Medich Giant Platelet Syndrome: An Evolving Qualitative and Quantitative Platelet Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Platelet Preparation Methods for Electron Microscopy
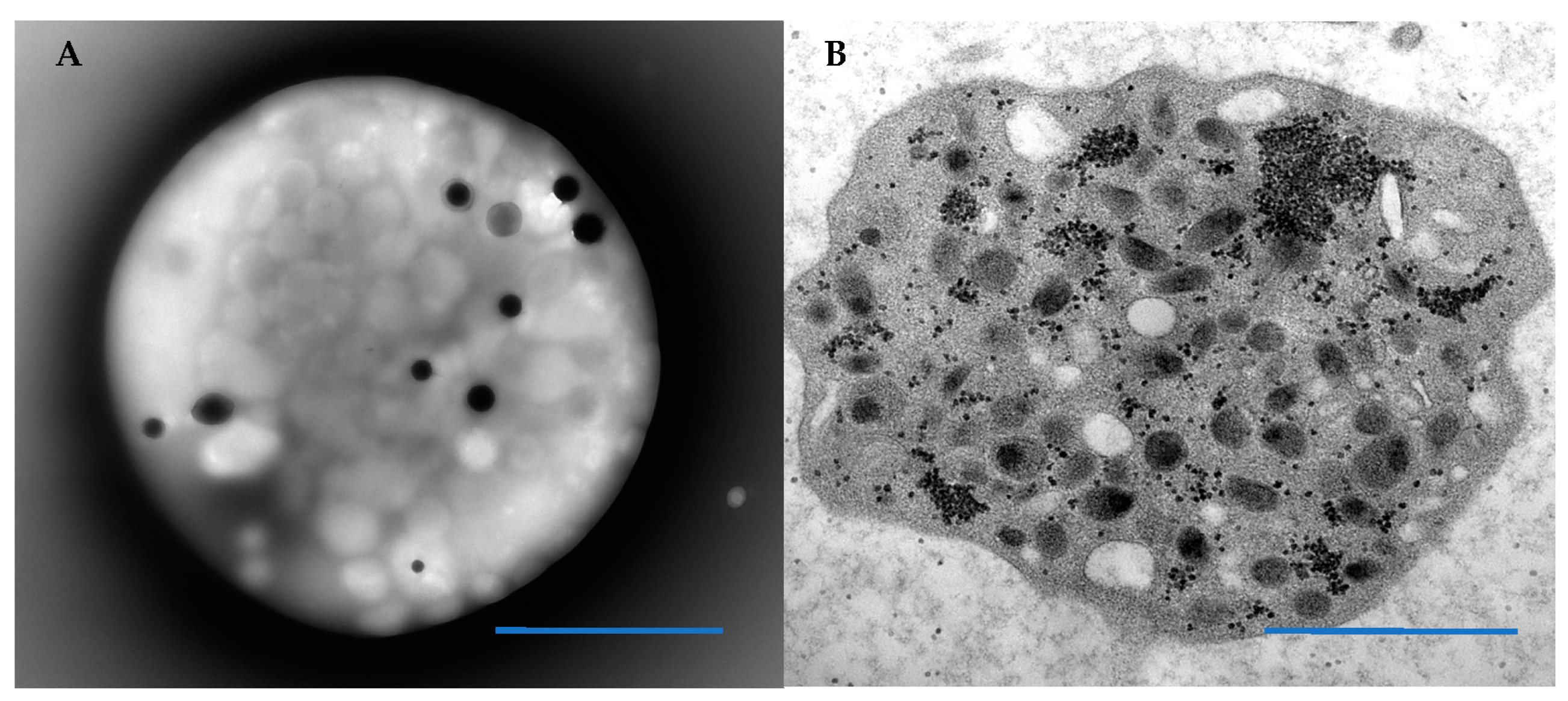
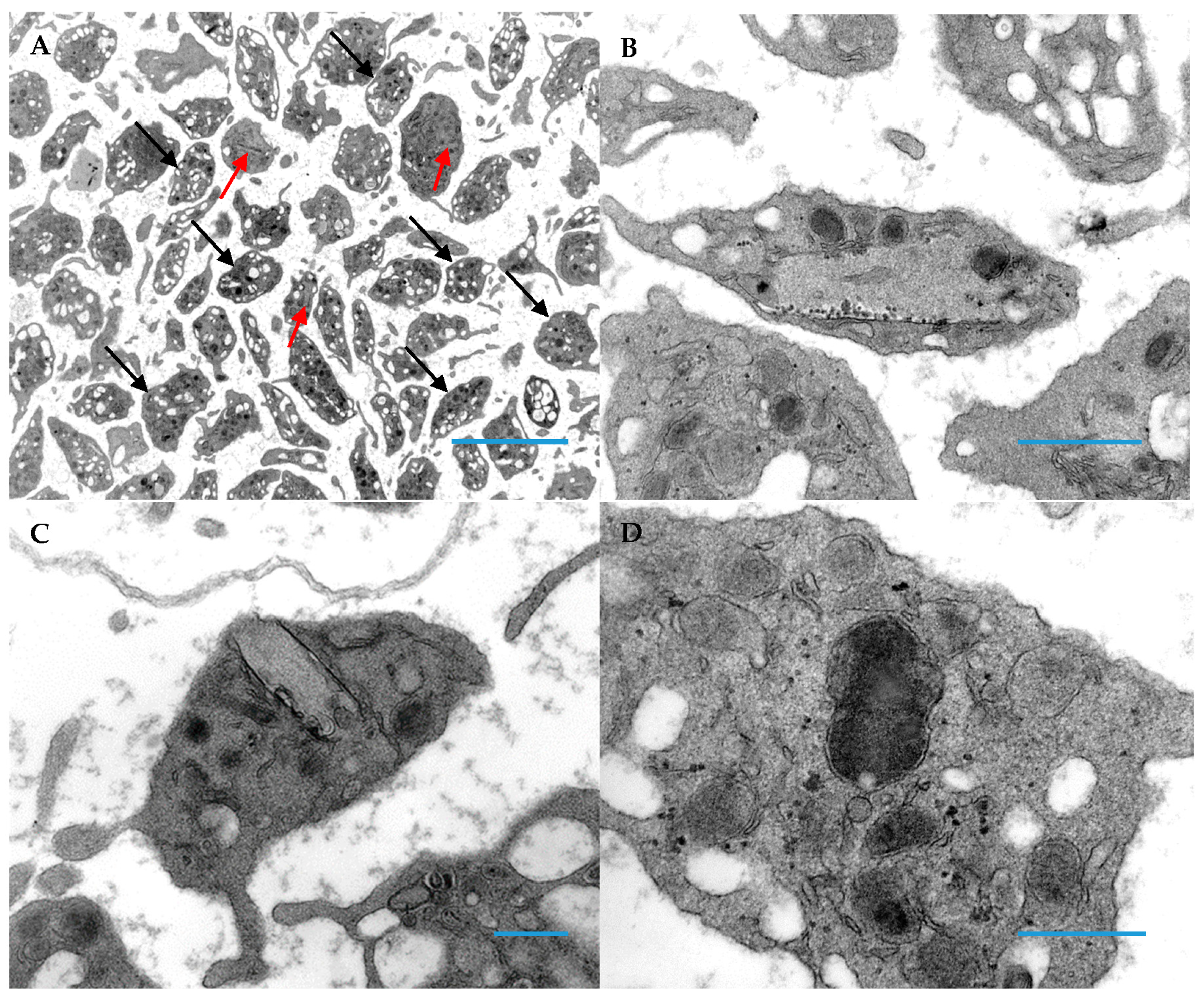
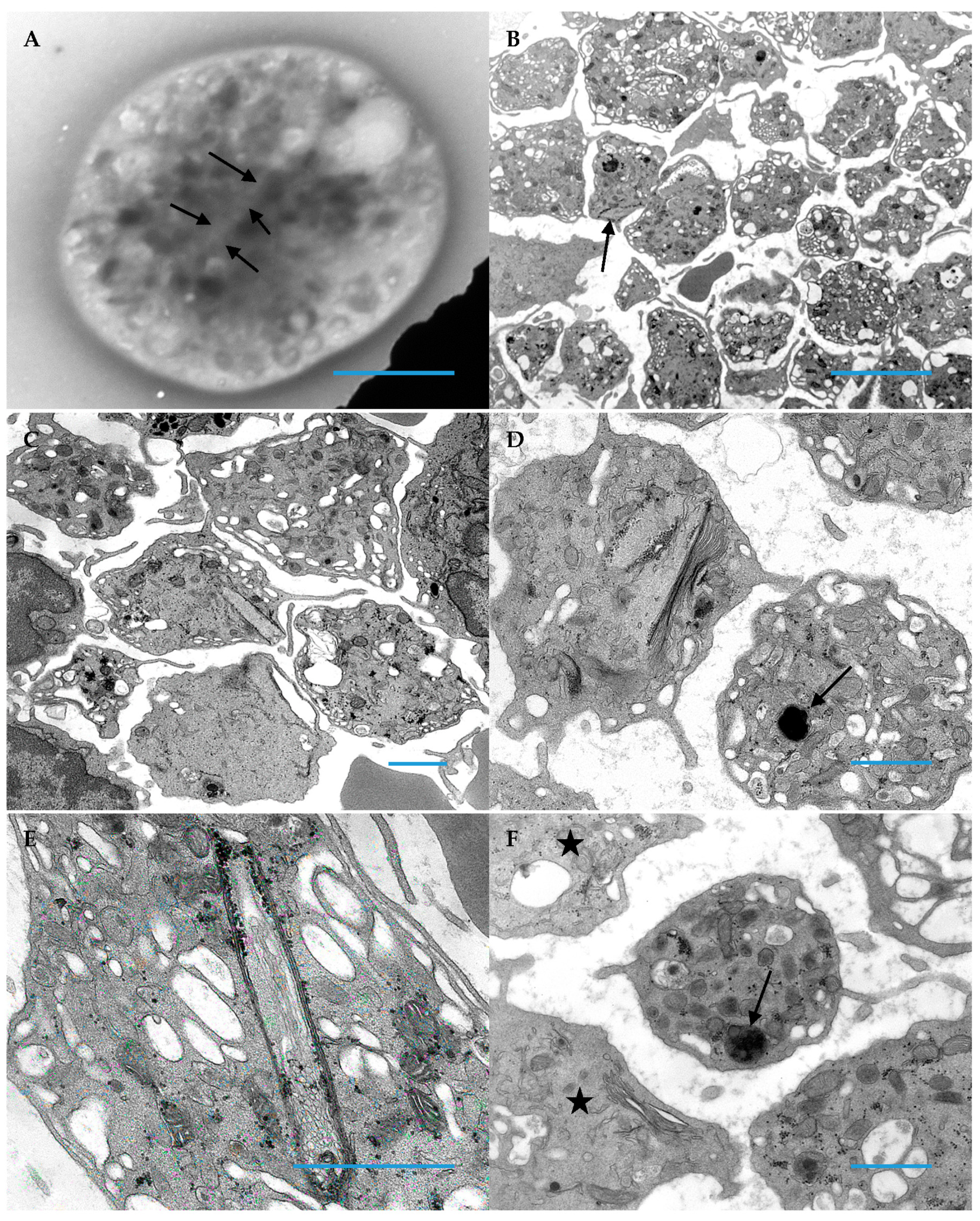
3. Results
3.1. Patient Platelets
3.2. Clinical Characteristics of Patients
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cox, K.; Price, V.; Kahr, W.H. Inherited platelet disorders: A clinical approach to diagnosis and management. Expert Rev. Hematol. 2011, 4, 455–472. [Google Scholar] [CrossRef] [PubMed]
- Israels, S.J.; Kahr, W.H.; Blanchette, V.S.; Luban, N.L.; Rivard, G.E.; Rand, M.L. Platelet disorders in children: A diagnostic approach. Pediatr. Blood Cancer 2011, 56, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Nurden, A.T.; Nurden, P.; Bermejo, E.; Combrie, R.; McVicar, D.W.; Washington, A.V. Phenotypic heterogeneity in the Gray platelet syndrome extends to the expression of TREM family member, TLT-1. Thromb. Haemost. 2008, 100, 45–51. [Google Scholar] [PubMed]
- White, J.G. Ultrastructural studies of the gray platelet syndrome. Am. J. Pathol. 1979, 95, 445–462. [Google Scholar]
- Witkop, C.J.; Krumwiede, M.; Sedano, H.; White, J.G. Reliability of absent platelet dense bodies as a diagnostic criterion for Hermansky-Pudlak syndrome. Am. J. Hematol. 1987, 26, 305–311. [Google Scholar] [CrossRef]
- Buchanan, G.R.; Handin, R.I. Platelet function in the Chediak-Higashi syndrome. Blood 1976, 47, 941–948. [Google Scholar] [CrossRef]
- Clauser, S.; Cramer-Borde, E. Role of platelet electron microscopy in the diagnosis of platelet disorders. Semin. Thromb. Hemost. 2009, 35, 213–223. [Google Scholar] [CrossRef]
- White, J.G. Platelet storage pool deficiency in Jacobsen syndrome. Platelets 2007, 18, 522–527. [Google Scholar] [CrossRef]
- Zahavi, J.; Gale, R.; Kakkar, V.V. Storage pool disease of platelets in an infant with thrombocytopenic absent radii (TAR) syndrome simulating Fanconi’s anaemia. Haemostasis 1981, 10, 121–133. [Google Scholar] [CrossRef]
- Glembotsky, A.C.; Bluteau, D.; Espasandin, Y.R.; Goette, N.P.; Marta, R.F.; Marin Oyarzun, C.P.; Korin, L.; Lev, P.R.; Laguens, R.P.; Molinas, F.C.; et al. Mechanisms underlying platelet function defect in a pedigree with familial platelet disorder with a predisposition to acute myelogenous leukemia: Potential role for candidate RUNX1 targets. J. Thromb. Haemost. 2014, 12, 761–772. [Google Scholar] [CrossRef]
- Hayward, C.P.; Weiss, H.J.; Lages, B.; Finlay, M.; Hegstad, A.C.; Zheng, S.; Cowie, A.; Massé, J.M.; Harrison, P.; Cramer, E.M.; et al. The storage defects in grey platelet syndrome and alphadelta-storage pool deficiency affect alpha granule factor V and multimerin storage without altering their proteolytic processing. Br. J. Haematol. 2001, 113, 871–877. [Google Scholar] [CrossRef] [PubMed]
- White, J.G.; Keel, S.; Reyes, M.; Burris, S.M. Alpha-delta platelet storage pool deficiency in three generations. Platelets 2007, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- White, J.G. Golgi complexes in hypogranular platelet syndromes. Platelets 2005, 16, 51–60. [Google Scholar] [CrossRef] [PubMed]
- White, J.G. Medich giant platelet disorder: A unique alpha granule deficiency I. Structural abnormalities. Platelets 2004, 15, 345–353. [Google Scholar] [CrossRef]
- Gunning, W.; Dole, M.; Brecher, M.; White, J.G. The Medich giant platelet syndrome: Two new cases. Platelets 2013, 24, 107–112. [Google Scholar] [CrossRef]
- Bull, B.J. The ultrastructure of negatively stained platelets. Blood 1966, 28, 901–912. [Google Scholar] [CrossRef]
- White, J.G.; Edson, J.R.; Desnick, S.J.; Witkop, C.J., Jr. Studies of platelets in a variant of the Hermansky-Pudlak syndrome. Am. J. Pathol. 1971, 63, 319–332. [Google Scholar]
- Gunning, W.T.; Raghavan, M.; Calomeni, E.P.; Turner, J.N.; Roysam, B.; Roysam, S.; Smith, M.R.; Kouides, P.A.; Lachant, N.A. A Morphometric Analysis of Platelet Dense Granules of Patients with Unexplained Bleeding: A New Entity of Microgranular Storage Pool Deficiency. J. Clin. Med. 2020, 9, 1734. [Google Scholar] [CrossRef]
- White, J.G. Electron dense chains and clusters in human platelets. Platelets 2002, 13, 317–325. [Google Scholar] [CrossRef]
- Weiss, H.J.; Lages, B.; Vicic, W.; Tsung, L.Y.; White, J.G. Heterogeneous abnormalities of platelet dense granule ultrastructure in patients with congenital storage pool deficiency. Br. J. Haematol. 1993, 83, 282–295. [Google Scholar] [CrossRef]
- Lewinson, D. Application of the ferrocyanide-reduced osmium method for mineralizing cartilage: Further evidence for the enhancement of intracellular glycogen and visualization of matrix components. Histochem. J. 1989, 21, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, S.A.; Johnson, B.; Lowe, G.C.; Bem, D.; Drake, S.; Lordkipanidzé, M.; Guiú, I.S.; Dawood, B.; Rivera, J.; Simpson, M.A.; et al. SLFN14 mutations underlie thrombocytopenia with excessive bleeding and platelet secretion defects. J. Clin. Investig. 2015, 125, 3600–3605. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.P. Inherited Platelet Disorders: A Modern Approach to Evaluation and Treatment. Hematol. Oncol. Clin. North Am. 2019, 33, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Pluthero, F.G.; Kahr, W.H.A. Recent advances in inherited platelet disorders. Curr. Opin. Hematol. 2019, 26, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Kim, B. Diagnostic workup of inherited platelet disorders. Blood Res. 2022, 57 (Suppl. S1), 11–19. [Google Scholar] [CrossRef]
- Collins, J.; Astle, W.J.; Megy, K.; Mumford, A.D.; Vuckovic, D. Advances in understanding the pathogenesis of hereditary macrothrombocytopenia. Br. J. Haematol. 2021, 195, 25–45. [Google Scholar] [CrossRef]
- Marconi, C.; Di Buduo, C.A.; Barozzi, S. SLFN14-related thrombocytopenia: Identification within a large series of patients with inherited thrombocytopenia. Thromb. Haemost. 2016, 115, 1076–1079. [Google Scholar] [CrossRef]
- Savoia, A. Molecular basis of inherited thrombocytopenias: An update. Curr. Opin. Hematol. 2016, 23, 486–492. [Google Scholar] [CrossRef]
| Patient | Sex | Ethnicity | Age of Initial Thrombocytopenia | Age at Diagnosis | Bleeding Symptoms | Therapies | Other Findings | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Female | Hispanic | 2 weeks | 8 years | Bruising Mucosal bleeding | IVIG Steroids Anti-CD20 Immunosuppressants Platelet transfusion Splenectomy Antifibrinolytics OCP+ | Duplication of alpha globin gene in one allele Missense in the SLFN14 gene, c.652A > G | |
| 2 | Male | African American | Birth | 6 years | Bruising Oral mucosal bleeding | Platelet transfusion Antifibrinolytics | Maternal thrombocytopenia | |
| 3 | Female | Caucasian | 1 year | 19 years | Bruising Bleeding with piercings and dental work Menorrhagia | Platelet transfusion Splenectomy OCP+ Antifibrinolytics | None | [14] |
| 4 | Male | Caucasian | Birth | 2 years | Platelet transfusion | CHD++ Craniosynostosis 4Ydel (11q23.3) | [15] | |
| 5 | Female | Caucasian | Birth | 18 months | Subdural hematoma | Platelet transfusion | EGA+++ 29 weeks one of fraternal triplets | [15] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massey, G.; Tyrrell, L.; Diab, Y.; Gunning, W.T., III. Medich Giant Platelet Syndrome: An Evolving Qualitative and Quantitative Platelet Disorder. Hematol. Rep. 2022, 14, 349-357. https://doi.org/10.3390/hematolrep14040049
Massey G, Tyrrell L, Diab Y, Gunning WT III. Medich Giant Platelet Syndrome: An Evolving Qualitative and Quantitative Platelet Disorder. Hematology Reports. 2022; 14(4):349-357. https://doi.org/10.3390/hematolrep14040049
Chicago/Turabian StyleMassey, Gita, Laura Tyrrell, Yaser Diab, and William T. Gunning, III. 2022. "Medich Giant Platelet Syndrome: An Evolving Qualitative and Quantitative Platelet Disorder" Hematology Reports 14, no. 4: 349-357. https://doi.org/10.3390/hematolrep14040049
APA StyleMassey, G., Tyrrell, L., Diab, Y., & Gunning, W. T., III. (2022). Medich Giant Platelet Syndrome: An Evolving Qualitative and Quantitative Platelet Disorder. Hematology Reports, 14(4), 349-357. https://doi.org/10.3390/hematolrep14040049






