Effects of Sirolimus Treatment on Fetal Hemoglobin Production and Response to SARS-CoV-2 Vaccination: A Case Report Study
Abstract
1. Introduction
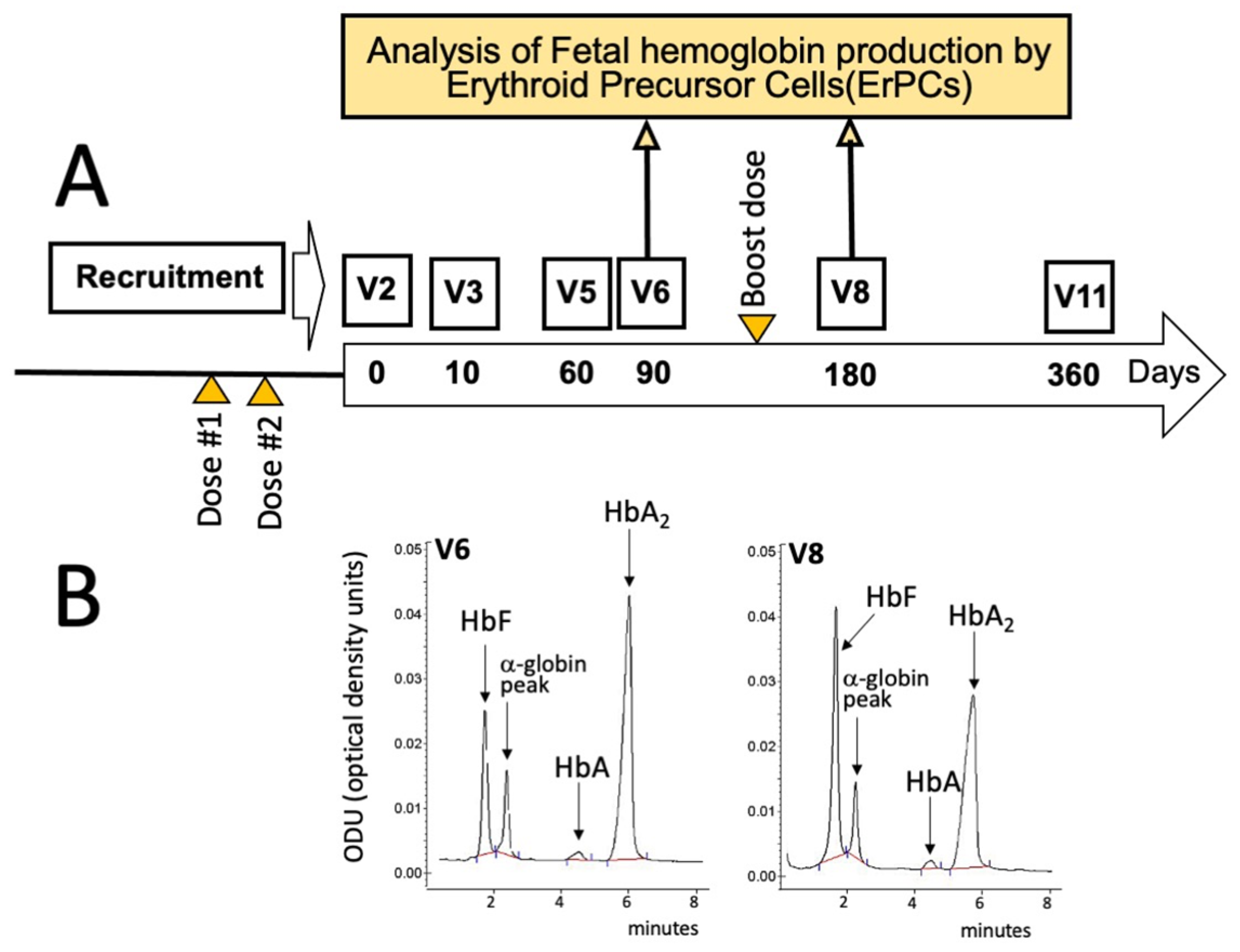
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weatherall, D.J. Phenotype-genotype relationships in monogenic disease: Lessons from the thalassaemias. Nat. Rev. Genet. 2001, 2, 245–255. [Google Scholar] [CrossRef]
- Galanello, R.; Origa, R. Β-thalassemia. Orphanet J. Rare Dis. 2010, 5, 11. [Google Scholar] [CrossRef]
- Origa, R. β-Thalassemia. Genet. Med. 2017, 19, 609–619. [Google Scholar] [CrossRef]
- Sripichai, O.; Fucharoen, S. Fetal hemoglobin regulation in β-thalassemia: Heterogeneity, modifiers and therapeutic approaches. Expert. Rev. Hematol. 2016, 9, 1129–1137. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, X.; Yu, L.; Cai, R.; Ma, X.; Zheng, C.; Zhou, Y.; Liu, Q.; Wei, X.; Lin, L.; et al. KLF1 mutations are relatively more common in a thalassemia endemic region and ameliorate the severity of β-thalassemia. Blood 2014, 124, 803–811. [Google Scholar] [CrossRef]
- Musallam, K.M.; Sankaran, V.G.; Cappellini, M.D.; Duca, L.; Nathan, D.G.; Taher, A.T. Fetal hemoglobin levels and morbidity in untransfused patients with β-thalassemia intermedia. Blood 2012, 119, 364–367. [Google Scholar] [CrossRef]
- Nuinoon, M.; Makarasara, W.; Mushiroda, T.; Setianingsih, I.; Wahidiyat, P.A.; Sripichai, O.; Kumasaka, N.; Takahashi, A.; Svasti, S.; Munkongdee, T.; et al. A genome-wide association identified the common genetic variants influence disease severity in β0-thalassemia/hemoglobin E. Hum. Genet. 2010, 127, 303–314. [Google Scholar] [CrossRef]
- Prosdocimi, M.; Zuccato, C.; Cosenza, L.C.; Borgatti, M.; Lampronti, I.; Finotti, A.; Gambari, R. A Rational Approach to Drug Repositioning in β-thalassemia: Induction of Fetal Hemoglobin by Established Drugs. Wellcome Open Res. 2022, 7, 150. [Google Scholar] [CrossRef]
- Sehgal, S.N. Sirolimus: Its discovery, biological properties, and mechanism of action. Transplant. Proc. 2003, 35 (Suppl. 3), 7S–14S. [Google Scholar] [CrossRef]
- Kahan, B.D. Sirolimus: A new agent for clinical renal transplantation. Transplant. Proc. 1997, 29, 48–50. [Google Scholar] [CrossRef]
- Mao, B.; Zhang, Q.; Ma, L.; Zhao, D.S.; Zhao, P.; Yan, P. Overview of Research into mTOR Inhibitors. Molecules 2022, 27, 5295. [Google Scholar] [CrossRef]
- Fibach, E.; Bianchi, N.; Borgatti, M.; Zuccato, C.; Finotti, A.; Lampronti, I.; Prus, E.; Mischiati, C.; Gambari, R. Effects of rapamycin on accumulation of alpha-, β- and gamma-globin mRNAs in erythroid precursor cells from β-thalassaemia patients. Eur. J. Haematol. 2006, 77, 437–441. [Google Scholar] [CrossRef]
- Pecoraro, A.; Troia, A.; Calzolari, R.; Scazzone, C.; Rigano, P.; Martorana, A.; Sacco, M.; Maggio, A.; Marzo, R.D. Efficacy of Rapamycin as Inducer of Hb F in Primary Erythroid Cultures from Sickle Cell Disease and β-Thalassemia Patients. Hemoglobin 2015, 39, 225–229. [Google Scholar] [CrossRef]
- Khaibullina, A.; Almeida, L.E.; Wang, L.; Kamimura, S.; Wong, E.C.; Nouraie, M.; Maric, I.; Albani, S.; Finkel, J.; Quezado, Z.M.N. Rapamycin increases fetal hemoglobin and ameliorates the nociception phenotype in sickle cell mice. Blood Cells Mol. Dis. 2015, 55, 363–372. [Google Scholar] [CrossRef]
- Wang, J.; Tran, J.; Wang, H.; Guo, C.; Harro, D.; Campbell, A.D.; Eitzman, D.T. mTOR Inhibition improves anaemia and reduces organ damage in a murine model of sickle cell disease. Br. J. Haematol. 2016, 174, 461–469. [Google Scholar] [CrossRef]
- Lechauve, C.; Keith, J.; Khandros, E.; Fowler, S.; Mayberry, K.; Freiwan, A.; Thom, C.S.; Delbini, P.; Romero, E.B.; Zhang, J.; et al. The autophagy-activating kinase ULK1 mediates clearance of free α-globin in β-thalassemia. Sci. Transl. Med. 2019, 11, eaav4881. [Google Scholar] [CrossRef]
- Gaudre, N.; Cougoul, P.; Bartolucci, P.; Dörr, G.; Bura-Riviere, A.; Kamar, N.; Del Bello, A. Improved Fetal Hemoglobin With mTOR Inhibitor-Based Immunosuppression in a Kidney Transplant Recipient with Sickle Cell Disease. Am. J. Transplant. 2017, 17, 2212–2214. [Google Scholar] [CrossRef]
- Al-Khatti, A.A.; Alkhunaizi, A.M. Additive effect of sirolimus and hydroxycarbamide on fetal haemoglobin level in kidney transplant patients with sickle cell disease. Br. J. Haematol. 2019, 185, 959–961. [Google Scholar] [CrossRef]
- Zuccato, C.; Cosenza, L.C.; Zurlo, M.; Gasparello, J.; Papi, C.; D’Aversa, E.; Breveglieri, G.; Lampronti, I.; Finotti, A.; Borgatti, M.; et al. Expression of γ-globin genes in β-thalassemia patients treated with sirolimus: Results from a pilot clinical trial (Sirthalaclin). Ther. Adv. Hematol. 2022, 13, 20406207221100648. [Google Scholar] [CrossRef]
- Sorrenti, V.; Benedetti, F.; Buriani, A.; Fortinguerra, S.; Caudullo, G.; Davinelli, S.; Zella, D.; Scapagnini, G. Immunomodulatory and Antiaging Mechanisms of Resveratrol, Rapamycin, and Metformin: Focus on mTOR and AMPK Signaling Networks. Pharmaceuticals 2022, 15, 912. [Google Scholar] [CrossRef]
- Jia, S.; Li, Y.; Fang, T. System dynamics analysis of COVID-19 prevention and control strategies. Environ. Sci. Pollut. Res. Int. 2022, 29, 3944–3957. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Ożarowski, M.; Seremak-Mrozikiewicz, A.; Wolski, H.; Wlodkowic, D. The 2020 race towards SARS-CoV-2 specific vaccines. Theranostics 2021, 11, 1690–1702. [Google Scholar] [CrossRef]
- Netti, G.S.; Infante, B.; Troise, D.; Mercuri, S.; Panico, M.; Spadaccino, F.; Catalano, V.; Gigante, M.; Simone, S.; Pontrelli, P.; et al. mTOR inhibitors improve both humoral and cellular response to SARS-CoV-2 messenger RNA BNT16b2 vaccine in kidney transplant recipients. Am. J. Transplant. 2022, 22, 1475–1482. [Google Scholar] [CrossRef]
- Tunbridge, M.; Perkins, G.B.; Singer, J.; Salehi, T.; Ying, T.; Grubor-Bauk, B.; Barry, S.; Sim, B.; Hissaria, P.; Chadban, S.J.; et al. Rapamycin and inulin for booster vaccine response stimulation (RIVASTIM)-rapamycin: Study protocol for a randomised, controlled trial of immunosuppression modification with rapamycin to improve SARS-CoV-2 vaccine response in kidney transplant recipients. Trials 2022, 23, 780. [Google Scholar] [CrossRef]
- Zurlo, M.; Nicoli, F.; Borgatti, M.; Finotti, A.; Gambari, R. Possible effects of sirolimus treatment on the long-term efficacy of COVID-19 vaccination in patients with β-thalassemia: A theoretical perspective. Int. J. Mol. Med. 2022, 49, 33. [Google Scholar] [CrossRef]
- Vasquez, E.M. Sirolimus: A new agent for prevention of renal allograft rejection. Am. J. Health Syst. Pharm. 2000, 57, 437–448. [Google Scholar] [CrossRef]
- Schaffer, S.A.; Ross, H.J. Everolimus: Efficacy and safety in cardiac transplantation. Expert. Opin. Drug Saf. 2010, 9, 843–854. [Google Scholar] [CrossRef]
- Tang, C.Y.; Shen, A.; Wei, X.F.; Li, Q.D.; Liu, R.; Deng, H.J.; Wu, Y.Z.; Wu, Z.J. Everolimus in de novo liver transplant recipients: A systematic review. Hepatobiliary Pancreat. Dis. Int. 2015, 14, 461–469. [Google Scholar] [CrossRef]
- Ji, L.; Xie, W.; Zhang, Z. Efficacy and safety of sirolimus in patients with systemic lupus erythematosus: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2020, 50, 1073–1080. [Google Scholar] [CrossRef]
- Li, H.; Ji, J.; Du, Y.; Huang, Y.; Gu, H.; Chen, M.; Wu, R.; Han, B. Sirolimus is effective for primary relapsed/refractory autoimmune cytopenia: A multicenter study. Exp. Hematol. 2020, 89, 87–95. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, M.; Xiang, B.; Chen, S.; Ji, Y. The efficacy and safety of pharmacological treatments for lymphangioleiomyomatosis. Respir. Res. 2020, 21, 55. [Google Scholar] [CrossRef]
- Gamberini, M.R.; Prosdocimi, M.; Gambari, R. Sirolimus for Treatment of β-Thalassemia: From Pre-Clinical Studies to the Design of Clinical Trials. Health Educ. 2021, 4, 425–435. [Google Scholar]
- Kaeberlein, T.L.; Green, A.S.; Haddad, G.; Hudson, J.; Isman, A.; Nyquist, A.; Rosen, B.S.; Suh, Y.; Zalzala, S.; Zhang, X.; et al. Evaluation of off-label rapamycin use to promote healthspan in 333 adults. Geroscience 2023, 1–12. [Google Scholar] [CrossRef]
- Mazzola, A.; Todesco, E.; Drouin, S.; Hazan, F.; Marot, S.; Thabut, D.; Varnous, S.; Soulié, C.; Barrou, B.; Marcelin, A.G.; et al. Poor Antibody Response After Two Doses of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine in Transplant Recipients. Clin. Infect. Dis. 2022, 74, 1093–1096. [Google Scholar] [CrossRef]
- Lee, W.C.; Hung, H.C.; Lee, J.C.; Huang, C.G.; Huang, P.W.; Gu, P.W.; Wang, Y.C.; Cheng, C.H.; Wu, T.H.; Lee, C.F.; et al. Adjustment of Immunosuppressants to Facilitate Anti-COVID-19 Antibody Production after mRNA Vaccination in Liver Transplant Recipients. Viruses 2023, 15, 678. [Google Scholar] [CrossRef]
- Rahbar, M.; Kazemi, R.; Salehi, H.; Ghasemi, P.; Naghizageh, M.; Dehghani, S.; Gholamnejad, M.; Pishkuhi, M.A.; Aghamir, S.M.K. Evaluation of SARS-CoV-2 Serum Level in Patients Vaccinated with Sinopharm/BBIBP-CorV With Kidney Transplantation. Transplant. Proc. 2022, 54, 2663–2667. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning Immunity After the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Tang, P.; Hasan, M.R.; AlMukdad, S.; Yassine, H.M.; Benslimane, F.M.; Khatib, H.A.A.; Coyle, P.; Ayoub, H.H.; Kanaani, Z.A.; et al. Waning of BNT162b2 Vaccine Protection Against SARS-CoV-2 Infection in Qatar. N. Engl. J. Med. 2021, 385, e83. [Google Scholar] [CrossRef]
- Rosenberg, E.S.; Dorabawila, V.; Easton, D.; Bauer, U.E.; Kumar, J.; Hoen, R.; Hoefer, D.; Wu, M.; Lutterloh, E.; Conroy, M.B.; et al. COVID-19 Vaccine Effectiveness in New York State. N. Engl. J. Med. 2021, 386, 116–127. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, Y.; Hu, D.; Zhou, W.; Liu, C.; Tian, X.; Zhang, H.; Xu, Y.-C.; Xu, K.-F. Humoral response to inactivated SARS-CoV-2 vaccines in patients on sirolimus alone. Sci. China Life Sci. 2022, 65, 2118–2120. [Google Scholar] [CrossRef]
- Banjongjit, A.; Phirom, S.; Phannajit, J.; Jantarabenjakul, W.; Paitoonpong, L.; Kittanamongkolchai, W.; Wattanatorn, S.; Prasithsirikul, W.; Eiam-Ong, S.; Avihingsanon, Y.; et al. Benefits of Switching Mycophenolic Acid to Sirolimus on Serological Response after a SARS-CoV-2 Booster Dose among Kidney Transplant Recipients: A Pilot Study. Vaccines 2022, 10, 1685. [Google Scholar] [CrossRef] [PubMed]
- De Boer, S.E.; Berger, S.P.; van Leer-Buter, C.C.; Kroesen, B.J.; van Baarle, D.; Sanders, J.F.; OPTIMIZE Study Group. Enhanced Humoral Immune Response After COVID-19 Vaccination in Elderly Kidney Transplant Recipients on Everolimus Versus Mycophenolate Mofetil-containing Immunosuppressive Regimens. Transplantation 2022, 106, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Delaporta, P.; Terpos, E.; Solomou, E.E.; Gumeni, S.; Nitsa, E.; Apostolakou, F.; Kyriakopoulou, D.; Ntanasis-Stathopoulos, I.; Papassotiriou, I.; Trougakos, I.P.; et al. Immune response and adverse events after vaccination against SARS-CoV-2 in adult patients with transfusion-dependent thalassaemia. Br. J. Haematol. 2022, 197, 576–579. [Google Scholar] [CrossRef]
- Carsetti, R.; Agrati, C.; Pinto, V.M.; Gianesin, B.; Gamberini, R.; Fortini, M.; Barella, S.; Denotti, R.; Perrotta, S.; Casale, M.; et al. Premature aging of the immune system affects the response to SARS-CoV-2 mRNA vaccine in β-thalassemia: Role of an additional dose. Blood 2022, 140, 1735–1738. [Google Scholar] [CrossRef] [PubMed]
- Anastasi, E.; Marziali, M.; Preziosi, A.; Berardelli, E.; Losardo, A.A.; Ribersani, R.; Pugliese, P.; Farina, A.; Mancini, P.; Angeloni, A. Humoral immune response to Comirnaty (BNT162b2) SARS-Cov2 mRNA vaccine in Thalassemia Major patients. Microbes Infect. 2022, 24, 104976. [Google Scholar] [CrossRef]
- Zurlo, M.; Nicoli, F.; Proietto, D.; Dallan, B.; Zuccato, C.; Cosenza, L.C.; Gasparello, J.; Papi, C.; d’Aversa, E.; Borgatti, M.; et al. Effects of Sirolimus treatment on patients with β-Thalassemia: Lymphocyte immunophenotype and biological activity of memory CD4+ and CD8+ T cells. J. Cell. Mol. Med. 2023, 27, 353–364. [Google Scholar] [CrossRef]

| Clinical parameters | Comments/ongoing therapies at the time of recruitment to the NCT04247750 trial |
| A. General parameters | |
| Genotype | Homozygous for the β039-Thalassemia mutation XmnI polymorphism: -/- |
| Start of regular transfusion therapy | 12 December 1983; age 2.8 years |
| Transfusion regime | In 2020, 38 units of red blood cells were infused. Mean pre-transfusional Hb: 9.4 g/dL; iron intake: 0.33 mg/kg/die |
| Start of regular chelation therapy | 1 January 1984; age 2.9 years |
| Chelation therapy | Various schemes were used, including chelating agents in monotherapy or in combination. Since 4 February 2021, alternate combination therapy with desferrioxamine sc (28 mg/kg 3/7) and deferasirox FC per os (20.2 mg/kg 4/7) is ongoing |
| Iron overload | Severe hepatic and cardiac accumulation was found in 2008 by RM-T2; progressive improvement up to normalization of the deposits was obtained on 6 June 2021 (MRI-T2: cardiac T2 40 ms, LIC 2.19 mg/g liver dry tissue) Serum ferritin: high mean annual values (>2000 ng/mL) from 2008 to 2011; <500 ng/mL from 2019; on 16 March 2021: ferritin 428 ng/mL |
| Splenectomy | 15 March 1996; age 15 years |
| Adenotonsillectomy | 15 September 2008; age 17 years |
| B. Clinical complications | |
| Allergic Chronic Asthma (Since pediatric age; allergy developed against alternaria and grasses) | Beclomethasone 200 mcg plus formoterol 2 mcg (Foster®): 2 inhalations per day |
| Piastrinosis (1996, after splenectomy) | Lysine acetylsalicylate (cardirene®)300 mg/day |
| Osteoporosis (2003) | Aledronic acid (dralenos®) 70 mg/week |
| Postpuberal hypogonatropic hypogonadism (2006) | Testosterone gel (tostrex®) 40 mg/day (4 pumps in a single dose) |
| Dilated cardiopathy with ventricular dysfunction secondary to cardiac siderosis (2006) | Bisoprolol 1.25 mg/day, losartan 50 mg plus hydrochlorothiazide 12.5 mg/day |
| Vitamin D deficiency (2012) | Cholecalciferol (dibase®) 1250 IU/day |
| Growth Hormone deficiency (2014) | Somatotropin (humatrope®) 6 mg/day, 6 days/week |
| Hyper calciuria (2016) | / |
| Paravertebral ectopic erythropoyesis mass (2017; diameter 2 cm, stable at follow-up) | / |
| SARS-CoV-2 infection | Never infected |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamberini, M.R.; Zuccato, C.; Zurlo, M.; Cosenza, L.C.; Finotti, A.; Gambari, R. Effects of Sirolimus Treatment on Fetal Hemoglobin Production and Response to SARS-CoV-2 Vaccination: A Case Report Study. Hematol. Rep. 2023, 15, 432-439. https://doi.org/10.3390/hematolrep15030044
Gamberini MR, Zuccato C, Zurlo M, Cosenza LC, Finotti A, Gambari R. Effects of Sirolimus Treatment on Fetal Hemoglobin Production and Response to SARS-CoV-2 Vaccination: A Case Report Study. Hematology Reports. 2023; 15(3):432-439. https://doi.org/10.3390/hematolrep15030044
Chicago/Turabian StyleGamberini, Maria Rita, Cristina Zuccato, Matteo Zurlo, Lucia Carmela Cosenza, Alessia Finotti, and Roberto Gambari. 2023. "Effects of Sirolimus Treatment on Fetal Hemoglobin Production and Response to SARS-CoV-2 Vaccination: A Case Report Study" Hematology Reports 15, no. 3: 432-439. https://doi.org/10.3390/hematolrep15030044
APA StyleGamberini, M. R., Zuccato, C., Zurlo, M., Cosenza, L. C., Finotti, A., & Gambari, R. (2023). Effects of Sirolimus Treatment on Fetal Hemoglobin Production and Response to SARS-CoV-2 Vaccination: A Case Report Study. Hematology Reports, 15(3), 432-439. https://doi.org/10.3390/hematolrep15030044







