerals in adults and children with haemophilia A: Association with disease severity, quality of life, joint health and functional status. Int. J. Hematol.-Oncol. Stem Cell Res. 2020, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Konkle, B.; Pierce, G.; Coffin, D.; Naccache, M.; Clark, R.C.; George, L.; Iorio, A.; O’mahony, B.; Pipe, S.; Skinner, M. Core data set on safety, efficacy, and durability of hemophilia gene therapy for a global registry: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2020, 18, 3074–3077. [Google Scholar] [CrossRef] [PubMed]
- De la Corte-Rodriguez, H.; Rodriguez-Merchan, E.C. The role of physical medicine and rehabilitation in haemophiliac patients. Blood Coagul. Fibrinolysis 2013, 24, 1–9. [Google Scholar] [CrossRef]
- Arnold, W.D.; Hilgartner, M. Hemophilic arthropathy. Current concepts of pathogenesis and management. J. Bone Jt. Surg. 1977, 59, 287–305. [Google Scholar] [CrossRef]
- Sherief, L.; Mansour, A. Epidemiological profile of Inherited Bleeding Disorders in Egyptian Pediatric Patients: 4 Centers Experience, Retrospective Analysis. Ann. Rom. Soc. Cell Biol. 2021, 25, 14152–14165. [Google Scholar]
- Albayrak, C.; Albayrak, D. Vitamin D levels in children with severe hemophilia A: An underappreciated deficiency. Blood Coagul. Fibrinolysis 2015, 26, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Rawat, S.; Kushwaha, R.; Jain, M.; Verma, S.P.; Singh, U. Clinicopathological Parameters of Haemophilia Patients at a Tertiary Care Centre in Northern India. Cureus 2023, 15, e41670. [Google Scholar] [CrossRef] [PubMed]
- Alioglu, B.; Selver, B.; Ozsoy, H.; Koca, G.; Ozdemir, M.; Dallar, Y. Evaluation of bone mineral density in Turkish children with severe haemophilia A: Ankara hospital experience. Haemophilia 2012, 18, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, M.; Argun, M.; Kilic, E.; Saraymen, R.; Yazar, S. Magnesium, zinc and copper status in osteoporotic, osteopenic and normal post-menopausal women. J. Int. Med. Res. 2007, 35, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Sanadhya, A.; Singh, J. Comparative study of vitamin D levels in hemophilia and healthy children. IOSRJDMS 2016, 15, 1–5. [Google Scholar]
- Gamal Andrawes, N.; Hashem Fayek, M.; Salah El-Din, N.; Atef Mostafa, R. Effect of low-dose factor VIII prophylaxis therapy on bone mineral density and 25 (OH) vitamin D level in children with severe haemophilia A. Haemophilia 2020, 26, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Eid, R.; Fathy, A.A.; Hamdy, N. Health-related quality of life in Egyptian children with nephrotic syndrome. Qual. Life Res. 2020, 29, 2185–2196. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.Y.; Hassan, M.K. Health-related quality of life in children and adolescents with hemophilia in Basra, Southern Iraq. J. Pediatr. Hematol./Oncol. 2014, 36, 179–184. [Google Scholar] [CrossRef] [PubMed]
| Study Group (n = 45) | Control Group (n = 45) | Test of Sig. | p-Value | |
|---|---|---|---|---|
| Age (years) | ||||
| (Min.–Max.) | (1–16) | (5–17) | ||
| Mean ± SD | 8.70 ± 3.62 | 9.82 ± 3.08 | t = −1.59 | 0.113 |
| Residence | ||||
| Rural | N:42 (93.3%) | N:45 (100%) | t = 3.1 | 0.242 |
| Urban | N:3 (6.7%) | N:0 (0%) |
| Parameter | N | % | Test-Used | Significance |
|---|---|---|---|---|
| Residence | ||||
| Urban | 3 | 6.7 | χ2 = 5.57 | 0.0012 ** |
| Rural | 42 | 93.3 | ||
| Family history of similar condition | ||||
| No | 24 | 53.3 | χ2 = 3.2 | 0.02 |
| Yes | 21 | 46.7 | ||
| Consanguinity | ||||
| No | 27 | 60 | χ2 = 3.15 | 0.02 |
| Yes | 18 | 40 | ||
| Parameter | N | % | Test-Value | Significance |
|---|---|---|---|---|
| The severity of hemophilia (Factor level IU/dL) | ||||
| Mild > 5 | 2 | 4.4 | χ2 = 4.28 | 0.022 * |
| Moderate 1–5 | 13 | 28.9 | ||
| Severe < 1 | 30 | 66.7 | ||
| Antibodies against factor 8 | ||||
| Detected | 8 | 17.8 | χ2 = 4.46 | 0.024 * |
| Not detected | 37 | 82.2 | ||
| Primary prophylaxis | ||||
| No | 37 | 82.2 | χ2 = 3.22 | 0.05 * |
| Emicizumab(Hemilibra) | 8 | 17.8 | ||
| Variables | N = 45 |
|---|---|
| Number of bleeding events per month | |
| Min.–Max. | 1.00–7.00 |
| Mean ± SD | 3.689 ± 1.24 |
| Number of joint bleedings per month | |
| Min.–Max. | 1.00–4.00 |
| Mean ± SD | 2.5333 ± 0.94388 |
| Frequency of joint bleeding per month | N (%) |
| 1 time | 5 (11.1%) |
| 2 times | 20 (44.5%) |
| 3 times | 11 (24.4%) |
| 4 times | 9 (20%) |
| Group | Study Group | Control Group | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Range | Mean | ± | SD | Range | Mean | ± | SD | ||
| Serum Total Calcium (mg/dL) | 5.87–10.67 | 9.28 | ± | 1.12 | 5.85–10.98 | 9.73 | ± | 0.68 | 0.108 |
| Serum Phosphorus (mg/dL) | 3.41–8.40 | 5.69 | ± | 1.49 | 3.45–8.47 | 5.27 | ± | 1.08 | 0.195 |
| Alkaline Phosphatase (U/L) | 81–365 | 186.11 | ± | 93.71 | 70–301 | 121.86 | ± | 60.54 | <0.001 ** |
| Serum Vitamin D level (ng/mL) | 6.30–37.20 | 14.57 | ± | 6.90 | 6.35–38.26 | 29.05 | ± | 8.76 | <0.001 ** |
| Study Group n, (%) | Mean ± SD | Control Group n, (%) | Mean ± SD | p-Value | |
|---|---|---|---|---|---|
| Deficient < 20 ng/mL | 38 (84.4%) | 12.35 ± 4.11 | 8 (17.7%) | 18.39 ± 0.97 | 0.0002 ** |
| Insufficient ≥ 20 to > 30 ng/mL | 4 (8.8%) | 21.5 ± 1.11 | 16 (35.5%) | 24.3 ± 3.34 | 0.1308 |
| Sufficient ≥ 30 ng/mL | 3 (6.6%) | 33.4 ± 3.35 | 21 (46.6%) | 36.71 ± 5.89 | 0.3575 |
| Total Hemophilia Joint Health Score (HJHS) | ||
|---|---|---|
| Serum Total Calcium | r | −0.31 |
| p | 0.038 * | |
| Serum Phosphorus | r | 0.261 |
| p | 0.083 | |
| Alkaline Phosphatase | r | 0.834 |
| p | <0.001 ** | |
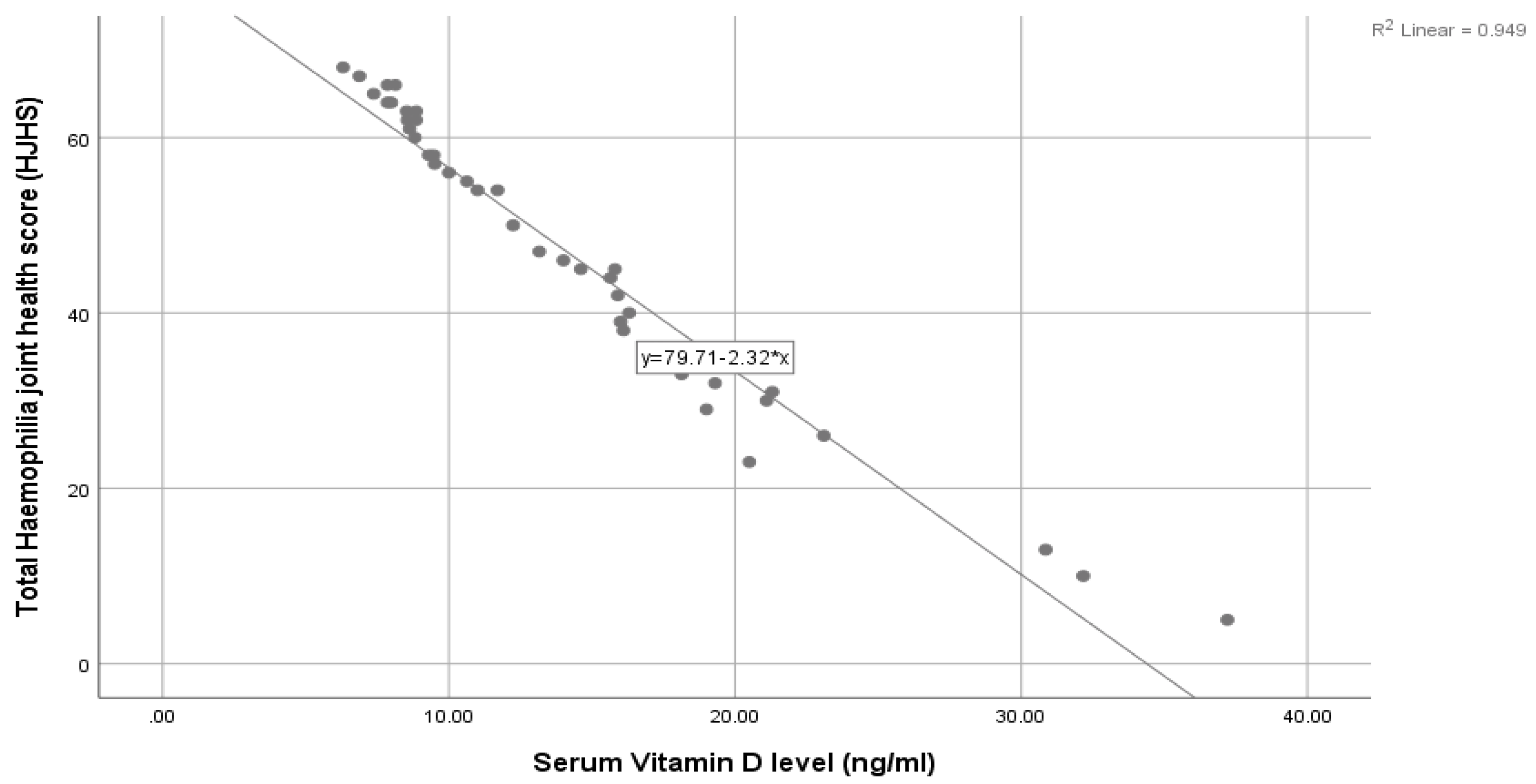
| Serum Vitamin D level (ng/mL) | r | −0.974 |
| p | <0.001 ** | |
| Variables | Group I 4–7 Years (n = 14) | Group II 8–12 Years (n = 24) | Group III 13–16 Years (n = 3) | Test of Significance | p Value |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |||
| Physical health | 18.071 ± 5.877 | 16.042 ± 6.617 | 12.667 ± 8.021 | F = 1.004 | 0.376 |
| Feelings | 5.786 ± 1.805 | 6.542 ± 3.050 | 4.000 ± 1.732 | F = 1.398 | 0.260 |
| View of yourself score | 4.357 ± 1.781 | 6.167 ± 2.615 | 4.667 ±2.082 | F = 2.832 | 0.071 |
| Sports and school score | 14.429 ± 4.702 | 10.625 ± 5.282 | 7.667 ± 3.512 | F = 3.601 | 0.037 * |
| Family score | 4.857 ± 1.748 | 5.917 ± 2.586 | 4.000 ± 2.000 | F = 1.546 | 0.226 |
| Friends score | 2.000 ± 0.555 | 3.083 ± 1.816 | 2.667 ± 2.082 | H = 5.524 | 0.063 |
| Treatment score | 2.071 ± 0.829 | 5.417 ± 2.701 | 4.333 ± 1.528 | H = 13.507 | 0.001 * |
| Dealing score | -- | 3.708 ± 2.053 | 2.000 ± 1.000 | H = 25.727 | <0.001 * |
| Perceived support score | -- | 4.000 ± 2.284 | 2.667 ± 2.309 | H = 22.648 | <0.001 * |
| Total hemophilia specific Quality of life index (Haemo-Qol) | 51.571 ± 15.441 | 61.500± 25.447 | 44.667 ± 24.111 | F = 1.343 | 0.273 |
| Total Hemophilia Specific Quality of Life Index (Haemo-Qol/Haem-A-QoL) | ||
|---|---|---|
| Total hemophilia joint health score (HJHS) | r | 0.934 |
| p | 0.000 ** | |
| Serum total calcium | r | −0.115 |
| p | 0.474 | |
| Serum phosphorus | r | 0.261 |
| p | 0.099 | |
| Alkaline phosphatase | r | 0.842 |
| p | 0.000 ** | |
| Serum vitamin D level (ng/mL) | r | −0.924 |
| p | 0.000 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salem, A.M.S.; AbdEltwwab, T.M.; Moawad, H.H.; Elgendy, M.O.; Al-Fakharany, R.S.; Khames, A.; Meabed, M.H. Serum Vitamin D in Children with Hemophilia A and Its Association with Joint Health and Quality of Life. Hematol. Rep. 2024, 16, 742-751. https://doi.org/10.3390/hematolrep16040071
Salem AMS, AbdEltwwab TM, Moawad HH, Elgendy MO, Al-Fakharany RS, Khames A, Meabed MH. Serum Vitamin D in Children with Hemophilia A and Its Association with Joint Health and Quality of Life. Hematology Reports. 2024; 16(4):742-751. https://doi.org/10.3390/hematolrep16040071
Chicago/Turabian StyleSalem, Aida M. S., Takwa Mohamed AbdEltwwab, Hanan Hosni Moawad, Marwa O. Elgendy, Reham S. Al-Fakharany, Ahmed Khames, and Mohamed Hussein Meabed. 2024. "Serum Vitamin D in Children with Hemophilia A and Its Association with Joint Health and Quality of Life" Hematology Reports 16, no. 4: 742-751. https://doi.org/10.3390/hematolrep16040071
APA StyleSalem, A. M. S., AbdEltwwab, T. M., Moawad, H. H., Elgendy, M. O., Al-Fakharany, R. S., Khames, A., & Meabed, M. H. (2024). Serum Vitamin D in Children with Hemophilia A and Its Association with Joint Health and Quality of Life. Hematology Reports, 16(4), 742-751. https://doi.org/10.3390/hematolrep16040071







