Molecular Tumor Boards: The Next Step towards Precision Therapy in Cancer Care
Abstract
1. Introduction
2. Materials and Methods
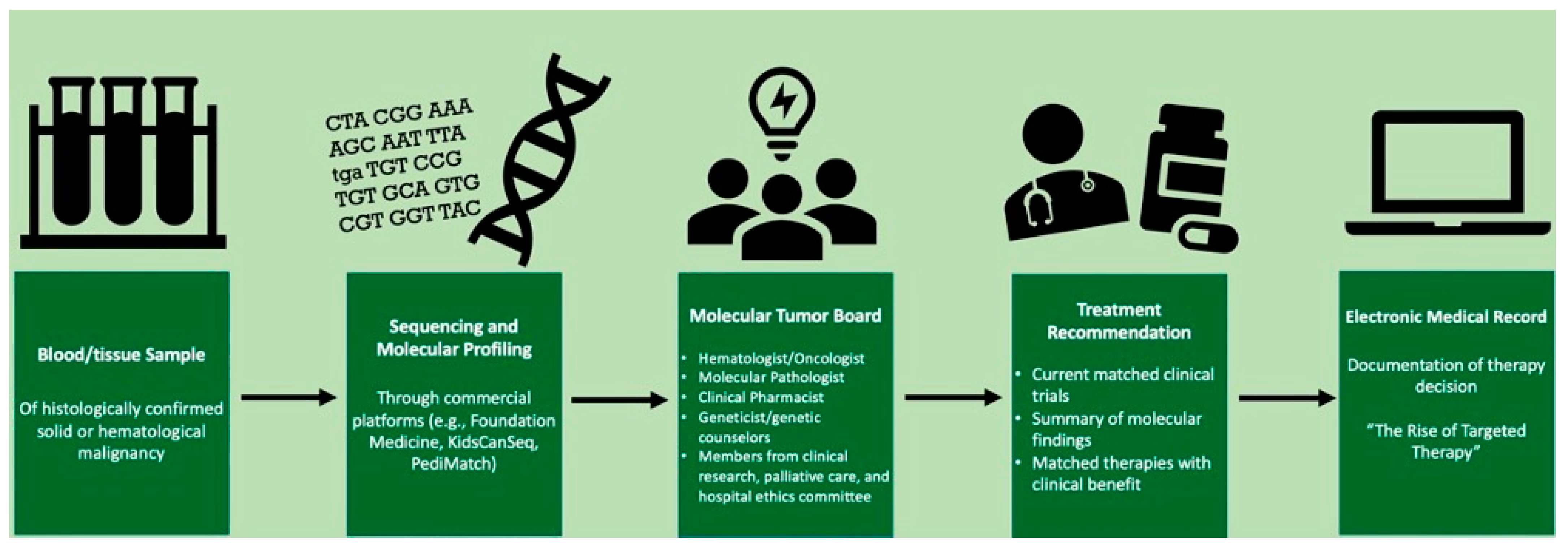
2.1. Our Interdisciplinary Approach to MTB Meetings
2.2. Role of Clinical Pharmacists
3. Results
3.1. Financial Impact
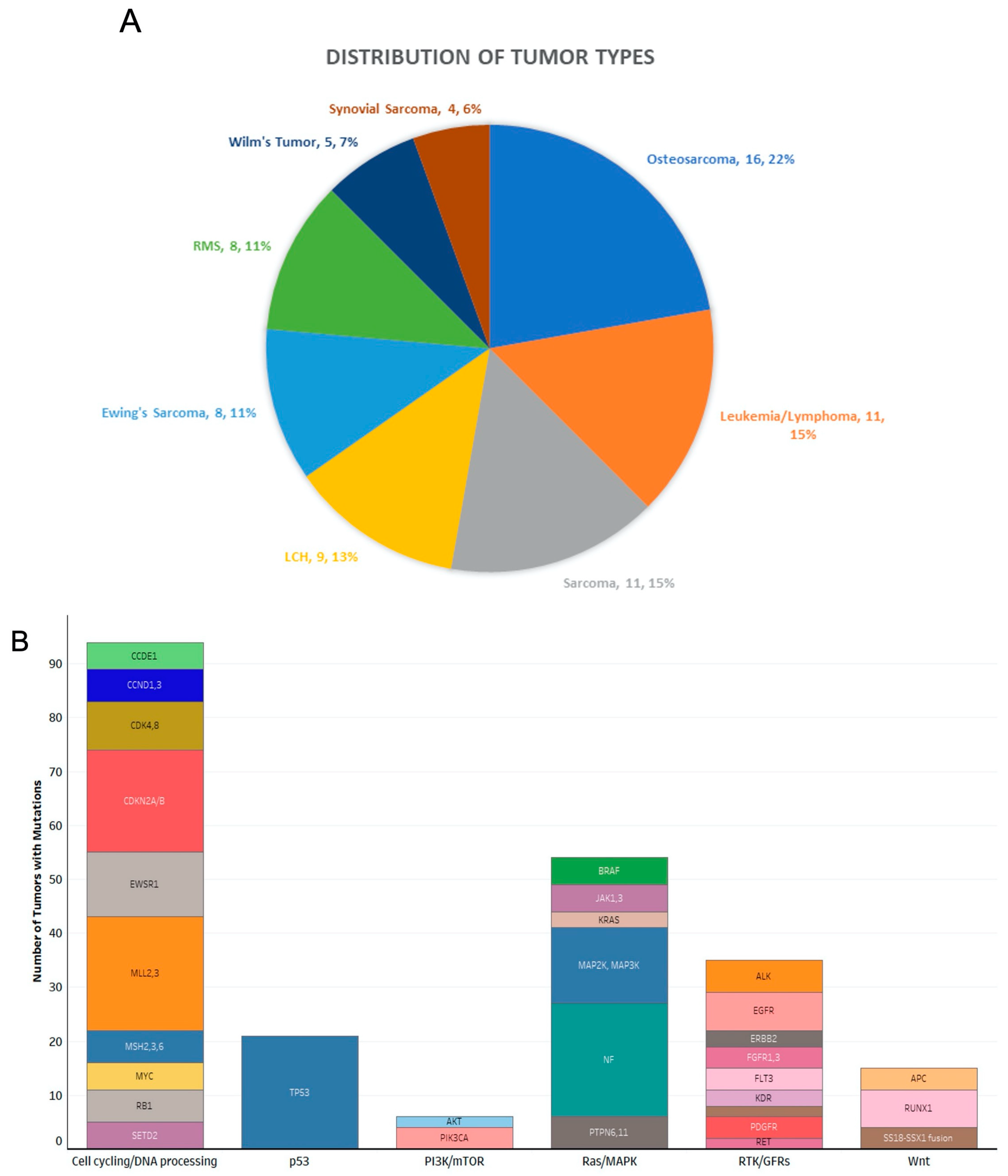
3.2. Disease Settings Evaluated by Molecular Tumor Board
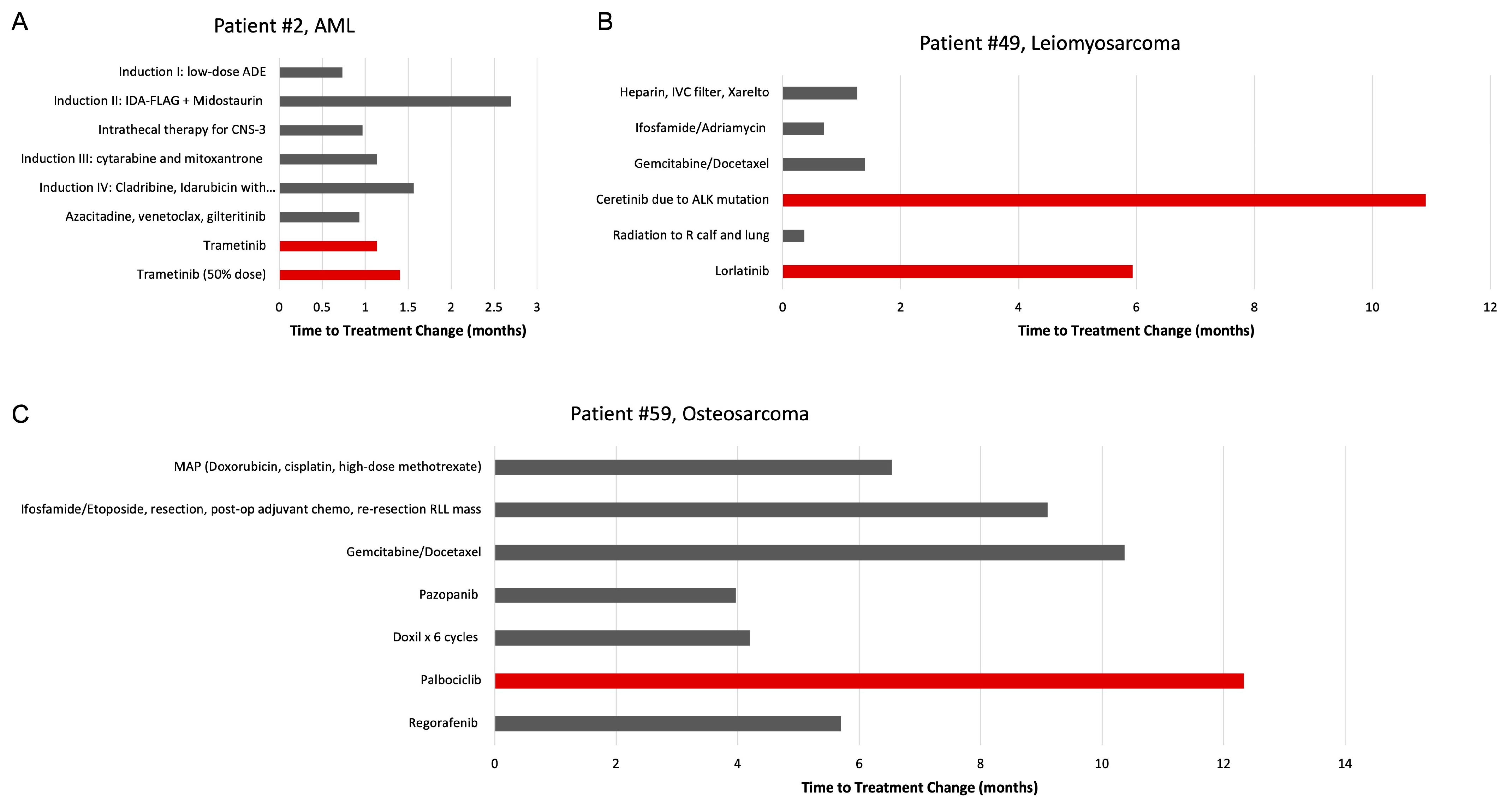
3.3. Impact of Molecular Tumor Board on Treatment Decisions
4. Discussion
4.1. Impact of MTB-Recommended Therapy on Patient Survival
4.2. Need for Tumor Re-Biopsy
4.3. Matching Therapy to MTB Recommendations
4.4. Standardization of MTB Recommendations
4.5. MTB Format and Turnaround Time
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forrester, J.V.; Kuffova, L.; Dick, A.D. Autoimmunity, Autoinflammation, and Infection in Uveitis. Am. J. Ophthalmol. 2018, 189, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Mody, R.J.; Prensner, J.R.; Everett, J.; Parsons, D.W.; Chinnaiyan, A.M. Precision medicine in pediatric oncology: Lessons learned and next steps. Pediatr. Blood Cancer 2017, 64, e26288. [Google Scholar] [CrossRef]
- Larson, K.L.; Huang, B.; Weiss, H.L.; Hull, P.; Westgate, P.M.; Miller, R.W.; Arnold, S.M.; Kolesar, J.M. Clinical Outcomes of Molecular Tumor Boards: A Systematic Review. JCO Precis. Oncol. 2021, 5, 1122–1132. [Google Scholar] [CrossRef]
- Huang, B.; Chen, Q.; Allison, D.; El Khouli, R.; Peh, K.H.; Mobley, J.; Anderson, A.; Durbin, E.B.; Goodin, D.; Villano, J.L.; et al. Molecular Tumor Board Review and Improved Overall Survival in Non-Small-Cell Lung Cancer. JCO Precis. Oncol. 2021, 5, 1530–1539. [Google Scholar] [CrossRef]
- Luchini, C.; Lawlor, R.T.; Milella, M.; Scarpa, A. Molecular Tumor Boards in Clinical Practice. Trends Cancer 2020, 6, 738–744. [Google Scholar] [CrossRef]
- Rieke, D.T.; Lamping, M.; Schuh, M.; Tourneau, C.L.; Basté, N.; Burkard, M.E.; Metzeler, K.H.; Leyvraz, S.; Keilholz, U. Comparison of Treatment Recommendations by Molecular Tumor Boards Worldwide. JCO Precis. Oncol. 2018, 2, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Kato, S.M.; Kurzrock, R. Molecular Tumor Boards: Realizing Precision Oncology Therapy. Clin. Pharm. 2018, 103, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Parsons, D.W.; Roy, A.; Yang, Y.; Wang, T.; Scollon, S.; Bergstrom, K.; Kerstein, R.A.; Gutierrez, S.; Petersen, A.K.; Bavle, A.; et al. Diagnostic Yield of Clinical Tumor and Germline Whole-Exome Sequencing for Children with Solid Tumors. JAMA Oncol. 2016, 2, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Balko, J.M.; Giltnane, J.M.; Wang, K.; Schwarz, L.J.; Young, C.D.; Cook, R.S.; Owens, P.; Sanders, M.E.; Kuba, M.G.; Sanchez, V.; et al. Molecular profiling of the residual disease of triple-negative breast cancers after neoadjuvant chemotherapy identifies actionable therapeutic targets. Cancer Discov. 2014, 4, 232–245. [Google Scholar] [CrossRef]
- Kim, E.S.; Herbst, R.S.; Wistuba, I.I.; Lee, J.J.; Blumenschein, G.R., Jr.; Tsao, A.; Stewart, D.J.; Hicks, M.E.; Erasmus, J., Jr.; Gupta, S.; et al. The BATTLE trial: Personalizing therapy for lung cancer. Cancer Discov. 2011, 1, 44–53. [Google Scholar] [CrossRef]
- Tsimberidou, A.M.; Iskander, N.G.; Hong, D.S.; Wheler, J.J.; Falchook, G.S.; Fu, S.; Piha-Paul, S.; Naing, A.; Janku, F.; Luthra, R.; et al. Personalized medicine in a phase I clinical trials program: The MD Anderson Cancer Center initiative. Clin. Cancer Res. 2012, 18, 6373–6383. [Google Scholar] [CrossRef] [PubMed]
- Viale, P.H. The American Cancer Society’s Facts & Figures: 2020 Edition. J. Adv. Pract. Oncol. 2020, 11, 135–136. [Google Scholar] [CrossRef] [PubMed]
- Younger, E.; Husson, O.; Asare, B.; Benson, C.; Judson, I.; Miah, A.; Zaidi, S.; Dunlop, A.; Al-Muderis, O.; van Houdt, W.J.; et al. Metastatic Soft Tissue Sarcomas in Adolescents and Young Adults: A Specialist Center Experience. J. Adolesc. Young Adult Oncol. 2020, 9, 628–638. [Google Scholar] [CrossRef]
- Smeland, S.; Bielack, S.S.; Whelan, J.; Bernstein, M.; Hogendoorn, P.; Krailo, M.D.; Gorlick, R.; Janeway, K.A.; Ingleby, F.C.; Anninga, J.; et al. Survival and prognosis with osteosarcoma: Outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur. J. Cancer 2019, 109, 36–50. [Google Scholar] [CrossRef]
- Johnson, B.E.; Mazor, T.; Hong, C.; Barnes, M.; Aihara, K.; McLean, C.Y.; Fouse, S.D.; Yamamoto, S.; Ueda, H.; Tatsuno, K.; et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science 2014, 343, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Wagle, N.; Emery, C.; Berger, M.F.; Davis, M.J.; Sawyer, A.; Pochanard, P.; Kehoe, S.M.; Johannessen, C.M.; Macconaill, L.E.; Hahn, W.C.; et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J. Clin. Oncol. 2011, 29, 3085–3096. [Google Scholar] [CrossRef]
- Wagle, N.; Grabiner, B.C.; Van Allen, E.M.; Amin-Mansour, A.; Taylor-Weiner, A.; Rosenberg, M.; Gray, N.; Barletta, J.A.; Guo, Y.; Swanson, S.J.; et al. Response and acquired resistance to everolimus in anaplastic thyroid cancer. N. Engl. J. Med. 2014, 371, 1426–1433. [Google Scholar] [CrossRef]
- Duffy, M.J.; O’Donovan, N.; Crown, J. Use of molecular markers for predicting therapy response in cancer patients. Cancer Treat Rev. 2011, 37, 151–159. [Google Scholar] [CrossRef]
- Kato, S.; Kim, K.H.; Lim, H.J.; Boichard, A.; Nikanjam, M.; Weihe, E.; Kuo, D.J.; Eskander, R.N.; Goodman, A.; Galanina, N.; et al. Real-world data from a molecular tumor board demonstrates improved outcomes with a precision N-of-One strategy. Nat. Commun. 2020, 11, 4965. [Google Scholar] [CrossRef]
- Ortiz, M.V.; Kobos, R.; Walsh, M.; Slotkin, E.K.; Roberts, S.; Berger, M.F.; Hameed, M.; Solit, D.; Ladanyi, M.; Shukla, N.; et al. Integrating Genomics into Clinical Pediatric Oncology Using the Molecular Tumor Board at the Memorial Sloan Kettering Cancer Center. Pediatr. Blood Cancer 2016, 63, 1368–1374. [Google Scholar] [CrossRef]
- Koopman, B.; Groen, H.J.M.; Ligtenberg, M.J.L.; Grunberg, K.; Monkhorst, K.; de Langen, A.J.; Boelens, M.C.; Paats, M.S.; von der Thusen, J.H.; Dinjens, W.N.M.; et al. Multicenter Comparison of Molecular Tumor Boards in The Netherlands: Definition, Composition, Methods, and Targeted Therapy Recommendations. Oncologist 2021, 26, e1347–e1358. [Google Scholar] [CrossRef] [PubMed]
- VanderWalde, A.; Grothey, A.; Vaena, D.; Vidal, G.; ElNaggar, A.; Bufalino, G.; Schwartzberg, L. Establishment of a Molecular Tumor Board (MTB) and Uptake of Recommendations in a Community Setting. J. Pers. Med. 2020, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Klek, S.; Heald, B.; Milinovich, A.; Ni, Y.; Abraham, J.; Mahdi, H.; Estfan, B.; Khorana, A.A.; Bolwell, B.J.; Grivas, P.; et al. Genetic Counseling and Germline Testing in the Era of Tumor Sequencing: A Cohort Study. JNCI Cancer Spectr. 2020, 4, pkaa018. [Google Scholar] [CrossRef] [PubMed]
- Schwaederle, M.; Parker, B.A.; Schwab, R.B.; Fanta, P.T.; Boles, S.G.; Daniels, G.A.; Bazhenova, L.A.; Subramanian, R.; Coutinho, A.C.; Ojeda-Fournier, H.; et al. Molecular tumor board: The University of California-San Diego Moores Cancer Center experience. Oncologist 2014, 19, 631–636. [Google Scholar] [CrossRef]
- Harada, S.; Arend, R.; Dai, Q.; Levesque, J.A.; Winokur, T.S.; Guo, R.; Heslin, M.J.; Nabell, L.; Nabors, L.B.; Limdi, N.A.; et al. Implementation and utilization of the molecular tumor board to guide precision medicine. Oncotarget 2017, 8, 57845–57854. [Google Scholar] [CrossRef]
- Cree, I.A.; Deans, Z.; Ligtenberg, M.J.; Normanno, N.; Edsjo, A.; Rouleau, E.; Sole, F.; Thunnissen, E.; Timens, W.; Schuuring, E.; et al. Guidance for laboratories performing molecular pathology for cancer patients. J. Clin. Pathol. 2014, 67, 923–931. [Google Scholar] [CrossRef]
| Drug | # of Patients Treated | # of Cycles Dispensed | Cost Avoidance (Estimated Medication Cost) |
|---|---|---|---|
| Ceritinib | 1 | 11 | USD 132,145 |
| Dabrafenib | 1 | 31 | USD 250,898.20 |
| Crizotinib | 3 | 4 | USD 84,072 |
| Lorlatinib | 2 | 26 | USD 645,568 |
| Alisertib | 2 | 29 | N/A |
| Entrectinib | 1 | 4 | USD 29,972 |
| Tazemetostat | 1 | 1 | USD 5425 |
| Selumetinib | 4 | 15 | USD 286,321 |
| Selinexor | 2 | 6 | USD 8726.58 |
| Nirogacestat | 2 | 2 | N/A |
| Total | USD 1,443,127.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, A.; Vicenzi, P.; Sharma, I.; Orr, K.; Teller, C.; Koentz, M.; Trinkman, H.; Vallance, K.; Ray, A. Molecular Tumor Boards: The Next Step towards Precision Therapy in Cancer Care. Hematol. Rep. 2023, 15, 244-255. https://doi.org/10.3390/hematolrep15020025
Liu A, Vicenzi P, Sharma I, Orr K, Teller C, Koentz M, Trinkman H, Vallance K, Ray A. Molecular Tumor Boards: The Next Step towards Precision Therapy in Cancer Care. Hematology Reports. 2023; 15(2):244-255. https://doi.org/10.3390/hematolrep15020025
Chicago/Turabian StyleLiu, Angela, Paige Vicenzi, Ishna Sharma, Kaci Orr, Christa Teller, Micha Koentz, Heidi Trinkman, Kelly Vallance, and Anish Ray. 2023. "Molecular Tumor Boards: The Next Step towards Precision Therapy in Cancer Care" Hematology Reports 15, no. 2: 244-255. https://doi.org/10.3390/hematolrep15020025
APA StyleLiu, A., Vicenzi, P., Sharma, I., Orr, K., Teller, C., Koentz, M., Trinkman, H., Vallance, K., & Ray, A. (2023). Molecular Tumor Boards: The Next Step towards Precision Therapy in Cancer Care. Hematology Reports, 15(2), 244-255. https://doi.org/10.3390/hematolrep15020025







