Heritability of Morpho-Agronomic Traits in Cocona (Solanum sessiliflorum Dunal) and Efficiency of Early Visual Selection for Fruit Yield
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Seedling Production
2.3. Field Experiment
2.4. Plant Selection
2.5. Statistical Analysis
3. Results
3.1. Heritability Estimates
3.2. Early Visual Selection for Fruit Yield
3.3. Correlations Among Fruit Traits
4. Discussion
4.1. Heritability
4.2. Early Visual Selection
4.3. Analysis of Correlations Among Fruit Traits
4.4. Implications, Limitations and Further Studies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pahlen, A.v.d. Cubiu [Solanum topiro (Humb. & Bonpl.)], uma fruteira da Amazônia. Acta Amazon. 1977, 7, 301–307. [Google Scholar]
- Pizzinato, J.R.; Schuelter, A.R.; do Amaral Júnior, A.T.; de Souza Rocha, A.C.; da Siva, J.M.; Volkweis, C.R.; de Freitas, M.B.; Ricardo, F.; Schwantes, D.O. Crossing and diagnostic methods of cubiu hybrid plants based on genetic markers. Crop Breed. Appl. Biot. 2008, 8, 283–290. [Google Scholar] [CrossRef][Green Version]
- Paiva, W.O. Taxa de Polinização cruzada em Cubiu. Pesqui Agropec. Bras. 1999, 34, 145–149. [Google Scholar] [CrossRef]
- Salick, J. Crop domestication and the evolutionary ecology of cocona (Solanum sessiliflorum Dunal). In Evolutionary Biology; Hecht, M., Wallace, B., Macintyre, R., Eds.; Evolutionary Biology Plenum Press: New York, NY, USA, 1992; Volume 26, pp. 247–285. [Google Scholar]
- Yuyama, L.K.O.; Pantoja, L.; Maeda, R.N.; Aguiar, J.P.L.; Silva, S.B.d. Desenvolvimento e aceitabilidade de geléia dietética de cubiu (Solanum sessiliflorum Dunal). Food Sci. Technol. 2008, 28, 929–934. [Google Scholar] [CrossRef]
- Chaverra, L.M.M.; Molina, M.C.; Cuesta, J.H.C. Elaboração de néctar de lulo (Solanum sessiliflorum Dunal). Rev. Sinerg. 2019, 1, 64–81. [Google Scholar]
- Vargas-Arana, G.; Merino-Zegarra, C.; Alva-Arevalo, A.; Panduro-Bendezu, P.; Orbe-Peixoto, R.; Simirgiotis, M.J. Physicochemical properties, metabolomic analysis, antioxidant and lipid-lowering activity of a functional beverage based on cocona (Solanum sessiliflorum Dunal). Bol. Latinoam. Caribe Plantas Med. Aromat. 2024, 23, 304–325. [Google Scholar] [CrossRef]
- Pires, A.M.B.; Silva, P.S.; Nardelli, P.M.; Gomes, J.C.; Ramos, A.M. Caracterização e processamento de cubiu (Solanum sessiliflorum). Rev. Ceres 2006, 53, 309–316. [Google Scholar]
- Quispe-Herrera, R.; Valverde, Y.P.; Huamani, J.R.R. Antioxidant capacity and proximal analysis of Solanum sessiliflorum Dunal and Chenopodium quinoa Willdenow nectar-based. Agron. Mesoam. 2022, 33, 47706. [Google Scholar] [CrossRef]
- Ticona-Benavente, C.A.; Guimarães, R.G.V.; Silva, T.M.P.; Silva, L.S.; Boeira, L.S. Mixed tropical juice of cocona and pineapple has market potential. Eur. Acad. Res. 2020, 8, 77–91. [Google Scholar]
- Piltz, M.T. Atividade Biológica e Efeito Hipoglicemiante do Extrato de Solanum sessiliflorum Dunal (Maná-Cubiu) em Ratos Com Diabetes Induzida. Master’s Dissertation, Universidade Federal do Paraná, Curitiba, Brazil, 2018. [Google Scholar]
- Alvarado Santiago, T.I.; Herencia Torres, V.R. Efecto hipolipemiante del extracto de cocona (Solanum sessiliflorum Dunal) en pacientes con hipercolesterolemia. REMCA 2022, 5, 196–200. [Google Scholar] [CrossRef]
- Vargas-Arana, G.; Merino-Zegarra, C.; Riquelme-Penaherrera, M.; Nonato-Ramirez, L.; Delgado-Wong, H.; Pertino, M.W.; Parra, C.; Simirgiotis, M.J. Antihyperlipidemic and Antioxidant Capacities, Nutritional Analysis and UHPLC-PDA-MS Characterization of Cocona Fruits (Solanum sessiliflorum Dunal) from the Peruvian Amazon. Antioxidants 2021, 10, 1566. [Google Scholar] [CrossRef]
- Andrade, M.T.P.d. Efeito do Extrato de Solanum sessiliflorum Dunal (Mana-Cubiu) em Modelos Experimentais Submetidos à dieta Hipercalórica e Hiperlipídica. Ph.D. Thesis, Universidade Federal do Paraná, Curitiba, Brazil, 2023. [Google Scholar]
- Montagner, G.F.F.d.S.; Barbisan, F.; Ledur, P.C.; Bolignon, A.; Motta, J.d.R.; Ribeiro, E.E.; Praia, R.d.S.; Azzolin, V.F.; Cadoná, F.C.; Machado, A.K.; et al. In vitro biological properties of Solanum sessiliflorum (Dunal), an Amazonian Fruit. J. Med. Food 2020, 23, 978–987. [Google Scholar] [CrossRef]
- UPOV. PLUTO Plant Variety Database. Available online: https://pluto.upov.int/search (accessed on 9 January 2025).
- Terrones, L.E.B.; Toribio, C.C.; Huayllas, O.C. El cultivo de cocona; Editorial Colecciones Jovic S.A.C.: Lima, Peru, 2011; p. 115. [Google Scholar]
- Blind, A.D.; Silva Filho, D.F.; Ticona-Benavente, C.A.; Figueiredo, J.N.R.; Guimarães, H.C.; Aguiar, M.R.B. Diagnóstico do Banco de Germoplasma de Hortaliças do Inpa—Uso e conservação. In Agricultura Tropical: Uso e Manejo Sustentável de Recursos Naturais nos Agrossistemas da Amazônia Central; Souza, L.A.G., Netto, R.A.C., Hanada, R.E., Eds.; Editora do INPA: Manaus, Brazil, 2024; pp. 180–202. [Google Scholar]
- Prajapati, P.J.; Patel, J.N.; Patel, P.; Gowda, D.; Solanki, R. Heritability studies in tomato (Lycopersicon esculentum L.) genotypes. Int. J. Plant Soil. Sci. 2024, 36, 255–263. [Google Scholar] [CrossRef]
- El-Gabry, M.A.H.; Solieman, T.I.H.; Abido, A.I.A. Combining ability and heritability of some tomato (Solanum lycopersicum L.) cultivars. Sci. Hortic. 2014, 167, 153–157. [Google Scholar] [CrossRef]
- Gibrel, G.; Boe, A.A.; Simpson, W.R.; Everson, D.O. Evaluation of F1 hybrid tomato cultivars for earliness, fruit Size, and yield using diallel analysis. J. Am. Soc. Hortic. Sci. 1982, 107, 243–247. [Google Scholar] [CrossRef]
- Ordás, B.; Caicedo, M.; Romay, M.C.; Revilla, P.; Ordás, A. Effect of Visual Selection During the Development of Inbred Lines of Maize. Crop Sci. 2012, 52, 2538–2545. [Google Scholar] [CrossRef]
- Escamilla, D.M.; Huang, M.; McHale, L.; Wang, D.; Diers, B.; Xavier, A.; Rainey, K.M. Canopy coverage phenotyping and field spatial variability adjustment as an efficient selection tool in soybean breeding. Crop Sci. 2023, 63, 3277–3291. [Google Scholar] [CrossRef]
- Teixeira, D.H.L.; Gonçalves, F.M.A.; Nunes, J.A.R.; Sobrinho, F.S.; Benites, F.R.G.; Dias, K.O.d.G. Visual selection of Urochloa ruziziensis genotypes for green biomass yield. Acta Scientiarum. Agron. 2020, 42, e42444. [Google Scholar] [CrossRef]
- Cutrim, V.d.A.; Ramalho, M.A.P.; Carvalho, A.M. Eficiência da seleção visual na produtividade de grãos de arroz (Oryza sativa) irrigado. Pesqui. Agropecu. Bras. 1997, 32, 601–606. [Google Scholar]
- Bradshaw, J.E.; Dale, M.F.B.; Swan, G.E.L.; Todd, D.; Wilson, R.N. Early-generation selection between and within pair crosses in a potato (Solanum tuberosum subsp. tuberosum) breeding programme. Theor. Appl. Genet. 1998, 97, 1331–1339. [Google Scholar] [CrossRef]
- Sobreira, F.M.; Sobreira, F.M.; Fialho, G.S.; Barrera Sánchez, C.F.; Matta, F.d.P. Análise de trilha em pós-colheita de tomate tipo salada. Rev. Fac. Nac. Agron. Medellín 2009, 62, 4983–4988. [Google Scholar]
- Rodrigues, G.B.; Marim, B.G.; Silva, D.J.H.d.; Mattedi, A.P.; Almeida, V.d.S. Análise de trilha de componentes de produção primários e secundários em tomateiro do grupo Salada. Pesqui Agropec. Bras. 2010, 45, 155–162. [Google Scholar] [CrossRef][Green Version]
- INMET. Banco de Dados Metereologicos. Available online: https://bdmep.inmet.gov.br/ (accessed on 1 October 2024).[Green Version]
- Triola, M.F. Statdisk 13.0.1. Triola Stats. 2016. Available online: https://www.statdisk.com/static/downloads/Statdisk13Installer.exe (accessed on 1 October 2024).[Green Version]
- Roach, D.A.; Wulff, R.D. Maternal effects in plants. Annu. Rev. Ecol. Syst. 1987, 18, 209–235. [Google Scholar] [CrossRef]
- Kuppuraj, S.A.M.; Chokkalingam, Y.; Jothiganapathy, K.; Vedachalam, V.; Bisht, D.S.; Cholin, S.; Saminadane, T. Maternal effect on the inheritance of pericarp colour and grain dimension in rice (Oryza sativa L.). J. Genet. 2025, 104, 5. [Google Scholar] [CrossRef] [PubMed]



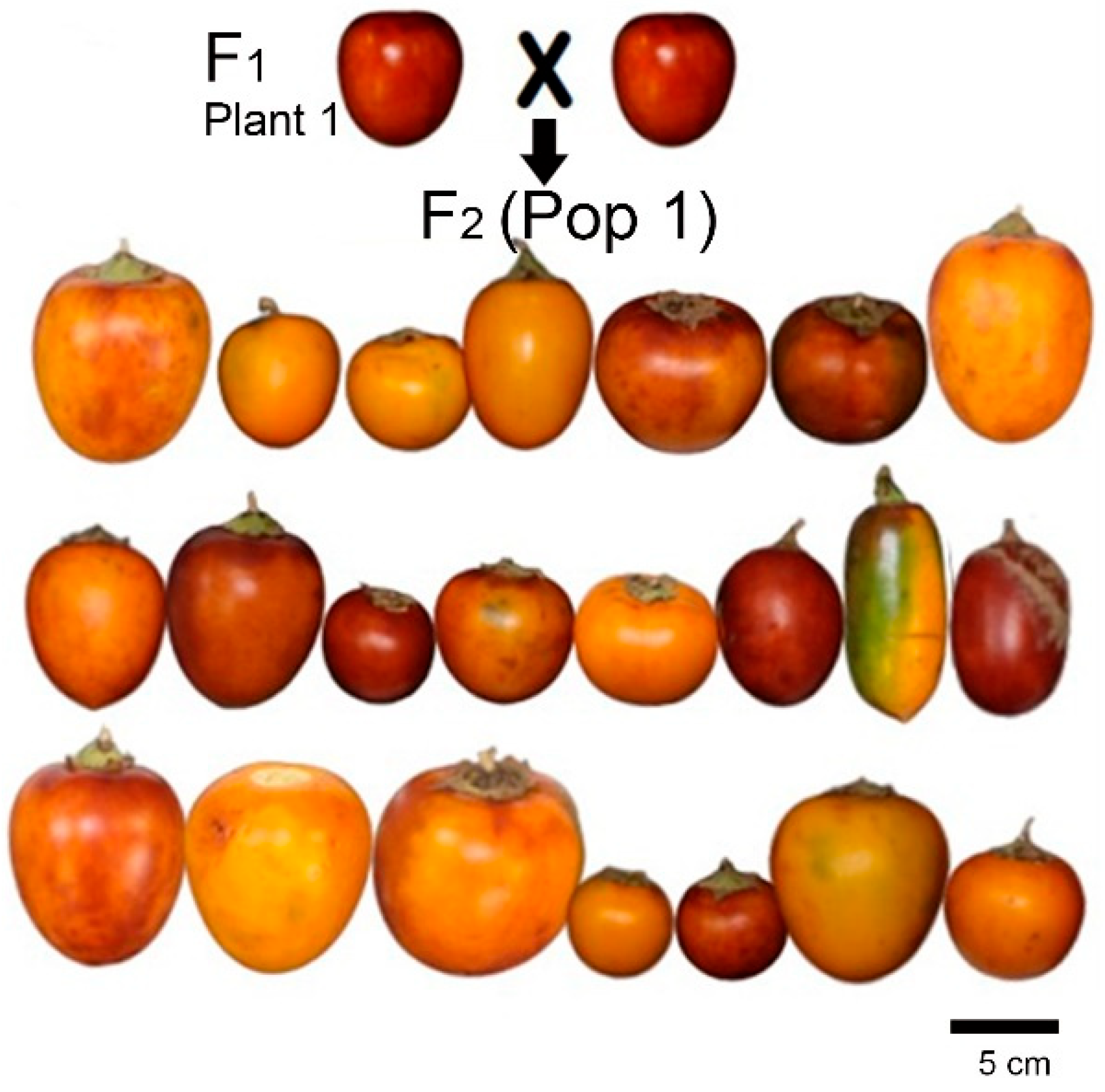
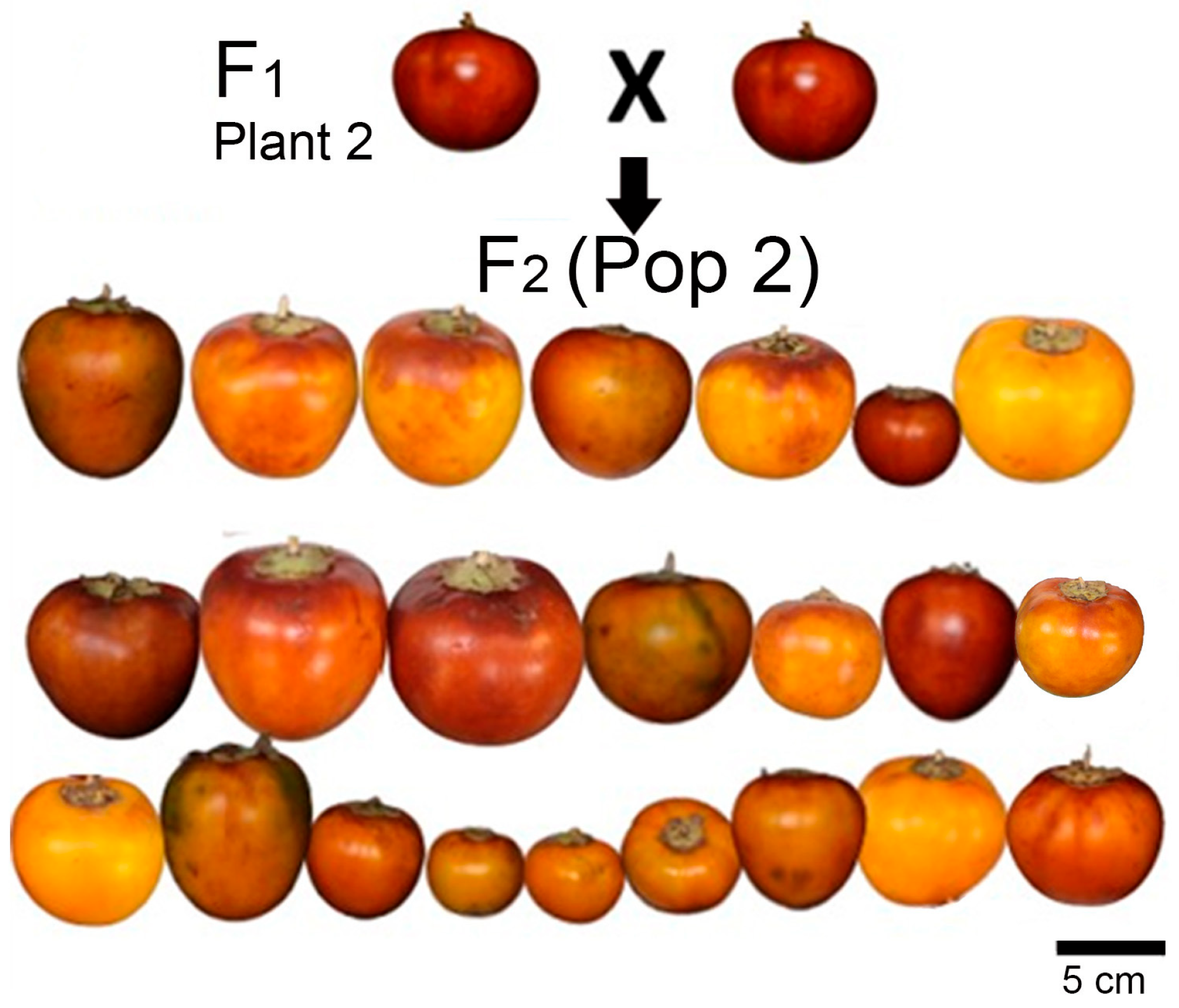
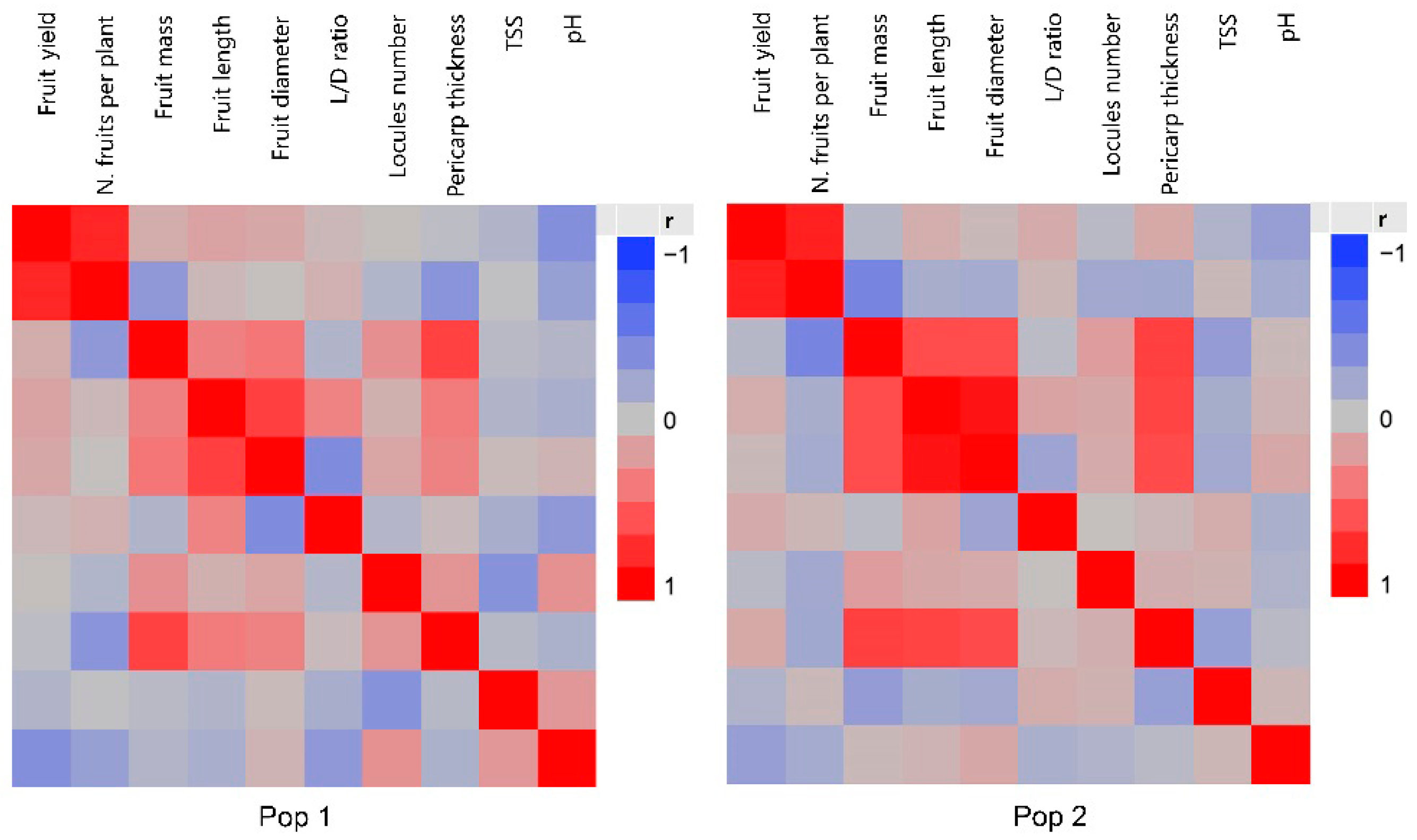
| Trait | P1 (n = 203) | P2 (n = 202) | CUB-4 (n = 7) | (Pop 1) § | (Pop 2) § | (Pop 1) | (Pop 2) |
|---|---|---|---|---|---|---|---|
| Plant height (cm) | 225.39 | 415.88 | 139.95 | 85.44 ** | 275.93 ** | 0.38 ** | 0.66 ** |
| Collar diameter (cm) | 0.18 | 0.28 | 0.08 | 0.10 ** | 0.20 ** | 0.55 ** | 0.71 ** |
| Leaf length (cm) | 55.04 | 67.36 | 31.81 | 23.23 ** | 35.55 ** | 0.42 ** | 0.52 ** |
| Leaf width (cm) | 32.82 | 39.93 | 105.81 | −72.99 | −65.88 | 0.00 | 0.00 |
| Petiole length (cm) | 5.67 | 13.18 | 1.62 | 4.05 ** | 11.56 ** | 0.71 ** | 0.87 ** |
| N° tillers | 0.70 | 0.85 | 1.00 | −0.30 | −0.15 | 0.00 | 0.00 |
| Canopy diameter (cm) | 382.82 | 431.82 | 179.67 | 203.15 ** | 252.15 ** | 0.53 ** | 0.58 ** |
| N° fruits per plant | 69.92 | 85.27 | 59.70 | 10.22 ** | 25.57 ** | 0.14 ** | 0.30 ** |
| N° flowers per plant | 574.26 | 570.80 | 67.81 | 506.45 ** | 502.99 ** | 0.88 ** | 0.88 ** |
| Plant vigor † | 0.64 | 2.06 | 4.07 | −3.43 | −2.01 | 0.00 | 0.00 |
| Fruit hairiness †† | 0.25 | 0.28 | <0.01 | 0.25 ** | 0.28 ** | 1.00 ** | 1.00 ** |
| Fruit length—L (cm) | 1.85 | 1.10 | 0.83 | 1.02 ** | 0.27 ** | 0.55 ** | 0.24 ** |
| Fruit diameter—D (cm) | 1.72 | 1.25 | 0.88 | 0.84 ** | 0.37 ** | 0.48 ** | 0.29 ** |
| L/D ratio | 0.0108 | 0.0128 | 0.0107 | 0.021 ** | 0.0002 ** | 0.38 ** | 0.27 ** |
| Trait | Pop 1 (n = 203) | Pop 2 (n = 202) | CUB-4 (C) (n = 7) | Δ Pop 1-C § | Δ Pop 2-C § |
|---|---|---|---|---|---|
| Plant height (cm) | 79.51 (15.01) ††† | 97.28 (20.39) | 61.57 (11.83) | 17.94 ** | 35.71 ** |
| Collar diameter (cm) | 2.50 (0.43) | 2.60 (0.53) | 2.20 (0.28) | 0.30 ** | 0.40 ** |
| Leaf length (cm) | 51.84 (7.24) | 55.41(8.21) | 46.86 (5.64) | 4.98 ** | 8.55 ** |
| Leaf width (cm) | 38.59 (5.73) | 40.72 (6.32) | 39.14 (10.29) | −0.55 | 1.58 |
| Petiole length (cm) | 9.16 (2.38) | 10.90 (3.63) | 8.43 (1.27) | 0.73 | 2.47 ** |
| N° tillers | 1.92 (0.84) | 1.61 (0.92) | 2.00 (1.00) | −0.08 | −0.39 |
| Canopy diameter (cm) | 117.15 (19.57) | 126.69 (20.78) | 116.00 (13.40) | 1.15 | 10.69 |
| N° fruits per plant | 12.05 (8.36) | 12.35 (9.23) | 12.71 (9.41) | −0.66 | −0.36 |
| N° flowers per plant | 60.45 (23.96) | 62.79 (23.89) | 48.86 (8.23) | 11.59 ** | 13.93 ** |
| Plant vigor † | 1.37 (0.80) | 1.80 (1.43) | 2.45 (2.02) | −1.08 | −0.65 |
| Fruit hairiness †† | 2.64 (0.50) | 2.56 (0.53) | 3.00 (0.00) | −0.36 | −0.44 ** |
| Fruit length—L (cm) | 4.81 (1.36) | 4.58 (1.05) | 3.23 (0.91) | 1.58 ** | 1.35 ** |
| Fruit diameter—D (cm) | 4.41 (1.31) | 4.70 (1.12) | 3.04 (0.94) | 1.37 ** | 1.66 ** |
| L/D ratio | 1.11 (0.10) | 0.98 (0.11) | 1.08 (0.10) | 0.03 | −0.10 ** |
| Plant | Code | Fruit Yield (t ha−1) | Fruit Number per Plant | Fruit Mass (g) | Fruit Length (L) (cm) | Fruit Diameter (D) (cm) | L/D Ratio | Locule Number | Pericarp Thickness (mm) | Total Soluble Solids (Brix) | pH |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | P1-198 | 12.99 | 14 | 139.21 | 6.25 | 7.25 | 0.86 | 4.67 | 0.73 | 5.10 | 3.11 |
| 2 | P1-113 | 11.61 | 18 | 96.76 | 6.63 | 6.15 | 1.08 | 4.67 | 0.71 | 4.90 | 3.73 |
| 3 | P1-18 | 11.37 | 29 | 58.79 | 5.30 | 4.96 | 1.07 | 4.67 | 0.40 | 5.07 | 5.00 |
| 4 | P1-15 | 10.57 | 29 | 54.66 | 4.83 | 4.43 | 1.09 | 4.00 | 0.44 | 4.73 | 2.88 |
| 5 | P1-215 | 9.63 | 16 | 90.31 | 6.25 | 4.75 | 1.32 | 4.00 | 0.80 | 4.10 | 2.94 |
| 6 | P1-98 | 9.60 | 23 | 62.61 | 5.80 | 5.53 | 1.05 | 4.00 | 0.47 | 4.93 | 5.50 |
| 7 | P1-195 | 8.63 | 20 | 64.75 | 6.28 | 4.70 | 1.34 | 5.00 | 0.43 | 5.10 | 6.30 |
| 8 | P1-79 | 8.13 | 19 | 64.21 | 4.20 | 3.50 | 1.20 | 4.00 | 0.47 | 4.77 | 3.22 |
| 9 | P1-17 | 7.78 | 7 | 166.67 | 4.25 | 4.43 | 0.96 | 4.67 | 0.77 | 5.03 | 5.70 |
| 10 | P1-16 | 7.43 | 13 | 85.77 | 6.40 | 5.65 | 1.13 | 3.00 | 0.65 | 4.95 | 3.16 |
| 11 | P1-214 | 7.33 | 18 | 61.11 | 4.24 | 6.24 | 0.68 | 4.00 | 0.56 | 5.03 | 4.17 |
| 12 | P1-95 | 7.27 | 7 | 155.71 | 8.40 | 7.50 | 1.12 | 5.00 | 1.14 | 3.95 | 3.06 |
| 13 | P1-196 | 7.10 | 15 | 71.00 | 6.00 | 4.80 | 1.25 | 4.00 | 0.60 | 5.00 | 3.26 |
| 14 | P1-219 | 6.87 | 14 | 73.57 | 5.90 | 5.40 | 1.09 | 4.00 | 0.50 | 4.97 | 5.70 |
| 15 | P1-267 | 6.87 | 15 | 68.67 | 4.60 | 4.00 | 1.15 | 4.00 | 0.60 | 4.70 | 2.98 |
| 16 | P1-252 | 6.83 | 18 | 56.94 | 5.63 | 5.23 | 1.08 | 4.00 | 0.47 | 5.03 | 3.87 |
| 17 | P1-197 | 6.72 | 22 | 45.85 | 6.28 | 4.70 | 1.34 | 4.67 | 0.60 | 5.33 | 4.70 |
| 18 | P1-13 | 6.43 | 16 | 60.31 | 6.00 | 6.37 | 0.94 | 4.00 | 0.67 | 4.97 | 5.43 |
| 19 | P1-206 | 6.09 | 14 | 65.31 | 5.28 | 5.12 | 1.03 | 4.67 | 0.53 | 5.00 | 4.00 |
| 20 | P1-178 | 6.05 | 9 | 100.91 | 5.33 | 4.77 | 1.12 | 4.67 | 0.57 | 4.77 | 2.90 |
| 21 | P1-199 | 5.81 | 8 | 108.87 | 6.56 | 6.28 | 1.04 | 4.00 | 0.63 | 5.03 | 4.06 |
| 22 | P1-168 | 5.80 | 22 | 39.55 | 7.33 | 7.50 | 0.98 | 4.00 | 0.33 | 5.03 | 4.53 |
| 23 | P1-181 | 5.40 | 14 | 57.86 | 5.66 | 4.54 | 1.25 | 4.00 | 0.40 | 4.43 | 3.75 |
| 24 | P1-220 | 5.38 | 7 | 115.29 | 6.23 | 6.30 | 0.99 | 4.00 | 0.60 | 4.77 | 5.70 |
| 25 | P1-190 | 5.30 | 14 | 56.79 | 5.27 | 4.67 | 1.13 | 4.00 | 0.53 | 5.03 | 4.57 |
| 26 | P1-214 | 5.00 | 8 | 93.75 | 7.50 | 4.70 | 1.60 | - | - | - | - |
| 27 | P1-228 | 4.21 | 8 | 78.92 | 6.35 | 5.50 | 1.15 | 4.00 | 0.50 | 4.60 | 4.11 |
| 28 | P1-173 | 3.93 | 5 | 118.00 | 5.20 | 5.50 | 0.95 | 4.67 | 0.53 | 5.00 | 5.20 |
| 29 | P1-275 | 3.73 | 8 | 70.00 | 5.50 | 4.65 | 1.18 | 4.00 | 0.40 | 5.10 | 4.90 |
| 30 | P1-32 | 2.95 | 12 | 36.82 | 4.46 | 4.52 | 0.99 | 4.00 | 0.49 | 5.00 | - |
| 31 | P1-175 | 2.66 | 10 | 39.91 | 7.17 | 7.33 | 0.98 | 4.00 | 0.33 | 5.10 | 5.73 |
| 32 | P1-274 | 2.57 | 2 | 192.50 | 6.85 | 7.70 | 0.89 | 5.00 | 1.15 | 5.05 | 5.75 |
| 33 | P1-97 | 1.97 | 4 | 73.75 | 4.35 | 4.15 | 1.05 | 4.00 | - | - | - |
| 34 | P1-176 | 1.50 | 3 | 75.00 | 5.20 | 5.50 | 0.95 | - | - | - | - |
| 35 | P1-62 | 1.37 | 3 | 68.33 | 5.57 | 5.13 | 1.08 | 4.00 | 0.43 | 5.40 | - |
| 36 | P1-255 | 1.03 | 2 | 77.50 | 4.80 | 5.00 | 0.96 | 4.00 | 0.60 | 5.10 | 5.30 |
| 37 | P1-145 | 0.90 | 1 | 135.00 | 5.50 | 5.80 | 0.95 | 4.00 | 0.80 | 6.00 | 5.00 |
| 38 | P1-133 | 0.87 | 1 | 130.00 | 7.20 | 5.60 | 1.29 | 5.00 | 0.80 | 4.10 | 4.90 |
| 39 | P1-254 | 0.80 | 2 | 60.00 | 3.07 | 3.17 | 0.97 | 4.67 | 0.33 | 4.10 | 5.00 |
| 40 | P1-34 | 0.70 | 3 | 35.00 | 5.20 | 5.70 | 0.91 | 6.00 | 0.60 | 4.10 | - |
| 41 | P1-36 | 0.70 | 2 | 52.50 | 5.20 | 4.60 | 1.13 | 4.00 | 0.85 | 5.70 | - |
| 42 | P1-177 | 0.70 | 1 | 105.00 | 6.90 | 5.50 | 1.25 | 4.00 | 0.70 | 5.10 | 2.93 |
| 43 | P1-158 | 0.60 | 2 | 45.00 | 4.50 | 4.00 | 1.13 | 4.00 | 0.45 | 5.10 | 5.00 |
| 44 | P1-61 | 0.33 | 1 | 50.00 | 5.20 | 4.60 | 1.13 | 4.00 | 0.40 | 4.90 | - |
| 45 | P1-101 | 0.33 | 1 | 50.00 | 5.00 | 4.50 | 1.11 | 4.00 | 0.90 | 5.00 | 5.20 |
| Mean | 5.11 | 10.67 | 80.19 | 5.68 | 5.29 | 1.09 | 4.26 | 0.59 | 4.91 | 4.41 | |
| s2 | 12.08 | 61.91 | 1308.18 | 1.09 | 1.11 | 0.03 | 0.24 | 0.04 | 0.16 | 1.08 | |
| CUB-4 | 3.60 | 5.23 | 35.70 | 3.98 | 4.06 | 0.98 | 4.00 | 0.36 | 5.10 | 4.41 |
| Plant | Code | Fruit Yield (t ha−1) | Fruit Number per Plant | Fruit Mass (g) | Fruit Length (L) (cm) | Fruit Diameter (D) (cm) | L/D Ratio | Locule Number | Pericarp Thickness (mm) | Total Soluble Solids (Brix) | pH |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | P2-27 | 15.62 | 29 | 80.80 | 5.54 | 5.38 | 1.03 | 4.00 | 0.53 | 5.23 | 3.19 |
| 2 | P2-117 | 14.87 | 22 | 101.36 | 6.14 | 6.04 | 1.02 | 4.00 | 0.60 | 4.77 | 4.10 |
| 3 | P2-177 | 14.63 | 14 | 156.79 | 6.40 | 6.60 | 0.97 | 6.00 | 0.75 | 4.10 | 3.15 |
| 4 | P2-4 | 13.53 | 18 | 112.78 | 5.32 | 5.24 | 1.02 | 4.67 | 0.63 | 5.03 | 5.45 |
| 5 | P2-87 | 12.50 | 28 | 66.96 | 5.60 | 5.86 | 0.96 | 4.00 | 0.77 | 5.10 | 3.38 |
| 6 | P2-115 | 12.23 | 31 | 59.19 | 5.28 | 4.90 | 1.08 | 4.00 | 0.53 | 4.77 | 3.59 |
| 7 | P2-173 | 12.13 | 20 | 91.00 | 5.93 | 5.68 | 1.04 | 4.00 | 0.80 | 4.50 | 3.03 |
| 8 | P2-206 | 12.12 | 27 | 67.36 | 5.36 | 5.44 | 0.99 | 4.00 | 0.61 | 5.07 | - |
| 9 | P2-204 | 11.80 | 29 | 61.03 | 5.30 | 5.36 | 0.99 | 4.00 | 0.47 | 5.00 | 5.70 |
| 10 | P2-198 | 11.53 | 34 | 50.85 | 4.64 | 4.74 | 0.98 | 4.00 | 0.33 | 5.10 | 4.44 |
| 11 | P2-269 | 11.50 | 17 | 101.47 | 6.68 | 5.83 | 1.15 | 4.00 | 0.77 | 3.77 | 5.32 |
| 12 | P2-29 | 11.13 | 17 | 98.24 | 5.80 | 6.00 | 0.97 | 4.50 | 0.75 | 4.60 | 3.12 |
| 13 | P2-84 | 11.07 | 18 | 92.22 | 5.94 | 5.66 | 1.05 | 4.00 | 0.60 | 4.90 | 5.67 |
| 14 | P2-175 | 10.77 | 19 | 85.00 | 6.20 | 5.15 | 1.20 | 4.67 | 0.69 | 4.97 | 3.25 |
| 15 | P2-33 | 10.50 | 34 | 46.32 | 4.60 | 4.96 | 0.93 | 4.00 | 0.40 | 5.40 | 4.90 |
| 16 | P2-256 | 10.37 | 20 | 77.75 | 5.72 | 5.56 | 1.03 | 4.00 | 0.63 | 4.77 | 5.60 |
| 17 | P2-163 | 10.20 | 17 | 90.00 | 5.65 | 5.10 | 1.11 | 4.00 | 0.81 | 5.00 | - |
| 18 | P2-151 | 9.87 | 13 | 113.85 | 6.10 | 6.13 | 1.00 | 4.67 | 0.67 | 4.93 | 4.14 |
| 19 | P2-153 | 9.87 | 30 | 49.33 | 4.70 | 4.64 | 1.01 | 4.00 | 0.48 | 5.10 | - |
| 20 | P2-236 | 9.70 | 13 | 111.92 | 6.63 | 6.38 | 1.04 | 4.00 | 0.66 | 4.80 | 3.37 |
| 21 | P2-202 | 9.66 | 16 | 90.56 | 5.80 | 7.03 | 0.83 | 4.00 | 0.81 | 5.05 | 4.70 |
| 22 | P2-234 | 9.53 | 16 | 89.38 | 5.58 | 5.58 | 1.00 | 4.00 | 0.53 | 5.10 | 4.70 |
| 23 | P2-34 | 9.30 | 13 | 107.31 | 5.86 | 6.12 | 0.96 | 4.00 | 0.77 | 3.77 | 3.68 |
| 24 | P2-23 | 9.23 | 12 | 115.42 | 6.07 | 6.23 | 0.97 | 5.00 | 0.73 | 4.43 | 3.00 |
| 25 | P2-122 | 9.09 | 20 | 68.21 | 5.46 | 5.50 | 0.99 | 4.67 | 0.70 | 4.90 | 4.97 |
| 26 | P2-54 | 8.70 | 22 | 59.32 | 5.00 | 5.30 | 0.94 | 4.33 | 0.53 | 5.10 | 4.20 |
| 27 | P2-28 | 8.37 | 18 | 69.72 | 5.35 | 5.38 | 1.00 | 4.00 | 0.57 | 4.43 | 4.90 |
| 28 | P2-268 | 8.37 | 14 | 89.64 | 5.68 | 5.92 | 0.96 | 4.67 | 0.63 | 4.43 | 5.47 |
| 29 | P2-105 | 8.33 | 15 | 83.33 | 5.83 | 5.80 | 1.01 | 4.00 | 0.53 | 5.00 | 4.34 |
| 30 | P2-220 | 7.93 | 16 | 74.38 | 5.70 | 5.85 | 0.97 | 4.00 | 0.63 | 4.43 | 3.70 |
| 31 | P2-5 | 7.80 | 15 | 78.00 | 5.50 | 5.47 | 1.01 | 5.00 | 0.60 | 5.43 | 2.98 |
| 32 | P2-6 | 7.63 | 25 | 45.80 | 4.40 | 4.75 | 0.93 | 4.00 | 0.49 | 5.00 | 5.47 |
| 33 | P2-79 | 7.53 | 20 | 56.50 | 4.77 | 4.90 | 0.97 | 4.00 | 0.47 | 4.97 | - |
| 34 | P2-190 | 7.51 | 15 | 75.13 | 4.24 | 4.12 | 1.03 | 4.00 | 0.63 | 5.13 | 5.00 |
| 35 | P2-162 | 7.47 | 19 | 58.95 | 5.13 | 4.58 | 1.12 | 4.00 | 0.52 | 4.93 | 6.67 |
| 36 | P2-138 | 7.30 | 22 | 49.77 | 4.40 | 4.60 | 0.96 | 4.00 | 0.50 | 4.90 | 2.98 |
| 37 | P2-217 | 7.17 | 13 | 82.69 | 5.46 | 5.04 | 1.08 | 4.67 | 0.71 | 4.97 | 2.82 |
| 38 | P2-170 | 6.96 | 12 | 87.00 | 6.10 | 6.24 | 0.98 | 5.67 | 0.83 | 5.10 | 3.60 |
| 39 | P2-92 | 6.93 | 17 | 61.18 | 5.08 | 5.42 | 0.94 | 4.33 | 0.37 | 3.03 | 4.50 |
| 40 | P2-93 | 6.93 | 9 | 115.56 | 6.35 | 6.63 | 0.96 | 5.00 | 0.73 | 5.10 | 5.26 |
| 41 | P2-180 | 6.87 | 14 | 73.59 | 5.20 | 5.25 | 0.99 | 5.50 | 0.50 | 4.90 | - |
| 42 | P2-25 | 6.87 | 15 | 68.67 | 4.04 | 4.10 | 0.99 | 5.00 | 0.51 | 5.17 | 4.56 |
| 43 | P2-114 | 6.87 | 11 | 93.64 | 5.63 | 6.13 | 0.92 | 4.00 | 0.83 | 3.77 | 6.30 |
| 44 | P2-187 | 6.80 | 23 | 44.35 | 4.20 | 4.40 | 0.95 | 4.00 | 0.40 | 4.97 | 5.30 |
| 45 | P2-148 | 6.59 | 11 | 89.88 | 3.17 | 3.37 | 0.94 | 4.00 | 0.60 | 4.43 | 3.02 |
| 46 | P2-161 | 6.00 | 20 | 45.00 | 3.85 | 4.10 | 0.94 | 4.00 | 0.51 | 4.95 | - |
| 47 | P2-179 | 5.30 | 13 | 61.15 | 4.86 | 5.00 | 0.97 | 4.67 | 0.53 | 5.20 | 6.05 |
| 48 | P2-288 | 5.13 | 5 | 154.00 | 6.75 | 5.90 | 1.14 | 5.33 | 0.80 | 4.77 | 2.97 |
| 49 | P2-52 | 5.06 | 12 | 63.25 | 5.46 | 5.38 | 1.01 | 5.33 | 0.47 | 5.13 | - |
| 50 | P2-8 | 5.03 | 14 | 53.93 | 4.86 | 4.72 | 1.03 | 5.00 | 0.47 | 5.10 | 4.90 |
| 51 | P2-121 | 4.53 | 6 | 113.33 | 5.50 | 6.93 | 0.79 | 4.00 | 0.70 | 5.00 | 6.03 |
| 52 | P2-86 | 3.73 | 6 | 93.33 | 5.60 | 5.86 | 0.96 | 4.00 | 0.63 | 4.77 | 4.30 |
| 53 | P2-82 | 3.57 | 8 | 66.88 | 5.83 | 6.00 | 0.97 | 4.00 | 0.50 | 4.10 | 3.78 |
| 54 | P2-19 | 3.10 | 7 | 66.43 | 5.24 | 5.16 | 1.02 | 4.00 | 0.47 | 5.10 | 4.87 |
| 55 | P2-287 | 3.03 | 3 | 151.67 | 6.67 | 6.33 | 1.05 | 4.67 | 0.87 | 4.87 | 5.67 |
| 56 | P2-189 | 2.90 | 5 | 87.00 | 5.20 | 5.40 | 0.96 | 4.67 | 0.57 | 5.20 | 6.30 |
| 57 | P2-2 | 2.73 | 4 | 102.50 | 5.10 | 5.23 | 0.97 | 6.00 | 0.50 | 4.77 | 4.90 |
| 58 | P2-144 | 2.67 | 9 | 44.44 | 4.50 | 4.45 | 1.01 | 4.00 | 0.45 | 5.50 | 3.03 |
| 59 | P2-61 | 2.50 | 7 | 53.57 | 4.64 | 4.72 | 0.98 | 4.67 | 0.50 | 5.10 | 4.37 |
| 60 | P-26 | 2.47 | 4 | 92.50 | 5.10 | 5.50 | 0.93 | 5.33 | 0.57 | 4.77 | 4.90 |
| 61 | P2-123 | 2.40 | 3 | 120.00 | 6.00 | 5.93 | 1.01 | 4.00 | 0.57 | 4.93 | 5.00 |
| 62 | P2-21 | 2.30 | 4 | 86.25 | 5.30 | 5.58 | 0.95 | 5.33 | 0.57 | 5.67 | 4.80 |
| 63 | P2-55 | 2.13 | 2 | 160.00 | 6.15 | 6.70 | 0.92 | 4.67 | 0.67 | 4.77 | - |
| 64 | P2-50 | 1.97 | 4 | 73.75 | 4.88 | 4.95 | 0.98 | 4.00 | 0.50 | - | - |
| 65 | P2-20 | 1.87 | 6 | 46.67 | 4.84 | 4.64 | 1.04 | 6.33 | 0.47 | 5.13 | 4.80 |
| 66 | P2-239 | 1.87 | 2 | 140.00 | 4.00 | 4.57 | 0.88 | 4.00 | 0.60 | 4.10 | - |
| 67 | P2-145 | 1.80 | 2 | 135.00 | 5.85 | 6.35 | 0.92 | 4.00 | 0.67 | 5.10 | 4.93 |
| 68 | P2-66 | 1.57 | 3 | 78.33 | 5.27 | 5.53 | 0.95 | 4.00 | 0.57 | 4.77 | 4.95 |
| 69 | P2-56 | 1.40 | 2 | 105.00 | 5.80 | 6.25 | 0.93 | 4.67 | 0.80 | 4.43 | - |
| 70 | P2-57 | 1.33 | 2 | 100.00 | 5.75 | 5.00 | 1.15 | 4.00 | 0.65 | 5.00 | - |
| 71 | P2-67 | 1.13 | 2 | 85.00 | 5.15 | 5.30 | 0.97 | 4.00 | 0.50 | 4.77 | 4.85 |
| 72 | P2-188 | 1.10 | 1 | 165.00 | 10.80 | 11.50 | 0.94 | - | - | - | - |
| 73 | P2-60 | 1.00 | 3 | 50.00 | 4.33 | 4.40 | 0.98 | 4.00 | 0.50 | 5.13 | - |
| 74 | P2-141 | 0.97 | 2 | 72.50 | 4.65 | 4.75 | 0.98 | 4.00 | 0.55 | 5.10 | 4.90 |
| 75 | P2-149 | 0.80 | 1 | 120.00 | 5.60 | 5.70 | 0.98 | 4.00 | 0.70 | 4.20 | 4.90 |
| 76 | P2-7 | 0.73 | 1 | 110.00 | 4.70 | 4.50 | 1.04 | 4.00 | 0.60 | 5.20 | 4.90 |
| 77 | P2-74 | 0.57 | 1 | 85.00 | 5.10 | 4.70 | 1.09 | 4.00 | 0.70 | 4.10 | 4.95 |
| 78 | P2-119 | 0.47 | 1 | 70.26 | 4.20 | 4.70 | 0.89 | - | - | - | - |
| 79 | P2-285 | 0.47 | 1 | 70.00 | 5.10 | 4.50 | 1.13 | 4.00 | 0.50 | 6.00 | - |
| 80 | P2-72 | 0.33 | 1 | 50.00 | 5.30 | 5.10 | 1.04 | 4.00 | 0.40 | 5.10 | 4.85 |
| Mean | 6.45 | 12.75 | 85.17 | 5.39 | 5.45 | 0.99 | 4.37 | 0.60 | 4.85 | 4.52 | |
| s2 | 17.16 | 81.10 | 865.14 | 0.87 | 0.99 | 0.00 | 0.33 | 0.02 | 0.21 | 0.97 | |
| CUB-4 | 3.60 | 5.23 | 35.70 | 3.98 | 4.06 | 0.98 | 4.00 | 0.36 | 5.10 | 4.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, L.S.e.; Ticona-Benavente, C.A. Heritability of Morpho-Agronomic Traits in Cocona (Solanum sessiliflorum Dunal) and Efficiency of Early Visual Selection for Fruit Yield. Int. J. Plant Biol. 2025, 16, 121. https://doi.org/10.3390/ijpb16040121
Silva LSe, Ticona-Benavente CA. Heritability of Morpho-Agronomic Traits in Cocona (Solanum sessiliflorum Dunal) and Efficiency of Early Visual Selection for Fruit Yield. International Journal of Plant Biology. 2025; 16(4):121. https://doi.org/10.3390/ijpb16040121
Chicago/Turabian StyleSilva, Leandro Sousa e, and César Augusto Ticona-Benavente. 2025. "Heritability of Morpho-Agronomic Traits in Cocona (Solanum sessiliflorum Dunal) and Efficiency of Early Visual Selection for Fruit Yield" International Journal of Plant Biology 16, no. 4: 121. https://doi.org/10.3390/ijpb16040121
APA StyleSilva, L. S. e., & Ticona-Benavente, C. A. (2025). Heritability of Morpho-Agronomic Traits in Cocona (Solanum sessiliflorum Dunal) and Efficiency of Early Visual Selection for Fruit Yield. International Journal of Plant Biology, 16(4), 121. https://doi.org/10.3390/ijpb16040121





