COVID-19-Associated Thrombotic Thrombocytopenic Purpura: A Case Report and Systematic Review
Abstract
:1. What Is the New Aspect of Your Work?
2. What Is the Central Finding of Your Work?
3. What Is (or Could Be) the Specific Clinical Relevance of Your Work?
4. Introduction
5. Case Presentation
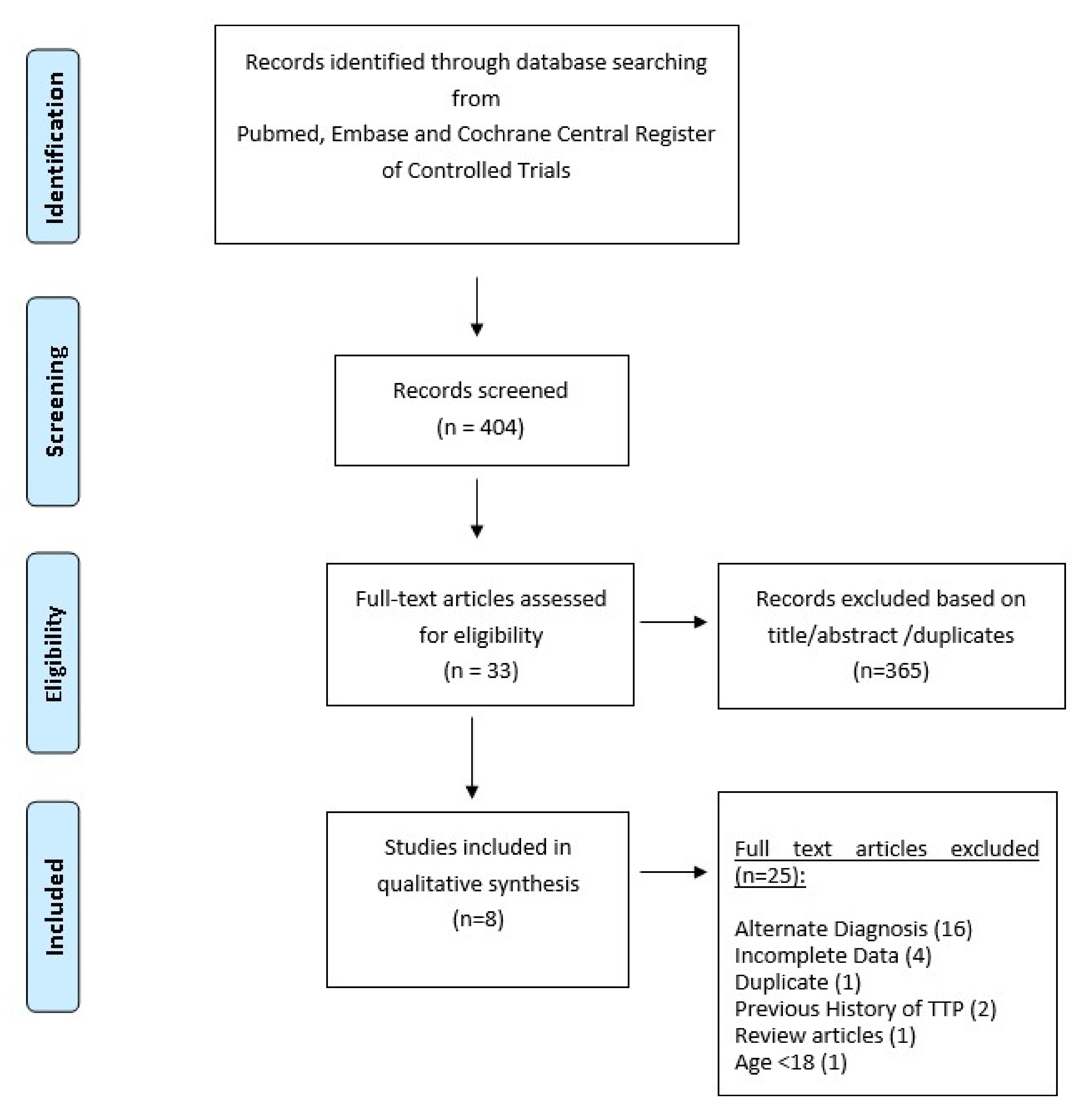
6. Methods
6.1. Search Strategy
6.2. Study Selection and Characteristics
6.3. Quality Assessment of Articles
7. Results
7.1. Patient Characteristics
7.2. Laboratory Parameters
7.3. Treatment
7.4. Outcome
7.5. Discussion
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Page, E.E.; Kremer Hovinga, J.A.; Terrell, D.R.; Vesely, S.K.; George, J.N. Thrombotic thrombocytopenic purpura: Diagnostic criteria, clinical features, and long-term outcomes from 1995 through 2015. Blood Adv. 2017, 1, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Reese, J.A.; Muthurajah, D.S.; Kremer Hovinga, J.A.; Vesley, S.K.; Terell, D.R.; George, J.N. Children and adults with thrombotic thrombocytopenic purpura associated severe, acquired ADAMTS13 deficiency: Comparison, incidence, demographic and clinical features. Pediatr. Blood Cancer 2013, 60, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- George, J.N. Clinical practice. Thrombotic thrombocytopenic purpura. N. Engl. J. Med. 2006, 354, 1927–1935. [Google Scholar] [CrossRef]
- Marietta, M.; Coluccio, V.; Luppi, M. COVID-19, coagulopathy and venous thromboembolism: more questions than answers. Intern. Emerg. Med. 2020, 15, 1375–1387. [Google Scholar] [CrossRef]
- Albiol, N.; Awol, R.; Martino, R. Autoimmune thrombotic thrombocytopenic purpura (TTP) associated with COVID-19. Ann Hematol. 2020, 99, 1673–1674. [Google Scholar] [CrossRef]
- Beaulieu, M.-C.; Mettelus, D.S.; Rioux-Massé, B.; Mahone, M. Thrombotic thrombocytopenic purpura as the initial presentation of COVID-19. J. Thromb. Haemost. 2021, 19, 1132–1134. [Google Scholar] [CrossRef]
- Dhingr ADhingra, G.; Maji, M.; Mandal, S.; Vaniyath, S.; Negi, G.; Nath, U.K. COVID-19 infection associated with thrombotic thrombocytopenic purpura. J. Thromb. Thrombolysis 2021, 52, 504–507. [Google Scholar] [CrossRef]
- Hindilerden, F.; Yonal-Hindilerden, I.; Akar, E.; Kart-Yasar, K. COVID-19 associated autoimmune thrombotic thrombocytopenic purpura: Report of a case. Thromb. Res. 2020, 195, 136–138. [Google Scholar] [CrossRef]
- Law, L.; Ho, G.; Cen, D.; Stenger, J. Atypical manifestations of coronavirus disease 2019 (COVID-19)-associated autoimmune thrombotic thrombocytopenic purpura. Clin. Case Rep. 2021, 9, 1402–1404. [Google Scholar] [CrossRef]
- Nicolotti, D.; Bignami, E.G.; Rossi, S.; Vezzani, A. A case of thrombotic thrombocytopenic purpura associated with COVID-19. J. Thromb. Thrombolysis 2021, 52, 468–470. [Google Scholar] [CrossRef]
- Shankar, K.; Huffman, D.L.; Peterson, C.; Yasir, M.; Kaplan, R. A Case of COVID-19 Induced Thrombotic Thrombocytopenic Purpura. Cureus 2021, 13, e16311. [Google Scholar] [CrossRef]
- Tehrani, H.A.; Darnahal, M.; Vaezi, M.; Haghighi, S. COVID-19 associated thrombotic thrombocytopenic purpura (TTP); A case series and mini-review. Int. Immunopharmacol. 2021, 93, 107397. [Google Scholar] [CrossRef]
- Farzana ASayani Charles, S. Abrams; How I treat refractory thrombotic thrombocytopenic purpura. Blood 2015, 125, 3860–3867. [Google Scholar] [CrossRef] [Green Version]
- Froissart, A.; Buffet, M.; Veyradier, A.; Poullin, P.; Provôt, F.; Malot, S.; Schwarzinger, M.; Galicier, L.; Vanhille, P.; Vernant, J.P.; et al. Efficacy and safety of first-line rituximab in severe, acquired thrombotic thrombocytopenic purpura with a suboptimal response to plasma exchange. Experience of the French Thrombotic Microangiopathies Reference Center. Crit. Care Med. 2012, 40, 104–111. [Google Scholar] [CrossRef]
- Scully, M.; Cataland, S.R.; Peyvandi, F.; Coppo, P.; Knöbl, P.; Kremer Hovinga, J.A.; Metjian, A.; de la Rubia, J.; Pavenski, K.; Callewaert, F.; et al. HERCULES Investigators. Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura. N. Engl. J. Med. 2019, 380, 335–346. [Google Scholar] [CrossRef]
- Coppo, P.; Bubenheim, M.; Azoulay, E.; Galicier, L.; Malot, S.; Bigé, N.; Poullin, P.; Provôt, F.; Martis, N.; Presne, C.; et al. A regimen with caplacizumab, immunosuppression, and plasma exchange prevents unfavorable outcomes in immune-mediated TTP. Blood 2021, 137, 733–742. [Google Scholar] [CrossRef]
- Magro, C.; Mulvey, J.J.; Berlin, D.; Nuovo, G.; Salvatore, S.; Harp, J.; Baxter-Stoltzfus, A.; Laurence, J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl. Res. 2020, 220, 32299776. [Google Scholar] [CrossRef]
- Lopes da Silva, R. Viral-associated thrombotic microangiopathies. Hematol/Oncol Stem Cell Ther. 2011, 4, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, T.A.; Kremer Hovinga, J.A.; Schatzberg, D.; Wagner, D.D.; Lämmle, B. Circulating DNA and myeloperoxidase indicate disease activity in patients with thrombotic microangiopathies. Blood 2012, 120, 1157–1164. [Google Scholar] [CrossRef] [Green Version]
- Fan, B.E.; Chong, V.C.L.; Chan, S.S.W.; Lim, G.H.; Lim, K.G.E.; Tan, G.B.; Mucheli, S.S.; Kuperan, P.; Ong, K.H. Hematologic parameters in patients with COVID-19 infection. Am. J. Hematol. 2020, 95, E131–E134, Erratum in: Am. J. Hematol. 2020, 95, 1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancini, I.; Baronciani, L.; Artoni, A.; Colpani, P.; Biganzoli, M.; Cozzi, G.; Novembrino, C.; Boscolo Anzoletti, M.; De Zan, V.; Pagliari, M.T.; et al. The ADAMTS13-von Willebrand factor axis in COVID-19 patients. J. Thromb. Haemost. 2020, 19, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Pascreau, T.; Zia-Chahabi, S.; Zuber, B.; Tcherakian, C.; Farfour, E.; Vasse, M. ADAMTS 13 deficiency is associated with abnormal distribution of von Willebrand factor multimers in patients with COVID-19. Thromb. Res. 2021, 204, 138–140. [Google Scholar] [CrossRef]
- Singhania, N.; Bansal, S.; Nimmatoori, D.P.; Ejaz, A.A.; McCullough, P.A.; Singhania, G. Current overview on hypercoagulability in COVID-19. Am. J. Cardiovasc. Drugs. 2020, 20, 393–403. [Google Scholar] [CrossRef]
- Murt, A.; Eskazan, A.E.; Yılmaz, U.; Ozkan, T.; Ar, M.C. COVID-19 presenting with immune thrombocytopenia: A case report and review of the literature. J. Med. Virol. 2021, 93, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Stanley, M.; Michalski, J.M. Thrombotic Thrombocytopenic Purpura; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
| Study/Year | Age (Years)/Gender | Comorbidities | Symptoms | Time from COVID-19 Illness to TTP Diagnosis | Steroids (Prednisone Equivalent Dose in mg/Day) | PLEX | RTX | CZB |
|---|---|---|---|---|---|---|---|---|
| Albiol et al. 2021 [5] | 57/F | Hypertension Breast Cancer in remission | Dry cough Anosmia Dysgeusia | Day 6 | Methylprednisone 1 mg/kg | 8 sessions | Yes | No |
| Beaulieu et al. 2021 [6] | 70/M | Peripheral arterial disease, dyslipidemia | Confusion, seizure, dark urine | Day 19 | Methylprednisone 1 mg/kg | 7 sessions | No | No |
| Dhingra et al. 2021 [7] | 35/F | None | Diarrhea, right hemiparesis, seizure | Day 15 | Methylprednisolone 1 g injection | 16 sessions | Yes | No |
| Hindilerden et al. 2020 [8] | 74/F | HTN | Dry cough, Fatigue | Day 5 | Methylprednisolone 1 mg/kg/day | 11 sessions | No | No |
| Law 2021 et al. [9] | 47/F | None | Fatigue, scleral icterus, dark urine | Day 17 | Dexamethasone (dose not mentioned) | 3 sessions | Yes | Yes |
| Nicolotti et al. 2021 [10] | 44/F | Obesity, Hx of DVTs | Weakness, dizziness, abdominal discomfort, respiratory distress | Day 3 | Methylprednisolone (1 mg/kg, 5 days) | 14 sessions | Yes | Yes |
| Shankar et al. 2021 [11] | 30/M | None | Low back pain, left flank pain, hematuria | Day 7 | Prednisone 1 mg/kg/day | 6 sessions | No | Yes |
| Tehrani 2021 [12] | ||||||||
| (i) | 25/F | Pregnant | Severe respiratory symptoms | Not mentioned | Dexamethasone 8 mg BID daily, 14 days | 10 days of sessions | No | No |
| (ii) | 56/F | Locally advanced breast cancer/In remission | Severe respiratory symptoms | Not mentioned | Dexamethasone 8 mg BID daily, 14 days | 14 days of sessions exchange | Yes | No |
| (iii) | 57/F | None | Severe respiratory symptoms | Not mentioned | Dexamethasone 8 mg BID daily, 14 days | 14 sessions | No | No |
| (iv) | 38/M | None | Rectal bleeding | Not mentioned | Dexamethasone 8 mg BID, 21 days | 21 sessions | Yes | No |
| Study/Year | Hb in g/dL | Plt Count × 103/m L | I NR | LDH (U/L) | ADAMTS13 Activity Ag (%) | ADAMTS13 Inhibitor Level (normal 12 U/mL; 0.5 BU) | ADAMTS13 Antibody Titer (Normal 15 U/mL) | CT/CXR of Lung Findings |
|---|---|---|---|---|---|---|---|---|
| Albiol et al. 2021 [5] | 6.9 | 13 | N/A | 1594 | 2.0% | 5.2 BU | - | CT thorax normal |
| Beaulieu et al. 2021 [6] | 6.0 | 18 | 1.1 | 1422 | 10% | - | 0.5 | CXR normal |
| Dhingra et al. 2021 [7] | 8.3 | 20 | N/A | 10,977 | undetectable | 3.0 BU | - | n/a |
| Hindilerden et al. 2020 [8] | 6.6 | 48 | Normal | 1108 | 0.2% | 90 U/mL | - | Patchy peripheral bibasilar ground glass opacities in both lungs. MRI brain normal |
| Law et al. 2021 [9] | 7.0 | 14 | 1.1 | 788 | 5.0% | 63 U/mL | - | n/a |
| Nicolotti et al. 2021 [10] | 6.0 | 7 | Normal | 2961 | 5.0% | 57 U/mL | - | Interstitial pneumonia involving 25% of lung parenchyma |
| Shankar et al. 2021 [11] | 13.7 | 9 | Normal | 1068 | 3.0% | 0.60 BU | - | n/a |
| Tehrani et al. [12] | ||||||||
| (i) | 7.0 | 10.5 | 1.4 | 3465 | 8.0% | - | 85 | Patchy infiltration |
| (ii) | 6.0 | 41 | 1.2 | 1520 | 0.01% | - | 36.2 | Patchy bilateral infiltration |
| (iii) | 7.9 | 98 | 1.5 | 1150 | 0.86% | - | 25.3 | Not mentioned |
| (iv) | 8.0 | 5.0 | 1.3. | 545 | 0.06% | - | 14 | Patchy infiltration in the right upper lobe |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaudhary, H.; Nasir, U.; Syed, K.; Labra, M.; Reggio, C.; Aziz, A.; Shah, P.; Reddy, R.; Sangha, N. COVID-19-Associated Thrombotic Thrombocytopenic Purpura: A Case Report and Systematic Review. Hematol. Rep. 2022, 14, 253-260. https://doi.org/10.3390/hematolrep14030035
Chaudhary H, Nasir U, Syed K, Labra M, Reggio C, Aziz A, Shah P, Reddy R, Sangha N. COVID-19-Associated Thrombotic Thrombocytopenic Purpura: A Case Report and Systematic Review. Hematology Reports. 2022; 14(3):253-260. https://doi.org/10.3390/hematolrep14030035
Chicago/Turabian StyleChaudhary, Haseeb, Usama Nasir, Khezar Syed, Maria Labra, Christopher Reggio, Ansar Aziz, Parin Shah, Roopika Reddy, and Navdeep Sangha. 2022. "COVID-19-Associated Thrombotic Thrombocytopenic Purpura: A Case Report and Systematic Review" Hematology Reports 14, no. 3: 253-260. https://doi.org/10.3390/hematolrep14030035
APA StyleChaudhary, H., Nasir, U., Syed, K., Labra, M., Reggio, C., Aziz, A., Shah, P., Reddy, R., & Sangha, N. (2022). COVID-19-Associated Thrombotic Thrombocytopenic Purpura: A Case Report and Systematic Review. Hematology Reports, 14(3), 253-260. https://doi.org/10.3390/hematolrep14030035






