Humoral and Cell-Mediated Responses to SARS-CoV-2 Vaccination in a Cohort of Immunodeficient Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of Patients
2.2. Sample Processing
3. Results
3.1. Antibody Response: Anti-SARS-CoV-2 IgG Value Evaluated Immediately after the Second Dose and after Six Months and Compared to Healthy Subjects
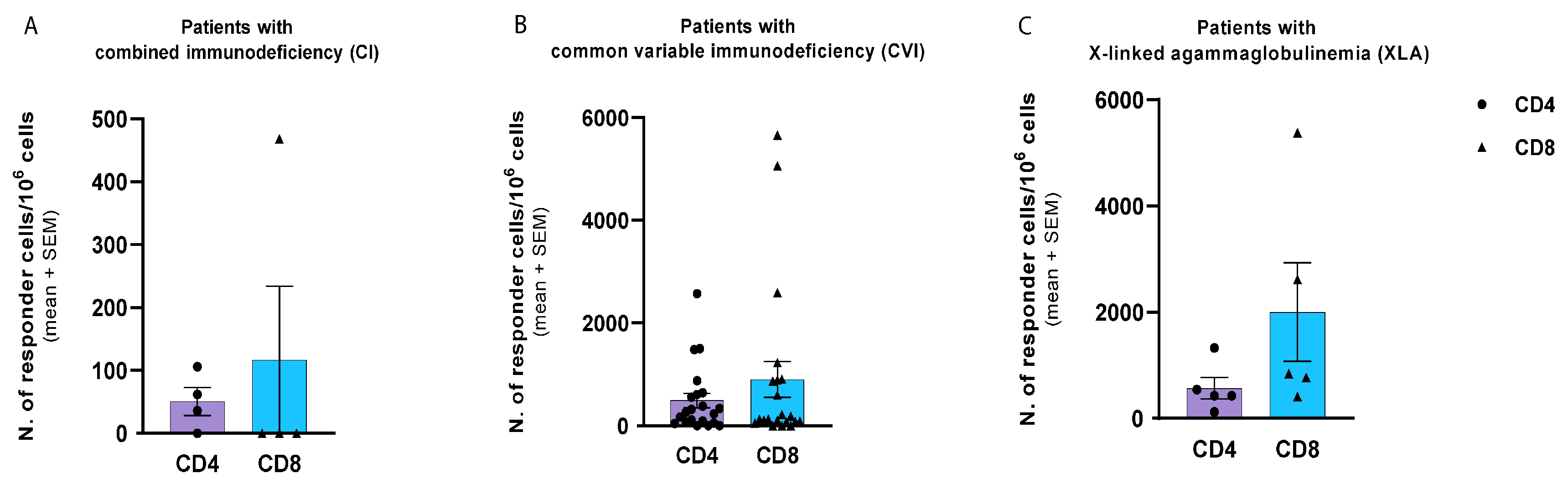
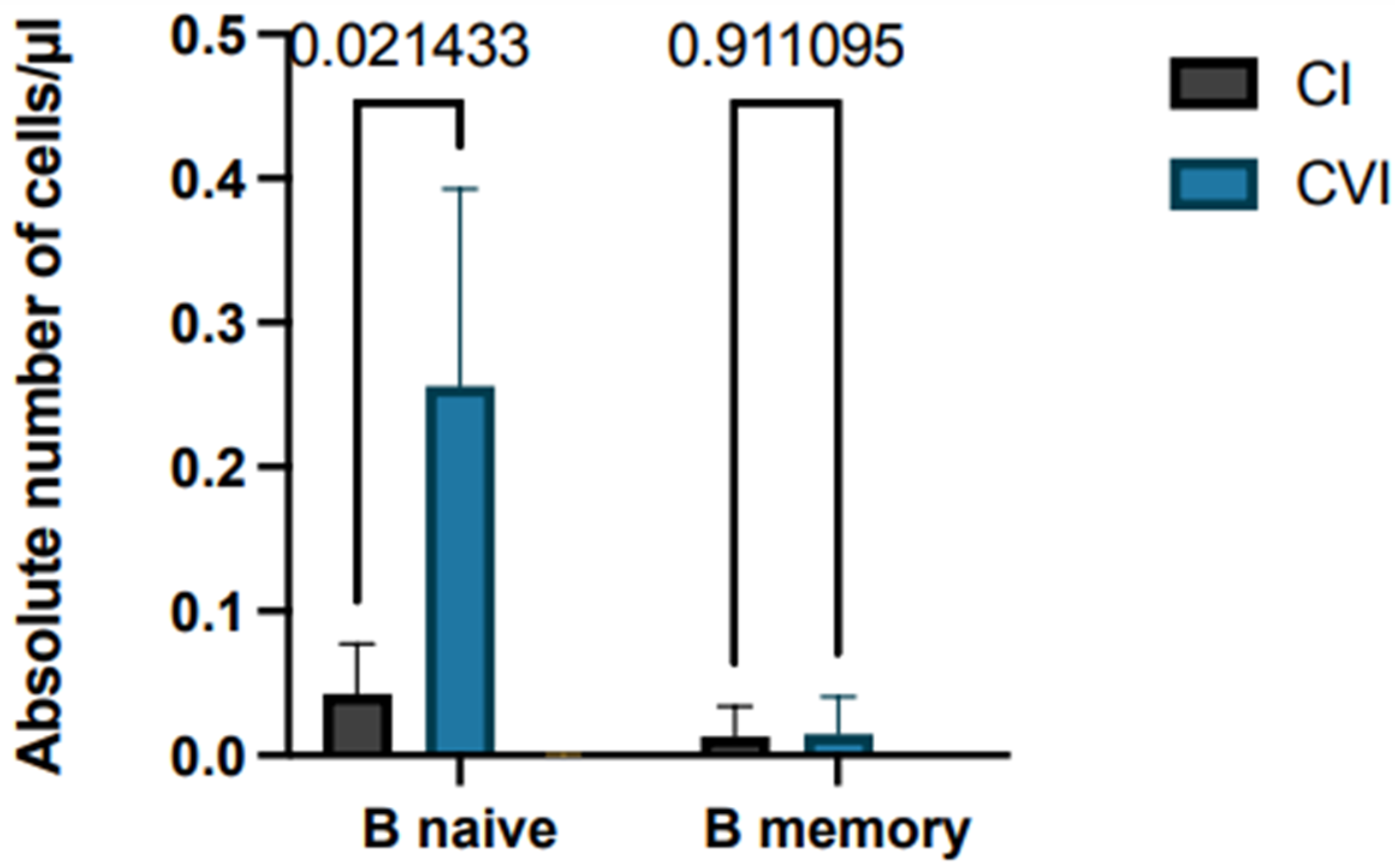
3.2. T-Cell-Mediated Response: Comparison of CD4 and CD8 T Cell Responses and Cytokine Production Assay to SARS-CoV-2 Protein S
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vitiello, A.; Ferrara, F.; Troiano, V.; La Porta, R. COVID-19 vaccines and decreased transmission of SARS-CoV-2. Inflammopharmacology 2021, 29, 1357–1360. [Google Scholar] [CrossRef] [PubMed]
- Shields, A.M.; Burns, S.O.; Savic, S.; Richter, A.G.; Anantharachagan, A.; Arumugakani, G.; Baker, K.; Bahal, S.; Bermingham, W.; Bhole, M.; et al. COVID-19 in patients with primary and secondary immunodeficiency: The United Kingdom experience. J. Allergy Clin. Immunol. 2021, 147, 870–875.e1. [Google Scholar] [CrossRef]
- Schulz, E.; Hodl, I.; Forstner, P.; Hatzl, S.; Sareban, N.; Moritz, M.; Fessler, J.; Dreo, B.; Uhl, B.; Url, C.; et al. CD19+IgD+CD27- Naïve B Cells as Predictors of Humoral Response to COVID-19 mRNA Vaccination in Immunocompromised Patients. Front. Immunol. 2021, 12, 803742. [Google Scholar] [CrossRef]
- Raje, N.; Dinakar, C. Overview of Immunodeficiency Disorders. Immunol. Allergy Clin. N. Am. 2015, 35, 599–623. [Google Scholar] [CrossRef] [PubMed]
- Al-Herz, W.; Bousfiha, A.; Casanova, J.-L.; Chatila, T.; Conley, M.E.; Cunningham-Rundles, C.; Etzioni, A.; Franco, J.L.; Gaspar, H.B.; Holland, S.M.; et al. Primary Immunodeficiency Diseases: An Update on the Classification from the International Union of Immunological Societies Expert Committee for Primary Immunodeficiency. Front. Immunol. 2014, 5, 162. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sanford, E.; Farnaes, L.; Batalov, S.; Bainbridge, M.; Laubach, S.; Worthen, H.M.; Tokita, M.; Kingsmore, S.F.; Bradley, J. Concomitant diagnosis of immune deficiency and Pseudomonas sepsis in a 19 month old with ecthyma gangrenosum by host whole-genome sequencing. Mol. Case Stud. 2018, 4, a003244. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, R.; Habibi, S.; Sharifi, L.; Azizi, G.; Abolhassani, H.; Olbrich, P.; Aghamohammadi, A. Common Variable Immunodeficiency: Epidemiology, Pathogenesis, Clinical Manifestations, Diagnosis, Classification, and Management. J. Investig. Allergol. Clin. Immunol. 2020, 30, 14–34. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Cunningham-Rundles, C. Role of B cells in common variable immune deficiency. Expert Rev. Clin. Immunol. 2009, 5, 557–564. [Google Scholar] [CrossRef]
- Warnatz, K.; Schlesier, M. Flowcytometric phenotyping of common variable immunodeficiency. Cytom. Part B Clin. Cytom. 2008, 74B, 261–271. [Google Scholar] [CrossRef]
- Carsetti, R.; Rosado, M.M.; Donnanno, S.; Guazzi, V.; Soresina, A.; Meini, A.; Plebani, A.; Aiuti, F.; Quinti, I. The loss of IgM memory B cells correlates with clinical disease in common variable immunodeficiency. J. Allergy Clin. Immunol. 2005, 115, 412–417. [Google Scholar] [CrossRef]
- Warnatz, K.; Denz, A.; Dräger, R.; Braun, M.; Groth, C.; Wolff-Vorbeck, G.; Eibel, H.; Schlesier, M.; Peter, H.H. Severe deficiency of switched memory B cells (CD27+IgM−IgD−) in subgroups of patients with common variable immunodeficiency: A new approach to classify a heterogeneous disease. Blood 2002, 99, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Giovannetti, A.; Pierdominici, M.; Mazzetta, F.; Marziali, M.; Renzi, C.; Mileo, A.M.; De Felice, M.; Mora, B.; Esposito, A.; Carello, R.; et al. Unravelling the Complexity of T Cell Abnormalities in Common Variable Immunodeficiency. J. Immunol. 2007, 178, 3932–3943. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.; Roberts, C.A.; Pryce, G.; Kang, A.S.; Marta, M.; Reyes, S.; Schmierer, K.; Giovannoni, G.; Amor, S. COVID-19 vaccine-readiness for anti-CD20-depleting therapy in autoimmune diseases. Clin. Exp. Immunol. 2020, 202, 149–161. [Google Scholar] [CrossRef]
- Aries, J.A.; Davies, J.K.; Auer, R.L.; Hallam, S.L.; Montoto, S.; Smith, M.; Sevillano, B.; Foggo, V.; Wrench, B.; Zegocki, K.; et al. Clinical outcome of coronavirus disease 2019 in haemato-oncology patients. Br. J. Haematol. 2020, 190, e64–e67. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, N.C.; López-Cortés, A.; González, E.V.; Grimaldos, A.B.; Prado, E.O. SARS-CoV-2 vaccines strategies: A comprehensive review of phase 3 candidates. npj Vaccines 2021, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Negahdaripour, M.; Shafiekhani, M.; Moezzi, S.M.I.; Amiri, S.; Rasekh, S.; Bagheri, A.; Mosaddeghi, P.; Vazin, A. Administration of COVID-19 vaccines in immunocompromised patients. Int. Immunopharmacol. 2021, 99, 108021. [Google Scholar] [CrossRef] [PubMed]
- Mrak, D.; Tobudic, S.; Koblischke, M.; Graninger, M.; Radner, H.; Sieghart, D.; Hofer, P.; Perkmann, T.; Haslacher, H.; Thalhammer, R.; et al. SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity. Rheumatology 2021, 80, 1345–1350. [Google Scholar] [CrossRef]
- Prendecki, M.; Clarke, C.; Edwards, H.; McIntyre, S.; Mortimer, P.; Gleeson, S.; Martin, P.; Thomson, T.; Randell, P.; Shah, A.; et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Rheumatology 2021, 80, 1322–1329. [Google Scholar] [CrossRef]
- Quinti, I.; Lougaris, V.; Milito, C.; Cinetto, F.; Pecoraro, A.; Mezzaroma, I.; Mastroianni, C.M.; Turriziani, O.; Bondioni, M.P.; Filippini, M.; et al. A possible role for B cells in COVID-19? Lesson from patients with agammaglobulinemia. J. Allergy Clin. Immunol. 2020, 146, 211–213.e4. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Quadeer, A.A.; McKay, M.R. SARS-CoV-2 T Cell Responses Elicited by COVID-19 Vaccines or Infection Are Expected to Remain Robust against Omicron. Viruses 2022, 14, 79. [Google Scholar] [CrossRef]
- Goldman, J.D.; Robinson, P.C.; Uldrick, T.S.; Ljungman, P. COVID-19 in immunocompromised populations: Implications for prognosis and repurposing of immunotherapies. J. Immunother. Cancer 2021, 9, e002630. [Google Scholar] [CrossRef] [PubMed]
- Malipiero, G.; Moratto, A.; Infantino, M.; D’agaro, P.; Piscianz, E.; Manfredi, M.; Grossi, V.; Benvenuti, E.; Bulgaresi, M.; Benucci, M.; et al. Assessment of humoral and cellular immunity induced by the BNT162b2 SARS-CoV-2 vaccine in healthcare workers, elderly people, and immunosuppressed patients with autoimmune disease. Immunol. Res. 2021, 69, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Shrotri, M.; van Schalkwyk, M.C.I.; Post, N.; Eddy, D.; Huntley, C.; Leeman, D.; Rigby, S.; Williams, S.V.; Bermingham, W.H.; Kellam, P.; et al. T cell response to SARS-CoV-2 infection in humans: A systematic review. PLoS ONE 2021, 16, e0245532. [Google Scholar] [CrossRef] [PubMed]
- Bange, E.M.; Han, N.A.; Wileyto, P.; Kim, J.Y.; Gouma, S.; Robinson, J.; Greenplate, A.R.; Hwee, M.A.; Porterfield, F.; Owoyemi, O.; et al. CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat. Med. 2021, 27, 1280–1289. [Google Scholar] [CrossRef]
- Yin, X.; Chen, S.; Eisenbarth, S.C. Dendritic Cell Regulation of T Helper Cells. Annu. Rev. Immunol. 2021, 39, 759–790. [Google Scholar] [CrossRef]
- Abbott, J.K.; Gelfand, E.W. Common Variable Immunodeficiency. Immunol. Allergy Clin. N. Am. 2015, 35, 637–658. [Google Scholar] [CrossRef]


| Number | Rate (%) | Mean | Range | |
|---|---|---|---|---|
| Total | 48 | 100 | ||
| Age | 29 | 6–65 | ||
| Sex | ||||
| Female | 21 | 44 | ||
| Male | 27 | 56 | ||
| Immunodeficiency | ||||
| Combined immunodeficiency | 4 | 9 | ||
| Common variable immunodeficiency | 39 | 81 | ||
| Agammaglobulinemia | 5 | 10 | ||
| SarsCov2 infection after vaccine | 26 | 54 | ||
| Symptoms during SarsCov2 infection | ||||
| Severe | 0 | 0 | ||
| Moderate | 3 | 12 | ||
| Mild/asymptomatic | 23 | 88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plano, F.; Shekarkar Azgomi, M.; Corsale, A.M.; Spoto, C.; Caccamo, N.; Meraviglia, S.; Dieli, F.; D’Angelo, P.; Trizzino, A.; Siragusa, S. Humoral and Cell-Mediated Responses to SARS-CoV-2 Vaccination in a Cohort of Immunodeficient Patients. Hematol. Rep. 2023, 15, 707-716. https://doi.org/10.3390/hematolrep15040071
Plano F, Shekarkar Azgomi M, Corsale AM, Spoto C, Caccamo N, Meraviglia S, Dieli F, D’Angelo P, Trizzino A, Siragusa S. Humoral and Cell-Mediated Responses to SARS-CoV-2 Vaccination in a Cohort of Immunodeficient Patients. Hematology Reports. 2023; 15(4):707-716. https://doi.org/10.3390/hematolrep15040071
Chicago/Turabian StylePlano, Federica, Mojtaba Shekarkar Azgomi, Anna Maria Corsale, Corinne Spoto, Nadia Caccamo, Serena Meraviglia, Francesco Dieli, Paolo D’Angelo, Antonino Trizzino, and Sergio Siragusa. 2023. "Humoral and Cell-Mediated Responses to SARS-CoV-2 Vaccination in a Cohort of Immunodeficient Patients" Hematology Reports 15, no. 4: 707-716. https://doi.org/10.3390/hematolrep15040071
APA StylePlano, F., Shekarkar Azgomi, M., Corsale, A. M., Spoto, C., Caccamo, N., Meraviglia, S., Dieli, F., D’Angelo, P., Trizzino, A., & Siragusa, S. (2023). Humoral and Cell-Mediated Responses to SARS-CoV-2 Vaccination in a Cohort of Immunodeficient Patients. Hematology Reports, 15(4), 707-716. https://doi.org/10.3390/hematolrep15040071








