Hemophagocytic Lymphohistiocytosis Triggered by Herpes Simplex Virus 1 and 2: A Narrative Review
Abstract
1. Introduction
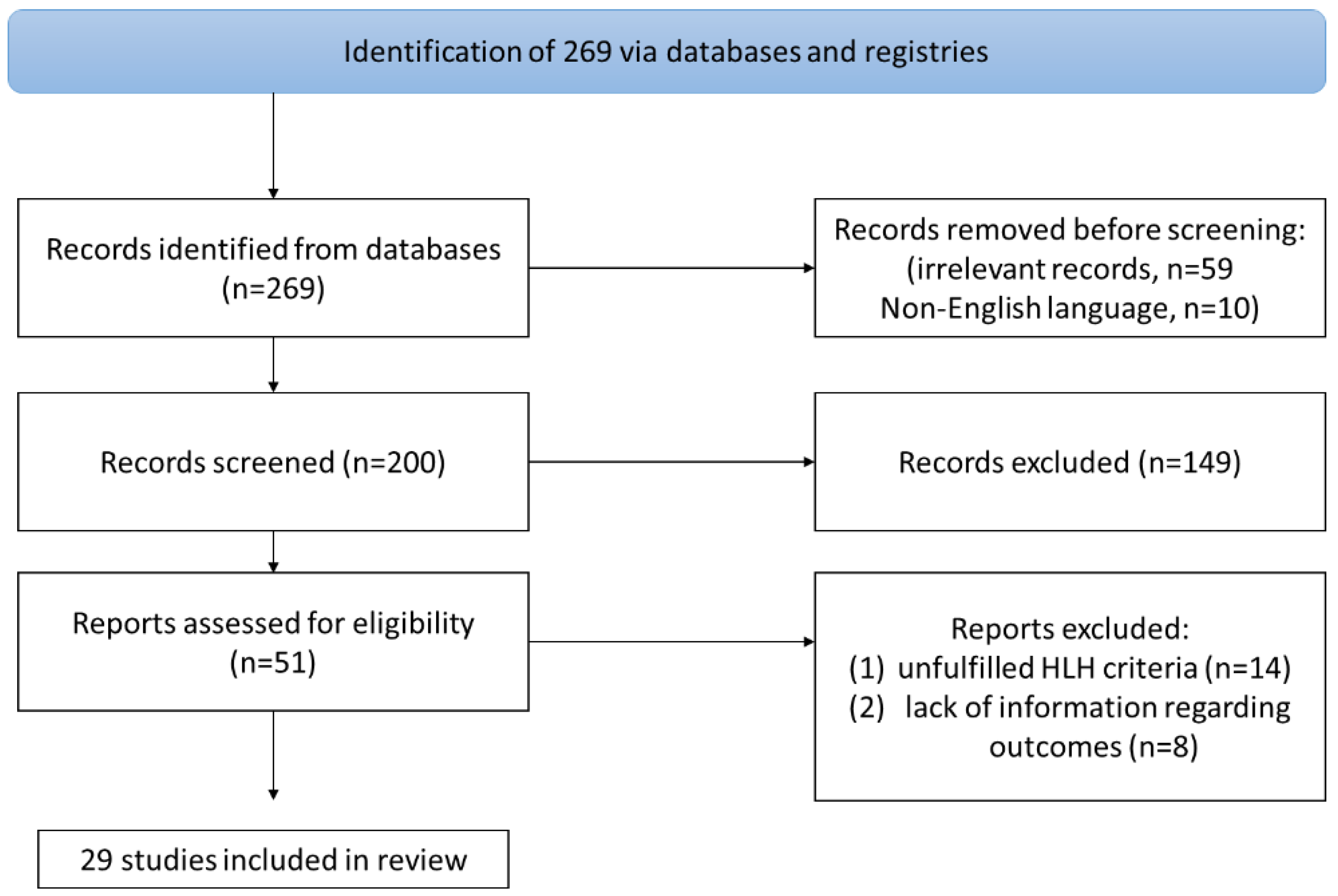
2. Materials and Methods
3. Results
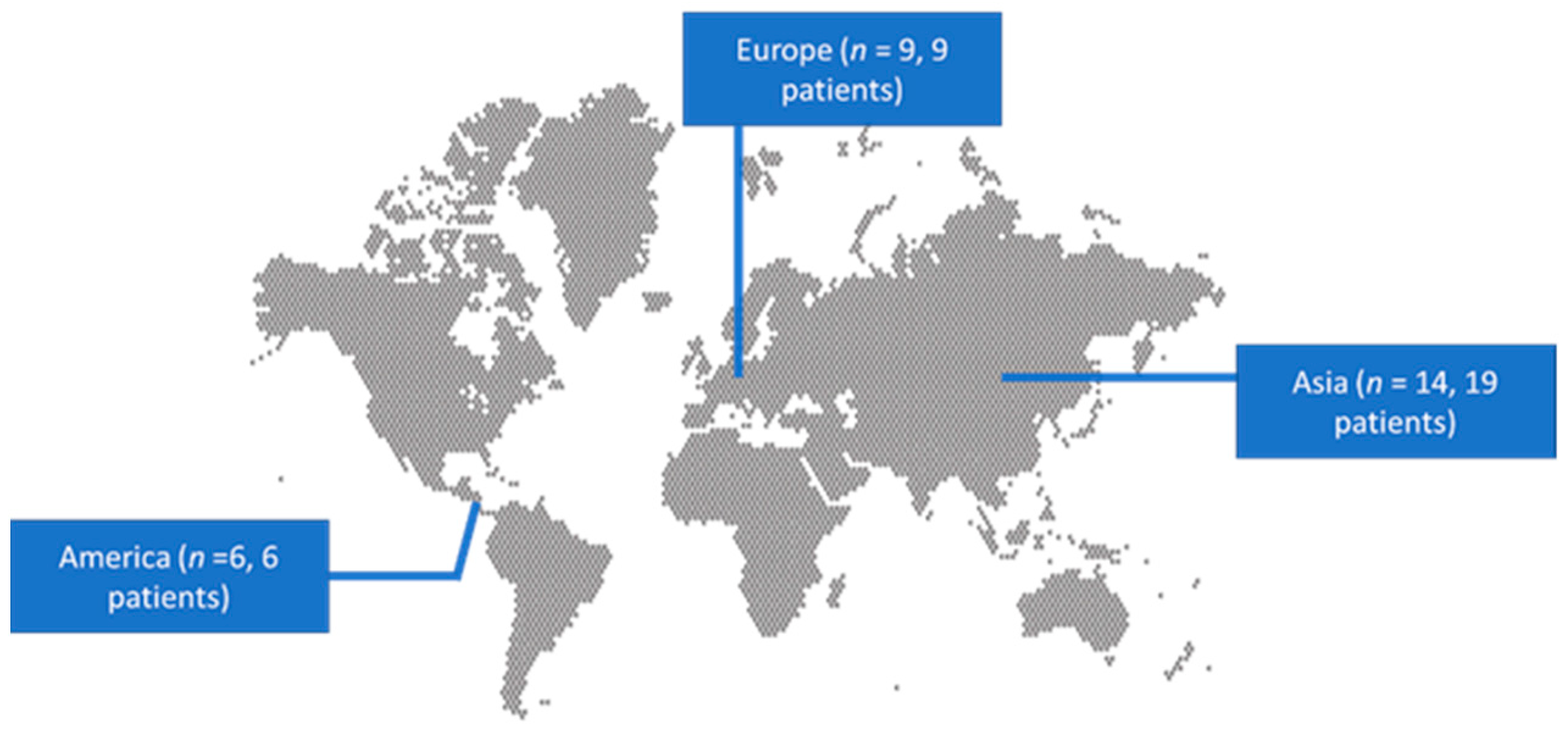
3.1. Included Studies’ Characteristics
3.2. Epidemiology and Clinical Characteristics of HLH Associated with HSV 1 and 2
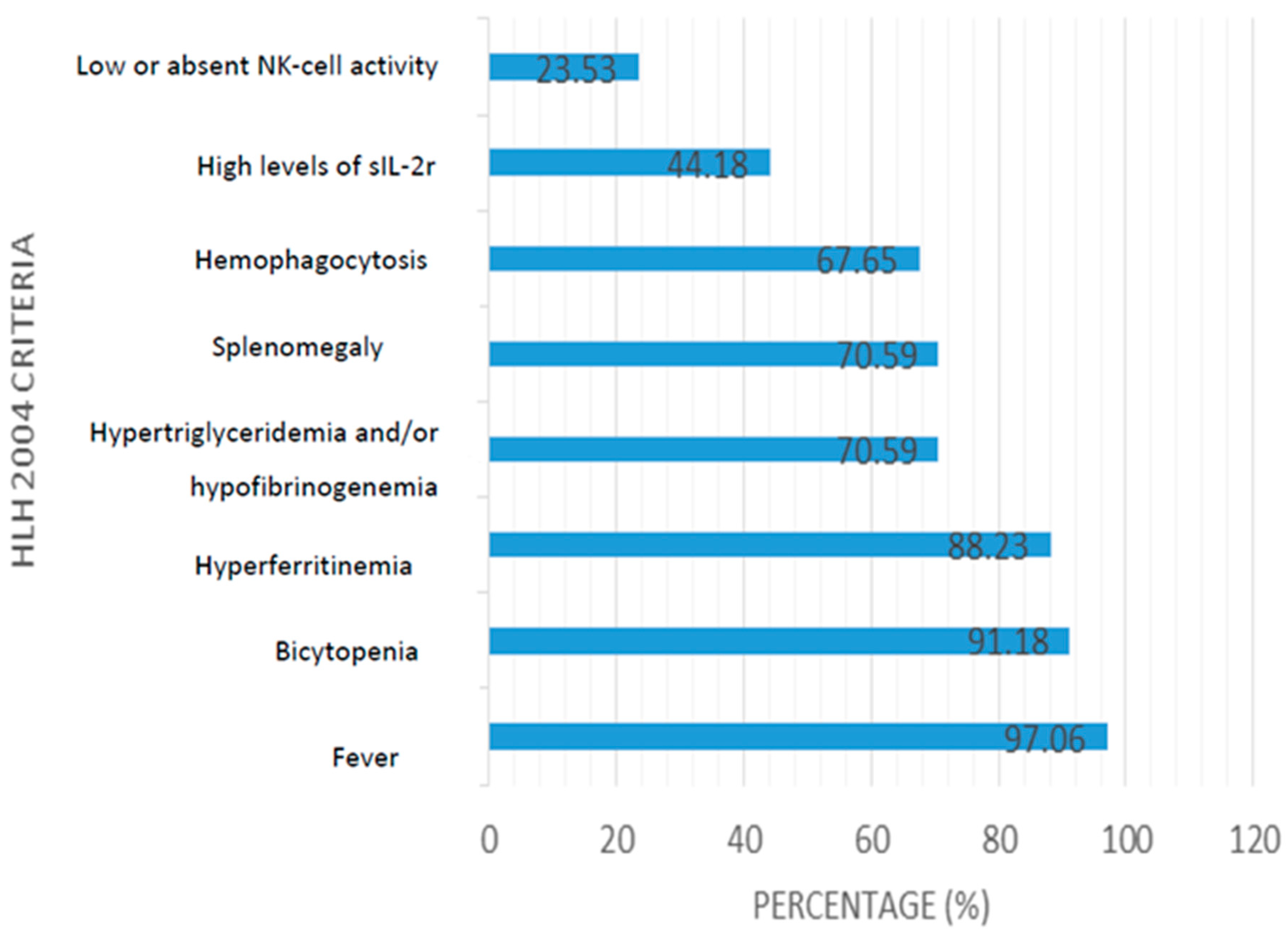
3.3. Microbiology and Diagnosis of HLH Associated with HSV 1 and 2
3.4. Treatment and Outcomes of HLH Associated with HSV 1 and 2
3.5. Statistical Analysis of HLH Associated with HSV 1 and 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Janka, G.E.; Lehmberg, K. Hemophagocytic Lymphohistiocytosis: Pathogenesis and Treatment. Hematology 2013, 2013, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Wang, Y.; Sun, Y.; Liu, L.; Zhang, R.; Fang, J.; Jin, R.; Yu, J.; Li, F.; Bai, J.; et al. Epidemiological Investigation of Hemophagocytic Lymphohistiocytosis in China. Orphanet J. Rare Dis. 2021, 16, 342. [Google Scholar] [CrossRef] [PubMed]
- West, J.; Card, T.R.; Bishton, M.J.; Lanyon, P.; Ban, L.; Bythell, M.; Elliss-Brookes, L.; Manson, J.J.; Nanduri, V.; Rankin, J.; et al. Incidence and Survival of Haemophagocytic Lymphohistiocytosis: A Population-based Cohort Study from England. J. Intern. Med. 2022, 291, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Hayden, A.; Park, S.; Giustini, D.; Lee, A.Y.Y.; Chen, L.Y.C. Hemophagocytic Syndromes (HPSs) Including Hemophagocytic Lymphohistiocytosis (HLH) in Adults: A Systematic Scoping Review. Blood Rev. 2016, 30, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Imashuku, S.; Morimoto, A.; Ishii, E. Virus-triggered Secondary Hemophagocytic Lymphohistiocytosis. Acta Paediatr. 2021, 110, 2729–2736. [Google Scholar] [CrossRef] [PubMed]
- Setiadi, A.; Zoref-Lorenz, A.; Lee, C.Y.; Jordan, M.B.; Chen, L.Y.C. Malignancy-Associated Haemophagocytic Lymphohistiocytosis. Lancet Haematol. 2022, 9, e217–e227. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, P.; Andanamala, H. Hemophagocytic Lymphohistiocytosis Secondary to Immune Checkpoint Inhibitor Therapy. World J. Oncol. 2022, 13, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, H.; Van Den Neste, E.; Defour, J.P.; Danse, E.; Yombi, J.C. Adult Haemophagocytic Lymphohistiocytosis: A Review. QJM Int. J. Med. 2022, 115, 205–213. [Google Scholar] [CrossRef]
- Yao, S.; He, L.; Zhang, R.; Liu, M.; Hua, Z.; Zou, H.; Wang, Z.; Wang, Y. Improved Hemophagocytic Lymphohistiocytosis Index Predicts Prognosis of Adult Epstein-Barr Virus-Associated HLH Patients. Ann. Med. 2023, 55, 89–100. [Google Scholar] [CrossRef]
- Balakumar, N.; Sendi, P.; Totapally, B.R. Epidemiology and Outcomes of Neonatal Hemophagocytic Lymphohistiocytosis. Front. Pediatr. 2022, 10, 848004. [Google Scholar] [CrossRef]
- Grabovac, V.; Kardum-Peric, M.; Sokol, S. Hemophagocytic Syndrome Associated with Human Herpesvirus I: A Case Report. Neurol.Croat. 2012, 61, 69–72. [Google Scholar]
- Henter, J.; Horne, A.; Aricó, M.; Egeler, R.M.; Filipovich, A.H.; Imashuku, S.; Ladisch, S.; McClain, K.; Webb, D.; Winiarski, J.; et al. HLH-2004: Diagnostic and Therapeutic Guidelines for Hemophagocytic Lymphohistiocytosis. Pediatr. Blood Cancer 2007, 48, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Griffin, G.; Shenoi, S.; Hughes, G.C. Hemophagocytic Lymphohistiocytosis: An Update on Pathogenesis, Diagnosis, and Therapy. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101515. [Google Scholar] [CrossRef]
- Sonoda, M.; Ishimura, M.; Eguchi, K.; Shiraishi, A.; Kanno, S.; Kaku, N.; Inoue, H.; Motomura, Y.; Ochiai, M.; Sakai, Y.; et al. Prognostic Factors for Survival of Herpes Simplex Virus-Associated Hemophagocytic Lymphohistiocytosis. Int. J. Hematol. 2020, 111, 131–136. [Google Scholar] [CrossRef]
- McKeone, D.J.; DeMartini, T.K.M.; Kavanagh, R.P.; Halstead, E.S. Case Report: Rapid Recognition and Immune Modulation of Secondary HLH Due to Disseminated HSV Infection. Front. Pediatr. 2021, 9, 681055. [Google Scholar] [CrossRef] [PubMed]
- Alidjinou, E.K.; Dewilde, A.; Terriou, L.; Lazrek, M.; Engelmann, I.; Hober, D. Persistent Viral DNA Detection in Blood after Primary Herpes Simplex 1 Infection Revealed by Hepatitis with Hemophagocytic Syndrome. J. Clin. Virol. 2015, 69, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Cheney-Peters, D.; Weber, D.M. Herpes Simplex Virus 1 Hepatitis Leading to Liver Failure and Hemophagocytic Lymphohistiocytosis: Case Report and Review of the Literature. Infect. Dis. Clin. Pract. 2019, 27, 321–324. [Google Scholar] [CrossRef]
- Cusini, A.; Günthard, H.F.; Stussi, G.; Schwarz, U.; Fehr, T.; Grueter, E.; Meerbach, A.; Bossart, W.; Schaer, D.J.; Rudiger, A. Hemophagocytic Syndrome Caused by Primary Herpes Simplex Virus 1 Infection: Report of a First Case. Infection 2010, 38, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Drori, A.; Ribak, Y.; Van Heerden, P.V.; Meir, K.; Wolf, D.; Safadi, R. Hemophagocytic Lymphohistiocytosis Due to Acute Primary Herpes Simplex Virus 1 Infection. J. Clin. Virol. 2015, 68, 6–10. [Google Scholar] [CrossRef]
- Freytag, M.R.; Jørgensen, S.E.; Thomsen, M.M.; Al-Mousawi, A.; Hait, A.S.; Olagnier, D.; Bay, J.T.; Helleberg, M.; Mogensen, T.H. Postpartum Disseminated Herpes Simplex Virus Type 1 Infection with Hemophagocytic Lymphohistiocytosis and Fulminant Neonatal Herpes Infection. J. Infect. Dis. 2022, 225, 157–162. [Google Scholar] [CrossRef]
- Honsig, C.; Beinhardt, S.; Tomasits, J.; Dienes, H.P. Haemophagocytic Lymphohistiocytosis Associated with Fulminant Hepatitis and Multiorgan Failure Following Primary Epstein–Barr Virus and Herpes Simplex Virus Type 1 Infection. BMJ Case Rep. 2017, 2017, bcr2016218310. [Google Scholar] [CrossRef] [PubMed]
- Halstead, E.S.; Rajasekaran, S.; Fitzgerald, J.C.; Weiss, S.L. Hyperferritinemic Sepsis: An Opportunity for Earlier Diagnosis and Intervention? Front. Pediatr. 2016, 4, 77. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ikumi, K.; Ando, T.; Katano, H.; Katsuno, M.; Sakai, Y.; Yoshida, M.; Saida, T.; Kimura, H.; Sobue, G. HSV-2–Related Hemophagocytic Lymphohistiocytosis in a Fingolimod-Treated Patient with MS. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e247. [Google Scholar] [CrossRef] [PubMed]
- Kojima, K.; Takahashi, N.; Yada, Y.; Koike, Y.; Matano, M.; Kono, Y.; Momoi, M.Y. White-matter Damage in a Neonate with Disseminated Herpes Simplex Virus Infection. Pediatr. Int. 2012, 54, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, S.; Sekiya, N.; Fukushima, K.; Ikeuchi, K.; Fukuda, A.; Takahashi, H.; Chen, F.; Hasegawa, H.; Katano, H.; Hishima, T.; et al. Unusual Manifestation of Disseminated Herpes Simplex Virus Type 2 Infection Associated with Pharyngotonsilitis, Esophagitis, and Hemophagocytic Lymphohisitocytosis without Genital Involvement. BMC Infect. Dis. 2019, 19, 65. [Google Scholar] [CrossRef] [PubMed]
- Nagamura, N.; Ishitobi, T. Hemophagocytic Syndrome Suspected to Be Caused by Herpes Simplex Virus Complicated with Severe Hepatitis during the Immunosuppressive Therapy for Dermatomyositis. Mod. Rheumatol. Case Rep. 2017, 1, 113–117. [Google Scholar] [CrossRef]
- Nasser, M.F.; Sharma, S.; Albers, E.; Sharma, S.; Duggal, A. Pregnancy-Related Hemophagocytic Lymphohistiocytosis Associated with Herpes Simplex Virus-2 Infection: A Diagnostic Dilemma. Cureus 2018, 10, e2352. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.H.; Jeong, D.Y.; Jeon, I.S.; Kim, H.M. Macrophage Activation Syndrome Triggered by Herpes Viral Infection as the Presenting Manifestation of Juvenile Systemic Lupus Erythematosus. Pediatr. Infect. Vaccine 2015, 22, 210. [Google Scholar] [CrossRef][Green Version]
- Otsubo, K.; Fukumura, A.; Hirayama, M.; Morimoto, T.; Kato, M.; Mochizuki, H. Hemophagocytic Lymphohistiocytosis Caused by Systemic Herpes Simplex Virus Type 1 Infection: Successful Treatment with Dexamethasone Palmitate. Pediatr. Int. 2016, 58, 390–393. [Google Scholar] [CrossRef]
- Saettini, F.; Radaelli, S.; Ocello, L.; Ferrari, G.M.; Corti, P.; Dell’Acqua, F.; Ippolito, D.; Foresti, S.; Gervasini, C.; Badolato, R.; et al. Secondary Hemophagocytic Lymphohystiocytosis in a Rubinstein Taybi Syndrome Patient. Pediatr. Hematol. Oncol. 2022, 39, 74–79. [Google Scholar] [CrossRef]
- Schwartz, M.; O’Brien, C.; Raya, N.; Reau, N. Acquired Hemophagocytic Lymphohistiocytosis Associated with Disseminated Herpes Simplex Virus in Immunocompetent Host. ACG Case Rep. J. 2019, 6, e00164. [Google Scholar] [CrossRef] [PubMed]
- Spinner, M.A.; Ker, J.P.; Stoudenmire, C.J.; Fadare, O.; Mace, E.M.; Orange, J.S.; Hsu, A.P.; Holland, S.M. GATA2 Deficiency Underlying Severe Blastomycosis and Fatal Herpes Simplex Virus–Associated Hemophagocytic Lymphohistiocytosis. J. Allergy Clin. Immunol. 2016, 137, 638–640. [Google Scholar] [CrossRef] [PubMed]
- States, V.A.; Kapp, M.E. Herpes Simplex Virus-1 Triggered Hemophagocytic Lymphohistiocytosis in a Patient with Granulomatosis with Polyangiitis. Autops. Case Rep. 2022, 12, e2021395. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Morimoto, A.; Ohga, S.; Kudo, K.; Ishida, Y.; Ishii, E. Characteristics of Hemophagocytic Lymphohistiocytosis in Neonates: A Nationwide Survey in Japan. J. Pediatr. 2009, 155, 235–238.e1. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Fujita, Y.; Otaka, T.; Kano, Y.; Fukushima, K.; Sato, Y.; Yoshihara, S. Postpartum Neonatal Disseminated Herpes Simplex Virus-1 Infection in Which Herpes Simplex Virus-1 Was Detected in Mother’s Breast Milk. Indian J. Pediatr. 2023, 90, 510–512. [Google Scholar] [CrossRef] [PubMed]
- Takehara, H.; Hirohata, K.; Mutoh, H.; Irisa, C.; Kakiuchi, S.; Nishimura, R.; Oka, A.; Takahashi, N. Critically Severe Case of Neonatal Herpes with High Viral Load and Hemophagocytic Syndrome. Tohoku J. Exp. Med. 2019, 247, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Vladescu, I.A.; Browning, W.L.; Thomsen, I.P. Massive Ferritin Elevation in Neonatal Herpes Simplex Virus Infection: Hemophagocytic Lymphohistiocytosis or Herpes Simplex Virus Alone? J. Pediatr. Infect. Dis. 2015, 4, e48–e52. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Kai, M.; Tanaka, H.; Shimizu, N.; Shimatani, M.; Oshima, T. Computed Tomography Findings of the Liver in a Neonate with Herpes Simplex Virus-associated Hemophagocytic Lymphohistiocytosis. Pediatr. Int. 2011, 53, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Yabushita, T.; Yoshioka, S.; Koba, Y.; Ono, Y.; Hiramoto, N.; Tabata, S.; Itou, M.; Shimizu, N.; Tomii, K.; Ishikawa, T. Successful Treatment of Herpes Simplex Virus (HSV)-1-Associated Hemophagocytic Lymphohistiocytosis (HLH) with Acyclovir: A Case Report and Literature Review. Intern. Med. 2017, 56, 2919–2923. [Google Scholar] [CrossRef][Green Version]
- Yamada, K.; Yamamoto, Y.; Uchiyama, A.; Ito, R.; Aoki, Y.; Uchida, Y.; Nagasawa, H.; Kimura, H.; Ichiyama, T.; Fukao, T.; et al. Successful Treatment of Neonatal Herpes Simplex-Type 1 Infection Complicated by Hemophagocytic Lymphohistiocytosis and Acute Liver Failure. Tohoku J. Exp. Med. 2008, 214, 1–5. [Google Scholar] [CrossRef][Green Version]
- Zhang, H.; Zhang, P.; Xiao, Z.; Gao, Y.; Han, N.; He, X.; Zhang, J.; Li, Y. Hemophagocytic Lymphohistiocytosis Caused by Herpes Simplex Virus Type 1 in a Young Adult: A Case Report with Literature Review. J. Hematop. 2024, 17, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Cole, S. Herpes Simplex Virus. Nurs. Clin. N. Am. 2020, 55, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Fatahzadeh, M.; Schwartz, R.A. Human Herpes Simplex Virus Infections: Epidemiology, Pathogenesis, Symptomatology, Diagnosis, and Management. J. Am. Acad. Dermatol. 2007, 57, 737–763. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Viejo-Borbolla, A. Pathogenesis and Virulence of Herpes Simplex Virus. Virulence 2021, 12, 2670–2702. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, M.P.; Hann, W.; Shivkumar, M.; Harman, L.E.R.; Connor, V.; Coleman, H.M.; Proença, J.T.; Efstathiou, S. The HSV-1 Latency-Associated Transcript Functions to Repress Latent Phase Lytic Gene Expression and Suppress Virus Reactivation from Latently Infected Neurons. PLoS Pathog. 2016, 12, e1005539. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, M.P.; Proença, J.T.; Efstathiou, S. The Molecular Basis of Herpes Simplex Virus Latency. FEMS Microbiol. Rev. 2012, 36, 684–705. [Google Scholar] [CrossRef]
- Abdelhay, A.; Mahmoud, A.A.; Al Ali, O.; Hashem, A.; Orakzai, A.; Jamshed, S. Epidemiology, Characteristics, and Outcomes of Adult Haemophagocytic Lymphohistiocytosis in the USA, 2006–2019: A National, Retrospective Cohort Study. eClinicalMedicine 2023, 62, 102143. [Google Scholar] [CrossRef] [PubMed]
- George, M.R. Hemophagocytic Lymphohistiocytosis: Review of Etiologies and Management. J. Blood Med. 2014, 5, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Zhang, J.; Chen, Q.; Xing, L.; Qiu, T.; Zhu, H.; Wang, L.; Fan, L.; Xu, W.; Li, J. Spectrum and Trigger Identification of Hemophagocytic Lymphohistiocytosis in Adults: A Single-Center Analysis of 555 Cases. Front. Immunol. 2022, 13, 970183. [Google Scholar] [CrossRef]
- Tantawy, A.A.; Elsherif, N.H.K.; Elsayed, S.M.; Ali, H.G.A.; Makkeyah, S.M.; Elsantiel, H.I.E.; De Saint Basile, G.; Ragab, I.A. Hemophagocytic Lymphohistiocytosis in Egyptian Children: Diagnosis, Treatment Challenges, and Outcome. Expert. Rev. Hematol. 2024, 17, 153–163. [Google Scholar] [CrossRef]
- Nienkemper, M.; Malherbe, J.; Barrett, C. Haemophagocytic Lymphohistiocytosis: Five Years’ Experience at Tertiary Hospitals in Free State Province, South Africa. S. Afr. J. Crit. Care 2020, 36, 115. [Google Scholar] [CrossRef]
- Koumadoraki, E.; Madouros, N.; Sharif, S.; Saleem, A.; Jarvis, S.; Khan, S. Hemophagocytic Lymphohistiocytosis and Infection: A Literature Review. Cureus 2022, 14, e22411. [Google Scholar] [CrossRef] [PubMed]
- Kwak, A.; Jung, N.; Shim, Y.J.; Kim, H.S.; Lim, H.J.; Lee, J.M.; Heo, M.H.; Do, Y.R. A Retrospective Analysis of Etiology and Outcomes of Hemophagocytic Lymphohistiocytosis in Children and Adults. Yeungnam Univ. J. Med. 2021, 38, 208–218. [Google Scholar] [CrossRef]
- Araki, S.; Shirahata, A. Vitamin K Deficiency Bleeding in Infancy. Nutrients 2020, 12, 780. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.-T.; Sheng, W.-H.; Lin, B.-H.; Lin, C.-W.; Wang, J.-T.; Chen, Y.-C.; Chang, S.-C. Causes, Clinical Symptoms, and Outcomes of Infectious Diseases Associated with Hemophagocytic Lymphohistiocytosis in Taiwanese Adults. J. Microbiol. Immunol. Infect. 2011, 44, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Park, S.; Jang, J.H.; Kim, K.; Kim, H.-J.; Kim, S.-H.; Kang, C.-I.; Chung, D.R.; Peck, K.R.; Lee, J.; et al. Clinical Significance of Bone Marrow Hemophagocytosis in Adult Patients with Malignancy and Non-Malignancy-Induced Hemophagocytic Lymphohistiocytosis. Ann. Hematol. 2016, 95, 325–335. [Google Scholar] [CrossRef]
- Lin, S.; Li, Y.; Long, J.; Liu, Q.; Yang, F.; He, Y. Acute Liver Failure Caused by Hemophagocytic Lymphohistiocytosis in Adults: A Case Report and Review of the Literature. Medicine 2016, 95, e5431. [Google Scholar] [CrossRef]
- Schram, A.M.; Comstock, P.; Campo, M.; Gorovets, D.; Mullally, A.; Bodio, K.; Arnason, J.; Berliner, N. Haemophagocytic Lymphohistiocytosis in Adults: A Multicentre Case Series over 7 Years. Br. J. Haematol. 2016, 172, 412–419. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Zheng, W.; Ma, J.; Zhang, W.; Wang, W.; Tian, X. Hemophagocytic Lymphohistiocytosis: Clinical Analysis of 103 Adult Patients. Medicine 2014, 93, 100–105. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Hsu, M.-H.; Kuo, H.-C.; Sheen, J.-M.; Cheng, M.-C.; Lin, Y.-J. Outcome Analysis of Pediatric Hemophagocytic Lymphohistiocytosis. J. Formos. Med. Assoc. 2021, 120, 172–179. [Google Scholar] [CrossRef]
- Nath, P.; Kabir, M.A.; Doust, S.K.; Ray, A. Diagnosis of Herpes Simplex Virus: Laboratory and Point-of-Care Techniques. Infect. Dis. Rep. 2021, 13, 518–539. [Google Scholar] [CrossRef] [PubMed]
- Kelesidis, T.; Humphries, R.; Terashita, D.; Eshaghian, S.; Territo, M.C.; Said, J.; Lewinski, M.; Currier, J.S.; Pegues, D. Epstein–Barr Virus-associated Hemophagocytic Lymphohistiocytosis in Los Angeles County. J. Med. Virol. 2012, 84, 777–785. [Google Scholar] [CrossRef]
- Okazaki, K.; Imadome, K.-I.; Nakao, H.; Miyairi, I.; Ishiguro, A. Quantitative PCR Assays of Cytomegalovirus and Epstein-Barr Virus in Hemophagocytic Lymphohistiocytosis. Indian J. Pediatr. 2018, 85, 593–594. [Google Scholar] [CrossRef] [PubMed]
- Zoref-Lorenz, A.; Ellis, M.; Jordan, M.B. Inpatient Recognition and Management of HLH. Hematology 2023, 2023, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.B.; Allen, C.E.; Greenberg, J.; Henry, M.; Hermiston, M.L.; Kumar, A.; Hines, M.; Eckstein, O.; Ladisch, S.; Nichols, K.E.; et al. Challenges in the Diagnosis of Hemophagocytic Lymphohistiocytosis: Recommendations from the North American Consortium for Histiocytosis (NACHO). Pediatr. Blood Cancer 2019, 66, e27929. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, M.D.; Prajescu, B.; Taras, R.; Popescu, N.; Vidlescu, R.; Smarandoiu, M.; Rosca, L.-E.; Enculescu, A.; Berghea, E.C.; Toma, C.L. Diagnostic Challenges in Hemophagocytic Lymphohistiocytosis, a Rare, Potentially Fatal Disease: Two Case Studies. J. Clin. Med. 2024, 13, 1643. [Google Scholar] [CrossRef]
- Liew, J.W.; Jones, B.L.; Hunter, A.J. Disseminated Herpes Simplex Masquerading as Hemophagocytic Lymphohistiocytosis: A Case Report. Perm. J. 2019, 23, 18–202. [Google Scholar] [CrossRef] [PubMed]
- Khandwalla, Z.; Gupta, C.; Jhala, H. P0109/#796: Diagnosis of hemophagocytic lymphohistiocytosis in disseminated neonatal herpes simplex virus infection and its influence on outcome. Pediatr. Crit. Care Med. 2021, 22, 85. [Google Scholar] [CrossRef]
- Averitt, G.; Al-Rahawan, M.M.; Levent, F. Neonatal Disseminated Herpes Simplex Virus Infection Triggering Extreme Hyperferritinemia Concerning for Hemophagocytic Lymphohistiocytosis. J. Investig. Med. High Impact Case Rep. 2019, 7, 2324709619862840. [Google Scholar] [CrossRef]
- Tabaja, H.; Kanj, A.; El Zein, S.; Comba, I.Y.; Chehab, O.; Mahmood, M. A Review of Hemophagocytic Lymphohistiocytosis in Patients with HIV. Open Forum Infect. Dis. 2022, 9, ofac071. [Google Scholar] [CrossRef]
- Geng, F.; Yang, M.; Zhang, X.; Zhao, H.; Zhou, D.; Hu, J. Typical Hemophagocytic Syndrome Associated with Cytomegalovirus Infection in an Immunocompetent Patient: A Case Report and Literature Review. J. Zhejiang Univ. Sci. B 2023, 24, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Rolsdorph, L.Å.; Mosevoll, K.A.; Helgeland, L.; Reikvam, H. Concomitant Hemophagocytic Lymphohistiocytosis and Cytomegalovirus Disease: A Case Based Systemic Review. Front. Med. 2022, 9, 819465. [Google Scholar] [CrossRef] [PubMed]
- Van Der Ven, A.J.A.M.; Netea, M.G.; Van Der Meer, J.W.M.; De Mast, Q. Ebola Virus Disease Has Features of Hemophagocytic Lymphohistiocytosis Syndrome. Front. Med. 2015, 2, 4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marsh, R.A. Epstein–Barr Virus and Hemophagocytic Lymphohistiocytosis. Front. Immunol. 2018, 8, 1902. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.-J.; Yoon, J.-H.; Park, K.H.; Bae, H.J.; Yun, S.J.; Min, G.J.; Park, S.-S.; Park, S.; Lee, S.-E.; Cho, B.-S.; et al. Natural-Killer Cell Cytotoxicity as a Diagnostic and Prognostic Marker for Adult Patients with Secondary Hemophagocytic Lymphohistiocytosis: A Prospective Phase II Observational Study. Ther. Adv. Hematol. 2021, 12, 204062072110205. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brito-Zerón, P.; López-Guillermo, A.; Khamashta, M.A.; Bosch, X. Adult Haemophagocytic Syndrome. Lancet 2014, 383, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Weitzman, S.; Abdelhaleem, M. The Role of Hemophagocytosis in Bone Marrow Aspirates in the Diagnosis of Hemophagocytic Lymphohistiocytosis. Pediatr. Blood Cancer 2008, 50, 192–194. [Google Scholar] [CrossRef] [PubMed]
- See, K.C. Dengue-Associated Hemophagocytic Lymphohistiocytosis: A Narrative Review of Its Identification and Treatment. Pathogens 2024, 13, 332. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-Y.; Huang, Y.-C.; Lin, T.-Y.; Huang, J.-L.; Yang, C.-P.; Hsueh, T.; Wu, C.-T.; Hsia, S.-H. Primary Epstein-Barr Virus Infection Associated with Kikuchi’s Disease and Hemophagocytic Lymphohistiocytosis: A Case Report and Review of the Literature. J. Microbiol. Immunol. Infect. 2010, 43, 253–257. [Google Scholar] [CrossRef]
- Gholam, C.; Grigoriadou, S.; Gilmour, K.C.; Gaspar, H.B. Familial Haemophagocytic Lymphohistiocytosis: Advances in the Genetic Basis, Diagnosis and Management. Clin. Exp. Immunol. 2011, 163, 271–283. [Google Scholar] [CrossRef]
- Allen, C.E.; McClain, K.L. Pathophysiology and Epidemiology of Hemophagocytic Lymphohistiocytosis. Hematology 2015, 2015, 177–182. [Google Scholar] [CrossRef]
- Canna, S.W.; Marsh, R.A. Pediatric Hemophagocytic Lymphohistiocytosis. Blood 2020, 135, 1332–1343. [Google Scholar] [CrossRef] [PubMed]
- Carvelli, J.; Piperoglou, C.; Farnarier, C.; Vely, F.; Mazodier, K.; Audonnet, S.; Nitschke, P.; Bole-Feysot, C.; Boucekine, M.; Cambon, A.; et al. Functional and Genetic Testing in Adults with HLH Reveals an Inflammatory Profile Rather than a Cytotoxicity Defect. Blood 2020, 136, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Chinnici, A.; Beneforti, L.; Pegoraro, F.; Trambusti, I.; Tondo, A.; Favre, C.; Coniglio, M.L.; Sieni, E. Approaching Hemophagocytic Lymphohistiocytosis. Front. Immunol. 2023, 14, 1210041. [Google Scholar] [CrossRef]
- Lehmberg, K.; Sprekels, B.; Nichols, K.E.; Woessmann, W.; Müller, I.; Suttorp, M.; Bernig, T.; Beutel, K.; Bode, S.F.N.; Kentouche, K.; et al. Malignancy-associated Haemophagocytic Lymphohistiocytosis in Children and Adolescents. Br. J. Haematol. 2015, 170, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Sieni, E.; Cetica, V.; Hackmann, Y.; Coniglio, M.L.; Da Ros, M.; Ciambotti, B.; Pende, D.; Griffiths, G.; Aricã2, M. Familial Hemophagocytic Lymphohistiocytosis: When Rare Diseases Shed Light on Immune System Functioning. Front. Immunol. 2014, 5, 167. [Google Scholar] [CrossRef] [PubMed]
- Bracaglia, C.; Prencipe, G.; De Benedetti, F. Macrophage Activation Syndrome: Different Mechanisms Leading to a One Clinical Syndrome. Pediatr. Rheumatol. 2017, 15, 5. [Google Scholar] [CrossRef]
- Brisse, E.; Wouters, C.H.; Andrei, G.; Matthys, P. How Viruses Contribute to the Pathogenesis of Hemophagocytic Lymphohistiocytosis. Front. Immunol. 2017, 8, 1102. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.B.; Allen, C.E.; Weitzman, S.; Filipovich, A.H.; McClain, K.L. How I Treat Hemophagocytic Lymphohistiocytosis. Blood 2011, 118, 4041–4052. [Google Scholar] [CrossRef]
- Imashuku, S.; Kuriyama, K.; Teramura, T.; Ishii, E.; Kinugawa, N.; Kato, M.; Sako, M.; Hibi, S. Requirement for Etoposide in the Treatment of Epstein-Barr Virus–Associated Hemophagocytic Lymphohistiocytosis. J. Clin. Oncol. 2001, 19, 2665–2673. [Google Scholar] [CrossRef]
- Chellapandian, D.; Das, R.; Zelley, K.; Wiener, S.J.; Zhao, H.; Teachey, D.T.; Nichols, K.E.; EBV-HLH Rituximab Study Group. Treatment of E Pstein B Arr Virus-induced Haemophagocytic Lymphohistiocytosis with Rituximab-containing Chemo-immunotherapeutic Regimens. Br. J. Haematol. 2013, 162, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Aleem, S.; Saleh, H.; Petts, J.; Ballas, Z.K. A Personalized Diagnostic and Treatment Approach for Macrophage Activation Syndrome and Secondary Hemophagocytic Lymphohistiocytosis in Adults. J. Clin. Immunol. 2017, 37, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Bichon, A.; Bourenne, J.; Allardet-Servent, J.; Papazian, L.; Hraiech, S.; Guervilly, C.; Pauly, V.; Kaplanski, G.; Mokart, D.; Gainnier, M.; et al. High Mortality of HLH in ICU Regardless Etiology or Treatment. Front. Med. 2021, 8, 735796. [Google Scholar] [CrossRef] [PubMed]
- Jongdee, P.; Julamanee, J.; Rattarittamrong, E.; Mukura, S.; Wanitpongpun, C.; Deoisares, R.; Surawong, A.; Chajuwan, T.; Chanswangphuwana, C. Prognostic Factors of Adult Hemophagocytic Lymphohistiocytosis and Clinical Utility of HLH-2004 Diagnostic Criteria and HScore: A Real-World Multicenter Study from Thailand. Acta Haematol. 2024, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.A.; Kapoor, P.; Letendre, L.; Kumar, S.; Wolanskyj, A.P. Prognostic Factors and Outcomes of Adults with Hemophagocytic Lymphohistiocytosis. Mayo Clin. Proc. 2014, 89, 484–492. [Google Scholar] [CrossRef]
- Oto, M.; Yoshitsugu, K.; Uneda, S.; Nagamine, M.; Yoshida, M. Prognostic Factors and Outcomes of Adult-Onset Hemophagocytic Lymphohistiocytosis: A Retrospective Analysis of 34 Cases. Hematol. Rep. 2015, 7, 5841. [Google Scholar] [CrossRef][Green Version]
| A | Molecular diagnosis consistent with HLH [pathologic mutations of Perforin (PRF1), SH2D1A/SAP, UNC13D, Syntaxin 11 (STX11), MUNC18-2, Ras-related protein Rab27a (RAB27a)] | |
| B | Any 5 of the 8 following clinical and laboratory criteria for HLH | |
| Fever | >38.5 °C | |
| Splenomegaly | ||
| Cytopenia (affecting ≥ 2 of 3 lineages in peripheral blood) | Hemoglobin < 9 g/dL (in infants < 4 weeks: Hb < 100 g/L) Platelets < 100 × 109/L Neutrophils < 1.0 × 109/L | |
| Hypertriglyceridemia and/or hypofibrinogenemia | Fasting triglycerides > 3.0 mmol/L (>265 mg/dL) Fibrinogen ≤ 1.5 g/L | |
| Hemophagocytosis in bone marrow, spleen, liver, lymph nodes, or other tissues | ||
| Low or absent natural killer cell activity | ||
| Serum ferritin concentration | ≥500 μg/L | |
| Soluble CD25 (soluble IL-2 receptor) | ≥2400 U/mL | |
| Survived (n = 9) | Died (n = 8) | p-Value | |
|---|---|---|---|
| Patient characteristics (neonates, n = 17): | |||
| Age, median (IQR) | 5 (4, 9) | 5.5 (3.5, 9.75) | 1.00 |
| Male sex, n (%) | 4 (44.4) | 3 (37.5) | 1.00 |
| Primary immunosuppression, n (%) | 0 | 1 (20.0) | 0.42 |
| Symptoms: | |||
| Fever, n (%) | 6 (66.7) | 3 (37.5) | 0.35 |
| Weight loss, n (%) | 0 | 2 (66.7) | 0.40 |
| Jaundice, n (%) | 2 (22.2) | 1 (12.0) | 1.00 |
| Hepatomegaly, n (%) | 6 (66.7) | 2 (25.0) | 0.15 |
| Splenomegaly, n (%) | 4 (44.4) | 2 (25.0) | 0.62 |
| Lethargy/drowsiness, n (%) | 1 (11.1) | 2 (25.0) | 0.58 |
| Mucocutaneous vesicles, n (%) | 2 (22.2) | 0 | 0.47 |
| Skin rash, n (%) | 1 (11.1) | 0 | 1.00 |
| Respiratory symptoms (tachypnea, dyspnea, apnea), n (%) | 1 (11.1) | 3 (37.5) | 0.29 |
| Median laboratory values (IQR): | |||
| Leukocytes (K/mL) | 4000 (2650, 8875) | 1200 (700, -) | 1.00 |
| Hemoglobin (g/dL) | 10.7 (8.6, 13.9) | 8.1 (7.0, -) | 0.17 |
| Platelets (×103 K/mL) | 96 (39, 104) | 17 (14, 65) | 0.24 |
| AST (U/L) | 3543 (2055, 3804) | 6966.5 (2439.5, 7403) | 0.24 |
| ALT (Ul/L) | 943 (308, 1226) | 2214 (539, 3127.5) | 1.00 |
| LDH (U/dL) | 6805 (4765.5, 85.3) | 8073 (3750, -) | 1.00 |
| Triglycerides (mg/dL) | 63 (40.5, 158.5) | 42.5 (41, -) | 0.43 |
| CRP (mg/dL) | 1.7 (0.84, 6.5) | 3.6 (1.7, 15.4) | 1.00 |
| Fibrinogen (mg/dL) | 61 (43, 104) | 92 (60, 98) | 1.00 |
| Ferritin (ng/mL) | 40,604.5 (14,387.8, 77,771.5) | 113,200 (76,650, 216,210) | 0.70 |
| sIL-2R (U/mL) | 3205 (2472.5, 4730.5) | 2503.5 (1068.3, 3979.3) | 1.00 |
| NK cell activity (%) | 10 (0, -) | 4 (4, 4) | 1.00 |
| PT (s) | 80 (13.1, -) | 74.7 (29.4, -) | 1.00 |
| Duration of hospitalization, median (IQR) | 42 (27.8, 70) | 21 (6, 39) | 0.29 |
| Survived (n = 11) | Died (n = 6) | p-Value | |
|---|---|---|---|
| Patient characteristics (adults, n =17): | |||
| Age, median (IQR) | 36 (19, 59) | 31.5 (25.5, 51.5) | 1.00 |
| Male sex, n (%) | 6 (54.5) | 4 (66.7) | 1.00 |
| Primary immunosuppression, n (%) | 3 (27.3) | 0 | 0.52 |
| Drug-induced immunosuppression, n (%) | 2 (18.2) | 2 (33.3) | 0.58 |
| Autoimmune disease, n (%) | 3 (27.3) | 2 (33.3) | 1.00 |
| Symptoms: | |||
| Fever, n (%) | 11 (100) | 6 (100) | |
| Jaundice, n (%) | 0 | 2 (33.3) | 0.11 |
| Hepatomegaly, n (%) | 7 (63.6) | 2 (33.3) | 0.34 |
| Splenomegaly, n (%) | 7 (63.6) | 5 (83.3) | 0.60 |
| Lethargy/drowsiness, n (%) | 3 (27.3) | 1 (16.7) | 1.00 |
| Mucocutaneous vesicles, n (%) | 5 (45.5) | 1 (16.7) | 0.33 |
| Skin rash, n (%) | 2 (18.2) | 1 (16.7) | 1.00 |
| Abdominal pain (GI symptoms), n (%) | 4 (36.4) | 0 | 0.24 |
| Median laboratory values (IQR): | |||
| Leukocytes (K/mL) | 1420 (850, 1600) | 1360 (822.5, 1775) | 1.00 |
| Hemoglobin (g/dL) | 10.7 (8.6, 13.9) | 7.5 (6.7, 10.4) | 1.00 |
| Platelets (×103 K/mL) | 81 (47.5, 1125) | 25.5 (14.8, 71.8) | 0.61 |
| AST (U/L) | 1807 (1398, 4000) | 11,306.5 (8166, 12,554.5) | 0.03 |
| ALT (Ul/L) | 1803.5 (751, 3331.5) | 3076 (839, 4819.5) | 0.57 |
| LDH (U/dL) | 2284 (1345, 4248) | 11,100 (6000, -) | 0.17 |
| Triglycerides (mg/dL) | 281 (128.8, 502.3) | 79 (61.6, -) | 1.00 |
| CRP (mg/dL) | 9.8 (5.7, 10.4) | 2.5 (1.8, -) | 1.00 |
| Fibrinogen (mg/dL) | 29,726 (5296.8, 62,427) | 140 (99, -) | 1.00 |
| Ferritin (ng/mL) | 29,726 (5296.8, 62,427) | 30,524 (9264.5, 6200) | 0.85 |
| sIL-2R (U/mL) | 4590 (1574.5, 12,428) | 7214.5 (3075, 20,867) | 1.00 |
| NK cell activity (%) | 2.4 (2.4, 2.4) | - | - |
| PT (s) | 15.1 (10.8, -) | 13.02 (2.04, -) | 0.88 |
| Duration of hospitalization, median (IQR) | 21 (14, 57.5) | 6 (4, 12.5) | 0.30 |
| First Author(s) | Age | Sex | Type of HSV | HLH Criteria | Complications | Need for ICU | Treatment(s) Administered | Outcome |
|---|---|---|---|---|---|---|---|---|
| Alidjinou et al., 2015 [16] | 59 years | Male | Hsv1 | 5 | No | N/A | ACV, etoposide | Clinical cure |
| Cheney-Peters and Weber, 2019 [17] | 65 years | Male | Hsv1 | 6 | No | N/A | ACV | Clinical cure |
| Cusini et al., 2010 [18] | 57 years | Female | Hsv1 | 5 | Yes | Yes | ACV, steroids, etoposide, IVIG, platelets and erythrocyte replacement | Clinical cure |
| Drori et al., 2015 [19] | 50 years | Male | Hsv1 | 5 | Yes | Yes | ACV, etoposide, FFP, doxycycline/ceftazidime, hemodialysis | Death |
| Freytag et al., 2022 [20] | 19 years | Female | Hsv1 | 5 | Yes | Yes | ACV, steroids, foscarnet, IVIG, tocilizumab | Clinical cure |
| Grabovac et al., 2012 [11] | 36 years | Male | Hsv1 | 5 | Yes | Yes | ACV, steroids, IVIG, etoposide, FFP, blood transfusion, albumin, G-CSF, broad-spectrum antibiotics, vasopressors | Death |
| Honsig et al., 2017 [21] | 21 years | Male | Hsv1 | 5 | Yes | Yes | ACV | Death |
| Halstead et al., 2016 [22] | 5 days | Female | Hsv2 | 5 | Yes | Yes | ACV, steroids, vasopressors | Death |
| Ikumi et al., 2016 [23] | 56 years | Male | Hsv2 | 6 | Yes | N/A | ACV, steroids | Death |
| Kojima et al., 2012 [24] | 3 days | Male | Hsv1 | 5 | Yes | No | ACV, steroids, CsA | Clinical cure |
| Kurosawa et al., 2019 [25] | 46 years | Male | Hsv2 | 5 | No | No | Meropenem, ACV, steroids, | Clinical cure |
| Mckeone et al., 2021 [15] | 11 days | Female | Hsv1 | 5 | Yes | N/A | ACV, IVIG, steroids, etoposide, emapalumab, anakinra, FFP, hemodialysis | Death |
| Nagamura and Ishitobi, 2017 [26] | 34 years | Female | Hsv1 | 5 | Yes | N/A | Azathioprine, ACV, steroids, CsA, valacyclovir | Clinical cure |
| Nasser et al., 2018 [27] | 36 years | Female | Hsv2 | 5 | Yes | N/A | ACV, steroids | Clinical cure |
| Noh et al., 2015 [28] | 14 years | Female | Hsv1 | 5 | No | No | ACV, steroids | Clinical cure |
| Otsubo et al., 2016 [29] | 6 days | Female | Hsv1 | 7 | Yes | Yes | ACV, steroids, cefazolin, FFP, r-TM | Clinical cure |
| Saettini et al., 2021 [30] | 18 years | Male | Hsv1 | 5 | Yes | No | ACV, steroids, IVIG, ceftriaxone, amikacine, RBC transfusion | Clinical cure |
| Schwartz et al., 2019 [31] | 27 years | Female | Hsv2 | 6 | Yes | Yes | ACV, vasopressors | Death |
| Sonoda et al., 2019 [14] | 6 days | Male | Hsv2 | 7 | Yes | Yes | ACV, steroids, CsA, etoposide, IVIG, plasma exchange | Death |
| 4 days | Male | Hsv2 | 5 | Yes | Yes | ACV, steroids, cyclosporin, IVIG | Clinical cure | |
| Spinner et al., 2016 [32] | 18 days | Female | Hsv1 | 6 | Yes | N/A | ACV, steroids, vasopressors, vancomycin, meropenem, levofloxacin, liposomal amphotericin B | Death |
| States and Kapp, 2022 [33] | 27 years | Female | Hsv1 | 5 | Yes | Yes | ACV, steroids, etoposide, vasopressors | Death |
| Suzuki et al., 2009 [34] | 5 days | Female | Hsv1 | 7 | N/A | N/A | ACV, IVIG, steroids, CsA, etoposide | Death |
| 3 days | Male | Hsv1 | 6 | Yes | N/A | ACV, IVIG, steroids, CsA, etoposide | Death | |
| 8 days | Male | N/A | 5 | N/A | N/A | ACV, IVIG, steroids, CsA, etoposide | Clinical cure | |
| 6 days | Female | N/A | 6 | N/A | N/A | ACV, IVIG, steroids, etoposide | Death | |
| 4 days | Female | N/A | 5 | N/A | N/A | ACV, IVIG, steroids, CsA, etoposide | Clinical cure | |
| Takagi et al., 2023 [35] | 17 days | Female | Hsv1 | 6 | Yes | N/A | ACV, steroids | Clinical cure |
| Takehara et al., 2019 [36] | 2 days | Male | Hsv1 | 5 | Yes | Yes | ACV, steroids, foscarnet, CRRT, vasopressors | Death |
| Vladescu et al., 2015 [37] | 10 days | Female | Hsv2 | 6 | Yes | Yes | ACV, ampicillin, gentamicin | Clinical cure |
| Wada et al., 2011 [38] | 5 days | Female | Hsv2 | 5 | Yes | N/A | ACV, IVIG, antibiotics | Clinical cure |
| Yabushita et al., 2017 [39] | 69 years | Male | Hsv1 | 5 | No | N/A | ACV | Clinical cure |
| Yamada et al., 2008 [40] | 4 days | Male | Hsv1 | 6 | Yes | Yes | Platelet replacement, FFP, ACV, steroids, CsA, IVIG, vasopressors | Clinical cure |
| Zhang et al., 2023 [41] | 19 years | Male | Hsv1 | 8 | Yes | Yes | ACV, steroids, etoposide, IVIG, imipenem | Clinical cure |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papazachariou, A.; Ioannou, P. Hemophagocytic Lymphohistiocytosis Triggered by Herpes Simplex Virus 1 and 2: A Narrative Review. Hematol. Rep. 2024, 16, 487-503. https://doi.org/10.3390/hematolrep16030047
Papazachariou A, Ioannou P. Hemophagocytic Lymphohistiocytosis Triggered by Herpes Simplex Virus 1 and 2: A Narrative Review. Hematology Reports. 2024; 16(3):487-503. https://doi.org/10.3390/hematolrep16030047
Chicago/Turabian StylePapazachariou, Andria, and Petros Ioannou. 2024. "Hemophagocytic Lymphohistiocytosis Triggered by Herpes Simplex Virus 1 and 2: A Narrative Review" Hematology Reports 16, no. 3: 487-503. https://doi.org/10.3390/hematolrep16030047
APA StylePapazachariou, A., & Ioannou, P. (2024). Hemophagocytic Lymphohistiocytosis Triggered by Herpes Simplex Virus 1 and 2: A Narrative Review. Hematology Reports, 16(3), 487-503. https://doi.org/10.3390/hematolrep16030047







