A Rare Onset of T-Lymphoid Blast Crisis in Chronic Myeloid Leukemia with Two Distinct Blast Populations
Abstract
1. Introduction
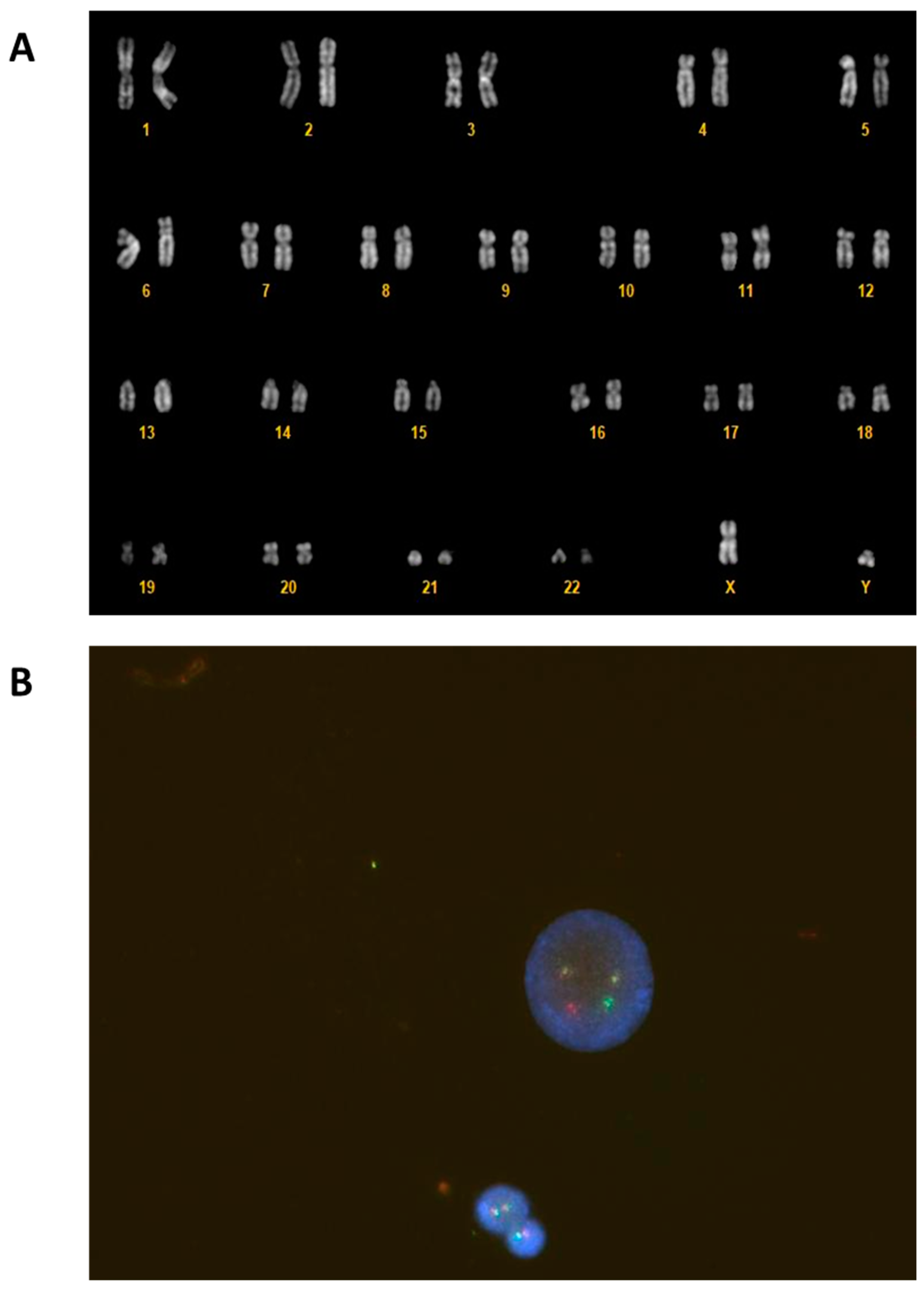
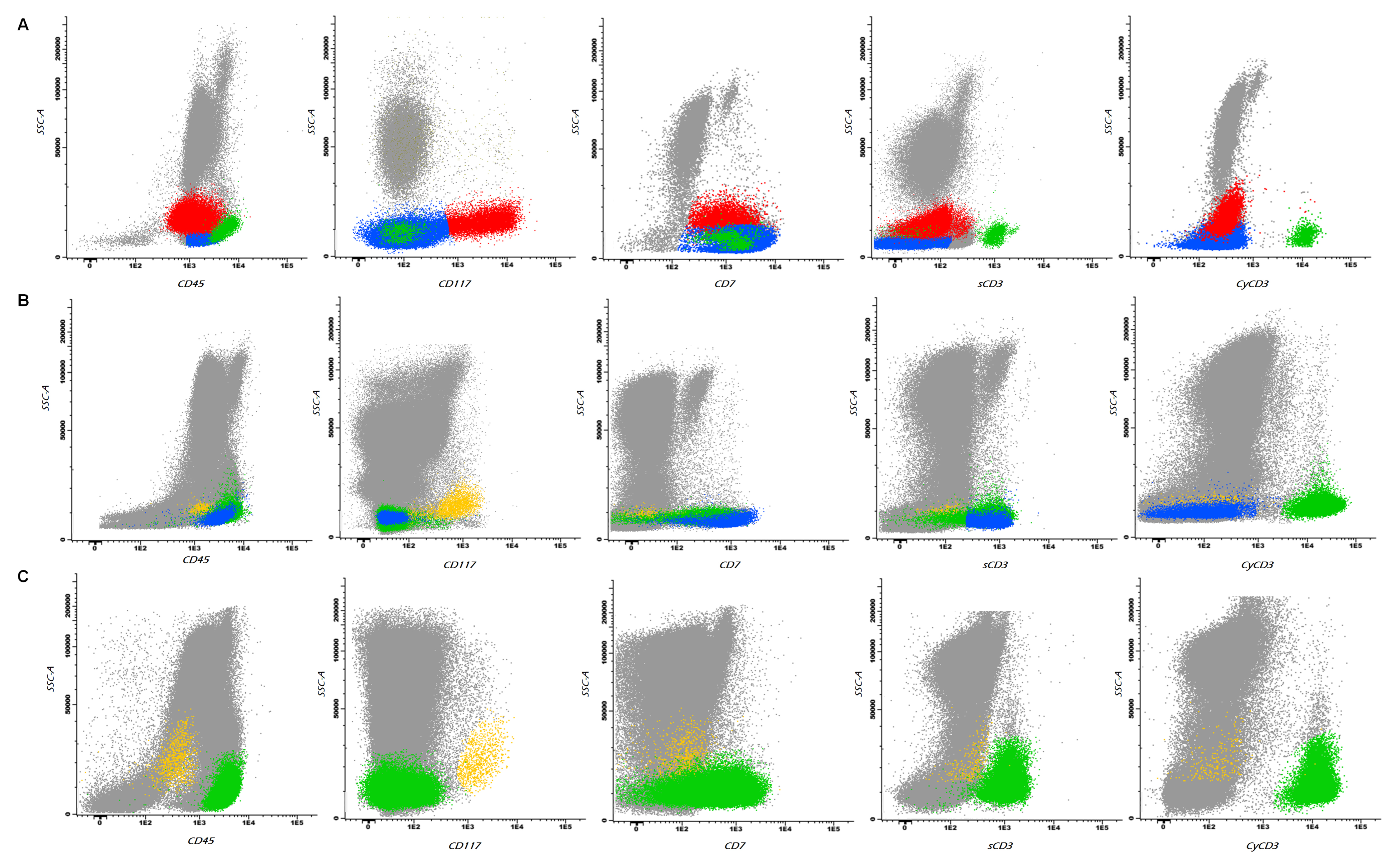
2. Case Report
3. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faderl, S.; Talpaz, M.; Estrov, Z.; O’Brien, S.; Kurzrock, R.; Kantarjian, H.M. The biology of chronic myeloid leukemia. N. Engl. J. Med. 1999, 341, 164–172. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, X.; Yan, Q.; Lin, Y.; Liu, E.; Mi, Y.; Liang, S.; Wang, H.; Xu, J.; Ru, K. The Diagnosis of Chronic Myeloid Leukemia with Deep Adversarial Learning. Am. J. Pathol. 2022, 192, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Perrotti, D.; Jamieson, C.; Goldman, J.; Skorski, T. Chronic myeloid leukemia: Mechanisms of blastic transformation. J. Clin. Investig. 2010, 120, 2254–2264. [Google Scholar] [CrossRef]
- Sampaio, M.M.; Santos, M.L.C.; Marques, H.S.; Gonçalves, V.L.S.; Araújo, G.R.L.; Lopes, L.W.; Apolonio, J.S.; Silva, C.S.; Santos, L.K.S.; Cuzzuol, B.R.; et al. Chronic myeloid leukemia-from the Philadelphia chromosome to specific target drugs: A literature review. World J. Clin. Oncol. 2021, 12, 69–94. [Google Scholar] [CrossRef] [PubMed]
- Tsushima, H.; Iwanaga, M.; Miyazaki, Y. Late effect of atomic bomb radiation on myeloid disorders: Leukemia and myelodysplastic syndromes. Int. J. Hematol. 2012, 95, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Granatowicz, A.; Piatek, C.I.; Moschiano, E.; El-Hemaidi, I.; Armitage, J.D.; Akhtari, M. An overview and update of chronic myeloid leukemia for primary care physicians. Korean J. Fam. Med. 2015, 36, 197–202. [Google Scholar] [CrossRef] [PubMed]
- DeFilipp, Z.; Khoury, H.J. Management of advanced-phase chronic myeloid leukemia. Curr. Hematol. Malig. Rep. 2015, 10, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Bassan, R.; Chiaretti, S.; Della Starza, I.; Spinelli, O.; Santoro, A.; Paoloni, F.; Messina, M.; Elia, L.; De Propris, M.S.; Scattolin, A.M.; et al. Pegaspargase-modified risk-oriented program for adult acute lymphoblastic leukemia: Results of the GIMEMA LAL1913 trial. Blood Adv. 2023, 7, 4448–4461. [Google Scholar] [CrossRef] [PubMed]
- Dorfmann, D.M.; Longtine, J.A.; Fox, E.A.; Weinberg, D.S.; Pinkus, G.S. T-cell blast crisis in chronic myelogenous leukemia. Immunophenotypic and molecular biologic findings. Am. J. Clin. Pathol. 1997, 107, 168–176. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fabbiano, F.; Santoro, A.; Felice, R.; Catania, P.; Cannella, S.; Majolino, I. bcr-abl rearrangement in adult T-lineage acute lymphoblastic leukemia. Haematologica 1998, 83, 856–857. [Google Scholar] [PubMed]
- Jain, P.; Kantarjian, H.; Jabbour, E.; Kanagal-Shamanna, R.; Patel, K.; Pierce, S.; Garcia-Manero, G.; Borthakur, G.; Ravandi, F.; O’Brien, S.; et al. Clinical characteristics of Philadelphia positive T-cell lymphoid leukemias-(De novo and blast phase CML). Am. J. Hematol. 2017, 92, E3–E4. [Google Scholar] [CrossRef] [PubMed]
- Padhi, P.; Topalovski, M.; El Behery, R.; Cantu, E.S.; Medavarapu, R. A Rare Case of Chronic Myelogenous Leukemia Presenting as T-Cell Lymphoblastic Crisis. Case Rep. Oncol. Med. 2018, 2018, 7276128. [Google Scholar] [CrossRef]
- Xu, J.; Li, S. Unusual T-lymphoblastic blast phase of chronic myelogenous leukemia. Case Rep. Hematol. 2014, 2014, 304359. [Google Scholar] [CrossRef]
- Copland, M. Treatment of blast phase chronic myeloid leukaemia: A rare and challenging entity. Br. J. Haematol. 2022, 199, 665–678. [Google Scholar] [CrossRef] [PubMed]

| Months after Transplant | Complete Morphological Remission (CMR) | Multiparametric Flow Cytometry- Measurable Residual Disease (MFC-MRD) | BCR/ABL MR | Chimerism |
|---|---|---|---|---|
| 1 | Yes | Negative | MR4 (0.0037%) | 99.7% |
| 2 | Yes | Negative | MR4.5 (0.0015%) | 99.8% |
| 3 | Yes | Negative | MR4.5 (0.0016%) | 99.9% |
| 6 | Yes | Negative | MR5 | 99.7% |
| 12 | Yes | Negative | Undetected | 99.9% |
| 15 | Yes | Negative | Undetected | 99.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mongia, A.; Romano, F.; Ciullini Mannurita, S.; Peruzzi, B.; Bencini, S.; Parrini, D.; Fasano, L.; Fanelli, A. A Rare Onset of T-Lymphoid Blast Crisis in Chronic Myeloid Leukemia with Two Distinct Blast Populations. Hematol. Rep. 2024, 16, 413-420. https://doi.org/10.3390/hematolrep16030040
Mongia A, Romano F, Ciullini Mannurita S, Peruzzi B, Bencini S, Parrini D, Fasano L, Fanelli A. A Rare Onset of T-Lymphoid Blast Crisis in Chronic Myeloid Leukemia with Two Distinct Blast Populations. Hematology Reports. 2024; 16(3):413-420. https://doi.org/10.3390/hematolrep16030040
Chicago/Turabian StyleMongia, Alessandra, Francesca Romano, Sara Ciullini Mannurita, Benedetta Peruzzi, Sara Bencini, Daniela Parrini, Laura Fasano, and Alessandra Fanelli. 2024. "A Rare Onset of T-Lymphoid Blast Crisis in Chronic Myeloid Leukemia with Two Distinct Blast Populations" Hematology Reports 16, no. 3: 413-420. https://doi.org/10.3390/hematolrep16030040
APA StyleMongia, A., Romano, F., Ciullini Mannurita, S., Peruzzi, B., Bencini, S., Parrini, D., Fasano, L., & Fanelli, A. (2024). A Rare Onset of T-Lymphoid Blast Crisis in Chronic Myeloid Leukemia with Two Distinct Blast Populations. Hematology Reports, 16(3), 413-420. https://doi.org/10.3390/hematolrep16030040






