Risk Factors for Death or Cardiovascular Events after Acute Coronary Syndrome in Patients with Myeloproliferative Neoplasms
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Outcomes and Statistical Analysis
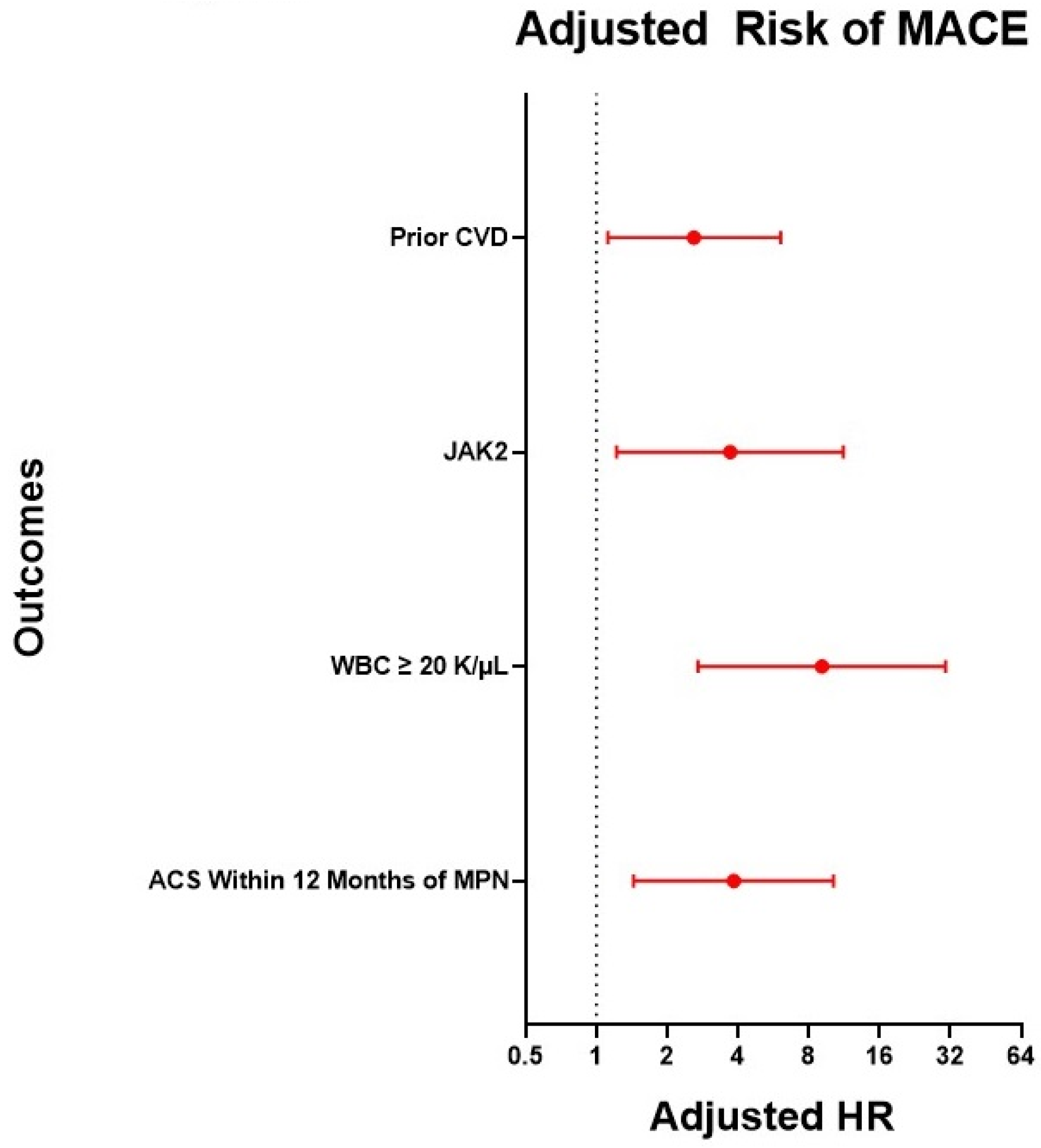
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leiva, O.; Hobbs, G.; Ravid, K.; Libby, P. Cardiovascular Disease in Myeloproliferative Neoplasms. JACC CardioOncol. 2022, 4, 166–182. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Natarajan, P.; Silver, A.J.; Gibson, C.J.; Bick, A.G.; Shvartz, E.; McConkey, M.; Gupta, N.; Gabriel, S.; Ardissino, D.; et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Sano, S.; Wang, Y.; Yura, Y.; Sano, M.; Oshima, K.; Yang, Y.; Katanasaka, Y.; Min, K.D.; Matsuura, S.; Ravid, K.; et al. JAK2 (V617F) -Mediated Clonal Hematopoiesis Accelerates Pathological Remodeling in Murine Heart Failure. JACC Basic Transl. Sci. 2019, 4, 684–697. [Google Scholar] [CrossRef] [PubMed]
- Carobbio, A.; Thiele, J.; Passamonti, F.; Rumi, E.; Ruggeri, M.; Rodeghiero, F.; Randi, M.L.; Bertozzi, I.; Vannucchi, A.M.; Antonioli, E.; et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: An international study of 891 patients. Blood 2011, 117, 5857–5859. [Google Scholar] [CrossRef] [PubMed]
- Cerquozzi, S.; Barraco, D.; Lasho, T.; Finke, C.; Hanson, C.A.; Ketterling, R.P.; Pardanani, A.; Gangat, N.; Tefferi, A. Risk factors for arterial versus venous thrombosis in polycythemia vera: A single center experience in 587 patients. Blood Cancer J. 2017, 7, 662. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology; American College of Cardiology; American Heart Association; et al. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef] [PubMed]
- Hultcrantz, M.; Bjorkholm, M.; Dickman, P.W.; Landgren, O.; Derolf, A.R.; Kristinsson, S.Y.; Andersson, T.M.L. Risk for Arterial and Venous Thrombosis in Patients with Myeloproliferative Neoplasms: A Population-Based Cohort Study. Ann. Intern. Med. 2018, 168, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.J.; Ardissino, D.; De Servi, S.; Murphy, S.A.; et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef] [PubMed]
- Montalescot, G.; Dallongeville, J.; Van Belle, E.; Rouanet, S.; Baulac, C.; Degrandsart, A.; Vicaut, E. STEMI and NSTEMI: Are they so different? 1 year outcomes in acute myocardial infarction as defined by the ESC/ACC definition (the OPERA registry). Eur. Heart J. 2007, 28, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Pendyala, L.K.; Torguson, R.; Loh, J.P.; Kitabata, H.; Minha, S.; Badr, S.; Dvir, D.; Barbash, I.M.; Satler, L.F.; Pichard, A.D.; et al. Comparison of adverse outcomes after contemporary percutaneous coronary intervention in women versus men with acute coronary syndrome. Am. J. Cardiol. 2013, 111, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, M.; Morrow, D.A.; Cannon, C.P.; Guo, W.; Murphy, S.A.; Gibson, C.M.; Sabatine, M.S. Association between baseline neutrophil count, clopidogrel therapy, and clinical and angiographic outcomes in patients with ST-elevation myocardial infarction receiving fibrinolytic therapy. Eur. Heart J. 2008, 29, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Kyne, L.; Hausdorff, J.M.; Knight, E.; Dukas, L.; Azhar, G.; Wei, J.Y. Neutrophilia and congestive heart failure after acute myocardial infarction. Am. Heart J. 2000, 139 Pt 1, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Lessard, D.; Yarzebski, J.; Furman, M.I.; Gore, J.M.; Goldberg, R.J. Leukocytosis and adverse hospital outcomes after acute myocardial infarction. Am. J. Cardiol. 2003, 92, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Carobbio, A.; Ferrari, A.; Masciulli, A.; Ghirardi, A.; Barosi, G.; Barbui, T. Leukocytosis and thrombosis in essential thrombocythemia and polycythemia vera: A systematic review and meta-analysis. Blood Adv. 2019, 3, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e4–e17. [Google Scholar] [CrossRef] [PubMed]
- Zwicker, J.I.; Paranagama, D.; Lessen, D.S.; Colucci, P.M.; Grunwald, M.R. Hemorrhage in patients with polycythemia vera receiving aspirin with an anticoagulant: A prospective, observational study. Haematologica 2021, 107, 1106. [Google Scholar] [CrossRef] [PubMed]
- Gangat, N.; Caramazza, D.; Vaidya, R.; George, G.; Begna, K.; Schwager, S.; Van Dyke, D.; Hanson, C.; Wu, W.; Pardanani, A.; et al. DIPSS plus: A refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J. Clin. Oncol. 2011, 29, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Rumi, E.; Finazzi, G.; Gisslinger, H.; Vannucchi, A.M.; Rodeghiero, F.; Randi, M.L.; Vaidya, R.; Cazzola, M.; Rambaldi, A.; et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: An international study. Leukemia 2013, 27, 1874–1881. [Google Scholar] [CrossRef] [PubMed]
| AllN = 41 | Death or CV EventN = 31 | No Death or CV EventN = 10 | p Value | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age at index, median (IQR) | 74 (67, 84) | 74 (64, 83) | 74 (68, 90) | 0.27 |
| Median time from MPN to index, months (IQR) | 47 (12, 84) | 32 (8, 75) | 82 (55, 107) | 0.029 |
| MedianfFollow-up, months (IQR) | 80 (27, 98) | 80 (27, 100) | 67 (13, 93) | 0.68 |
| BMI (IQR) | 23 (19, 26) | 23 (19, 27) | 23 (20, 26) | 0.22 |
| Male, N (%) | 26 (63) | 21 (68) | 5 (50) | 0.26 |
| MPN characteristics | ||||
| Age at MPN, median (IQR) | 68 (63, 79) | 71 (63, 79) | 68 (63, 81) | 0.95 |
| Type of MPN, N (%) | ||||
| PV | 15 (37) | 12 (39) | 3 (30) | 0.70 |
| ET | 19 (46) | 13 (42) | 6 (60) | |
| MF | 7 (17) | 6 (19) | 1 (10) | |
| JAK2V617F Positive, N (%) | 30 (73) | 26 (84) | 4 (40) | 0.013 |
| MPL Positive, N (%) | 1 (2.4) | 1 (3.2) | 0 | 1.00 |
| CALR Positive, N (%) | 3 (7.3) | 2 (6.4) | 1 (10.0) | 1.00 |
| Treatment for MPN, N (%) | 38 (93) | 29 (94) | 9 (90) | 1.00 |
| Hydroxyurea | 30 (73) | 22 (71) | 8 (80) | 0.70 |
| Ruxolitinib | 7 (17) | 3 (16) | 2 (20) | 1.00 |
| Phlebotomy | 13 (31.7) | 2 (20.0) | 11 (35.5) | 0.46 |
| Anagrelide | 4 (9.8) | 2 (20.0) | 2 (6.4) | 0.24 |
| Co-Morbidities, N (%) | ||||
| Prior CVD a | 24 (59) | 17 (55) | 7 (70) | 0.48 |
| CAD | 12 (29) | 9 (29) | 3 (30) | 1.00 |
| Prior bleed | 10 (24) | 7 (29) | 3 (18) | 0.48 |
| DM | 5 (12) | 4 (13) | 1 (10) | 1.00 |
| HTN | 31 (76) | 24 (77) | 7 (70) | 0.68 |
| Prior stroke/TIA | 8 (20) | 8 (26) | 0 | 0.16 |
| Atrial fibrillation | 12 (29) | 8 (26) | 4 (40) | 0.44 |
| HF | 15 (37) | 13 (42) | 2 (20) | 0.28 |
| Never smoking | 13 (32) | 11 (35) | 2 (20) | 0.46 |
| Medications 1 year post-index, N (%) | ||||
| Aspirin | 37 (90) | 28 (90) | 9 (90) | 1.00 |
| P2Y12 inhibitor | 19 (46) | 15 (48) | 4 (40) | 0.73 |
| DAPT | 19 (46) | 15 (48) | 4 (40) | 0.73 |
| Anticoagulation | 12 (29) | 10 (32) | 2 (20) | 0.69 |
| Statin | 30 (73) | 25 (81) | 5 (50) | 0.098 |
| Labs at index | ||||
| WBC (K/µL), median (IQR) | 11 (8, 20) | 14 (9, 25) | 8 (8, 11) | 0.008 |
| Hematocrit (%), median (IQR) | 39 (32, 42) | 41 (32, 43) | 38 (32, 41) | 0.53 |
| Platelets (K/µL), median (IQR) | 429 (311, 608) | 429 (266, 708) | 420 (347, 607) | 0.86 |
| Spleen size (cm), median (IQR) | 13 (11, 15) | 14 (12, 16) | 11 (8, 14) | 0.065 |
| Type of event | ||||
| STE-ACS | 2 (5) | 2 (6) | 0 | 1.00 |
| NSTE-ACS | 39 (95) | 29 (94) | 10 (100) | 1.00 |
| LVEF at index, median (IQR) | 58 (43, 68) | 55 (47, 70) | 60 (45, 68) | 0.98 |
| Treatment, N (%) | ||||
| PCI | 16 (39) | 13 (42) | 3 (30) | 0.71 |
| DES | 8 | 7 | 1 | N/A |
| BMS | 6 | 4 | 2 | N/A |
| POBA | 1 | 1 | 0 | N/A |
| Unknown | 1 | 1 | 0 | N/A |
| CABG | 7 (17) | 6 (19) | 1 (10) | 0.66 |
| Any revascularization | 23 (56) | 19 (61) | 4 (40) | 0.29 |
| Outcomes | ||||
| Acute coronary syndrome | 11 (27) | 11 (35) | 0 | N/A |
| Ischemic stroke | 6 (15) | 6 (19) | 0 | N/A |
| HF hospitalization | 17 (41) | 17 (55) | 0 | N/A |
| All-cause death | 17 (41) | 17 (55) | 0 | N/A |
| CV-related death | 8 (20) | 8 (26) | 0 | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leiva, O.; Jenkins, A.; Rosovsky, R.P.; Leaf, R.K.; Goodarzi, K.; Hobbs, G. Risk Factors for Death or Cardiovascular Events after Acute Coronary Syndrome in Patients with Myeloproliferative Neoplasms. Hematol. Rep. 2023, 15, 398-404. https://doi.org/10.3390/hematolrep15020040
Leiva O, Jenkins A, Rosovsky RP, Leaf RK, Goodarzi K, Hobbs G. Risk Factors for Death or Cardiovascular Events after Acute Coronary Syndrome in Patients with Myeloproliferative Neoplasms. Hematology Reports. 2023; 15(2):398-404. https://doi.org/10.3390/hematolrep15020040
Chicago/Turabian StyleLeiva, Orly, Andrew Jenkins, Rachel P. Rosovsky, Rebecca K. Leaf, Katayoon Goodarzi, and Gabriela Hobbs. 2023. "Risk Factors for Death or Cardiovascular Events after Acute Coronary Syndrome in Patients with Myeloproliferative Neoplasms" Hematology Reports 15, no. 2: 398-404. https://doi.org/10.3390/hematolrep15020040
APA StyleLeiva, O., Jenkins, A., Rosovsky, R. P., Leaf, R. K., Goodarzi, K., & Hobbs, G. (2023). Risk Factors for Death or Cardiovascular Events after Acute Coronary Syndrome in Patients with Myeloproliferative Neoplasms. Hematology Reports, 15(2), 398-404. https://doi.org/10.3390/hematolrep15020040






