The Qualitative and Quantitative Relationship of Lettuce Grown in Soilless Systems in a Mediterranean Greenhouse
Abstract
1. Introduction
2. Materials and Methods
2.1. Location, Plant Material, and Experimental Set-Up
2.2. Growing Conditions
2.3. Morphological and Physiological Measurements
2.4. Determination of Chlorophyll and Carotenoid Content in Lettuce Leaves
2.5. Antioxidant Compound Extraction
2.6. Phenol Content (PC), 2,2-Diphenyl-1-Picrylhydrazyl (DPPH), and 2,20-Azino-Bis Acid (3-Ethylbenzothiazolin-6-Sulfonic Acid (ABTS) Assays
2.7. Greenhouse Covering Materials and Measurements
2.8. Statistical Analysis
3. Results
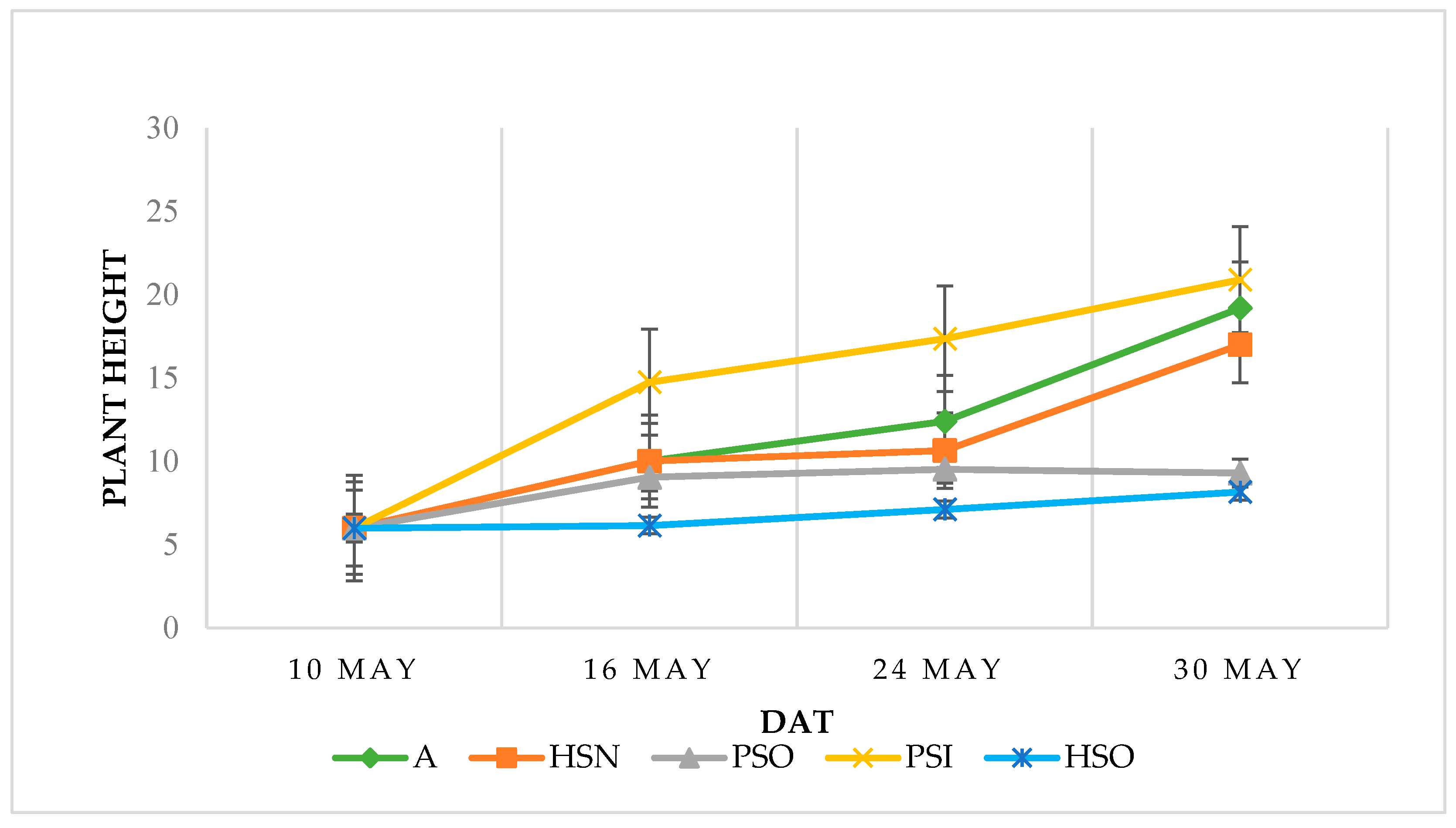
3.1. Morphological and Physiological Parameters
3.2. Chlorophyll, Carotenoid Content, and Antioxidant Activity
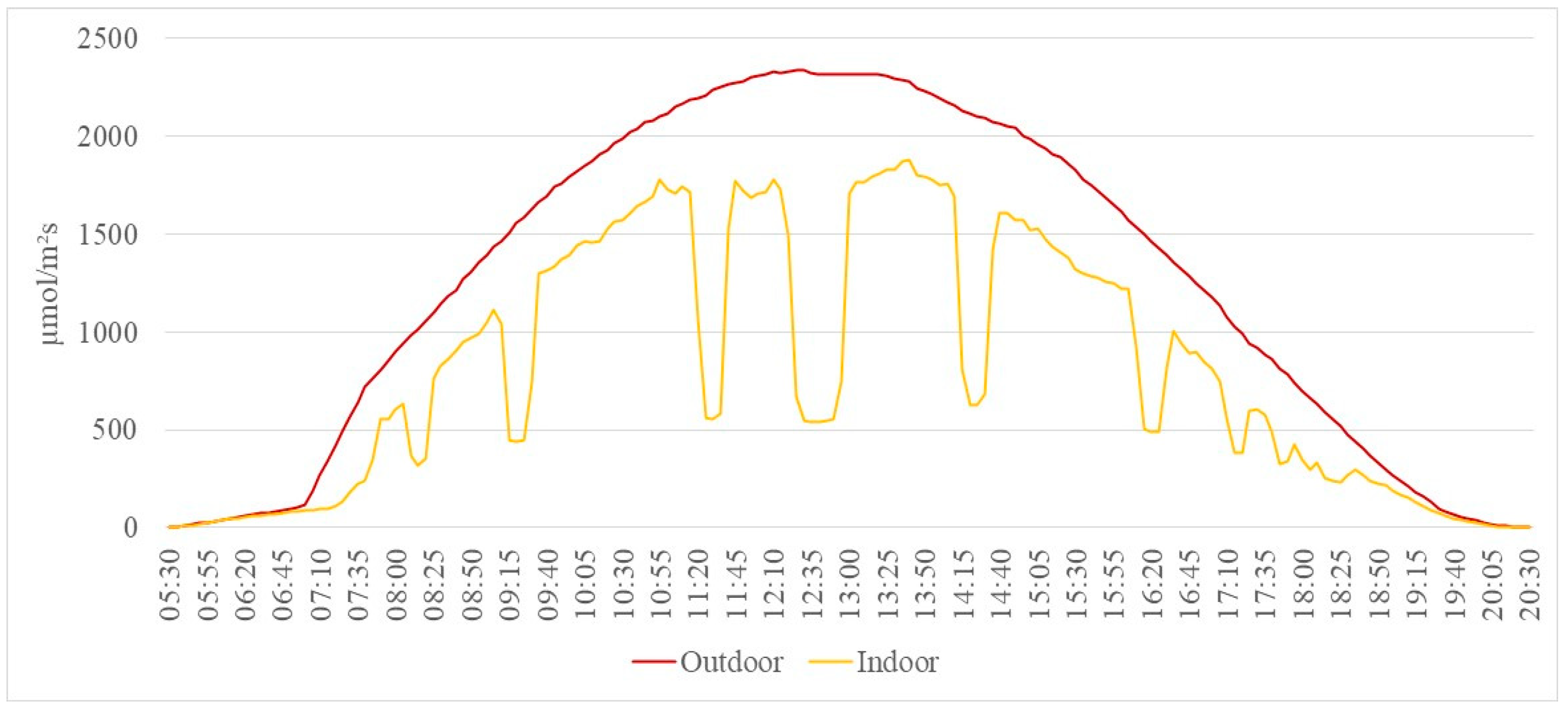
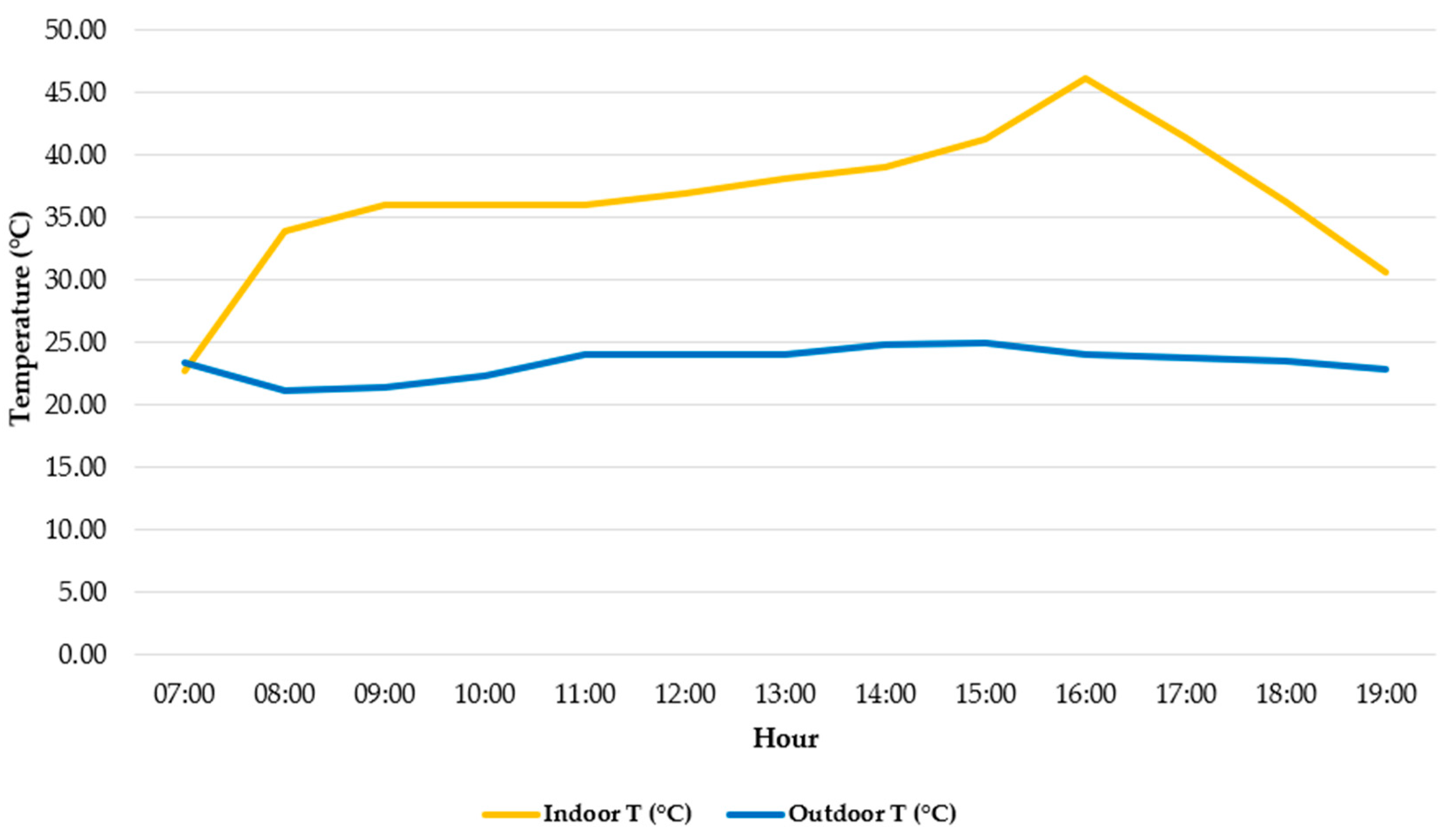
3.3. Total Irradiance, e-PAR, and Temperature
3.4. PCA Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Li, X.; Tang, B.; Gu, M. Growth Responses and Root Characteristics of Lettuce Grown in Aeroponics, Hydroponics, and Substrate Culture. Horticulturae 2018, 4, 35. [Google Scholar] [CrossRef]
- Impallomeni, G.; Filianoti, F.; Barreca, F. The Role of Agriculture for the European Energy Requests: Agrivoltaic Systems. In Proceedings of the Biosystems Engineering Promoting Resilience to Climate Change—AIIA 2024—Mid-Term Conference—Lecture Notes in Civil Engineering, Padova, Italy, 17–19 June 2024; Volume 586, pp. 776–782. [Google Scholar]
- Impallomeni, G.; Barreca, F. Agrivoltaic Systems towards the European Green Deal and Agricultural Policies: A Review. J. Agric. Eng. 2024, 56. [Google Scholar] [CrossRef]
- Majid, M.; Khan, J.N.; Ahmad Shah, Q.M.; Masoodi, K.Z.; Afroza, B.; Parvaze, S. Evaluation of Hydroponic Systems for the Cultivation of Lettuce (Lactuca sativa L., Var. Longifolia) and Comparison with Protected Soil-Based Cultivation. Agric. Water Manag. 2021, 245, 106572. [Google Scholar] [CrossRef]
- Lambin, E.F.; Meyfroidt, P. Global Land Use Change, Economic Globalization, and the Looming Land Scarcity. Proc. Natl. Acad. Sci. USA 2011, 108, 3465–3472. [Google Scholar] [CrossRef] [PubMed]
- Badji, A.; Benseddik, A.; Bensaha, H.; Boukhelifa, A.; Hasrane, I. Design, Technology, and Management of Greenhouse: A Review. J. Clean. Prod. 2022, 373, 133753. [Google Scholar] [CrossRef]
- Choab, N.; Allouhi, A.; El Maakoul, A.; Kousksou, T.; Saadeddine, S.; Jamil, A. Review on Greenhouse Microclimate and Application: Design Parameters, Thermal Modeling and Simulation, Climate Controlling Technologies. Sol. Energy 2019, 191, 109–137. [Google Scholar] [CrossRef]
- Peneyra, M.; Peneyra, M.B.; Peneyra, J.L. The Effect on the Growth and Yield of Green Leaf Romaine Lettuce (Lactuca sativa L.) in a Vertical Aeroponic System. Agric. Eng. Int. CIGR J. 2024, 26, 162–172. [Google Scholar]
- De Pascale, S.; Maggio, A.; Barbieri, G. La Sostenibilità Delle Colture Protette in Ambiente Mediterraneo: Limiti e Prospettive. Italus Hortus 2006, 13, 33–48. [Google Scholar]
- Abou Hadid, A.F. Strategic Crop and Greenhouse Management in Mild Winter Climate Areas. Acta Hortic. 2004, 633, 183–196. [Google Scholar] [CrossRef]
- Tunio, M.H.; Gao, J.; Mohamed, T.M.K.; Ahmad, F.; Abbas, I.; Shaikh, S.A. Comparison of Nutrient Use Efficiency, Antioxidant Assay, and Nutritional Quality of Butter-Head Lettuce (Lactuca sativa L.) in Five Cultivation Systems. Int. J. Agric. Biol. Eng. 2023, 16, 95–103. [Google Scholar] [CrossRef]
- Lee, J.S.; Chandra, D.; Son, J. Growth, Physicochemical, Nutritional, and Postharvest Qualities of Leaf Lettuce (Lactuca sativa L.) as Affected by Cultivar and Amount of Applied Nutrient Solution. Horticulturae 2022, 8, 436. [Google Scholar] [CrossRef]
- Selma, M.V.; Luna, M.C.; Martínez-Sánchez, A.; Tudela, J.A.; Beltrán, D.; Baixauli, C.; Gil, M.I. Sensory Quality, Bioactive Constituents and Microbiological Quality of Green and Red Fresh-Cut Lettuces (Lactuca sativa L.) Are Influenced by Soil and Soilless Agricultural Production Systems. Postharvest Biol. Technol. 2012, 63, 16–24. [Google Scholar] [CrossRef]
- Tittonell, P.; De Grazia, J.; Chiesa, A. Effect of Nitrogen Fertilization and Plant Population during Growth on Lettuce (Lactuca sativa L.) Postharvest Quality. Acta Hortic. 2001, 553, 67–68. [Google Scholar] [CrossRef]
- Kumar, T.V.; Verma, R. A Comprehensive Review on Soilless Cultivation for Sustainable Agriculture. J. Exp. Agric. Int. 2024, 46, 193–207. [Google Scholar] [CrossRef]
- Chowdhury, M.; Islam, N.; Reza, N.; Ali, M.; Rasool, K.; Kiraga, S.; Lee, D.-H.; Chung, S.-O. Sensor-Based Nutrient Recirculation for Aeroponic Lettuce Cultivation. J. Biosyst. Eng. 2021, 46, 81–92. [Google Scholar] [CrossRef]
- Scuderi, D.; Restuccia, C.; Chisari, M.; Barbagallo, R.N.; Caggia, C.; Giuffrida, F. Salinity of Nutrient Solution Influences the Shelf-Life of Fresh-Cut Lettuce Grown in Floating System. Postharvest Biol. Technol. 2011, 59, 132–137. [Google Scholar] [CrossRef]
- Gonnella, M.; Renna, M.; Serio, F. Yield and Quality of Greenhouse Multi-Leaf Lettuce Cultivars Grown in Soil and Soilless Culture under Mediterranean Conditions. Italus Hortus 2020, 27, 18–30. [Google Scholar] [CrossRef]
- Liška, M.; Tůmová, V.; Langhansová, L.; Mercl, F.; Tejnecký, V.; Petrová, Š.; Moťková, K.; Bureš, D.; Petrtýl, M.; Klouček, P. Enhancing Antioxidant Properties of Lettuce through Nutritional Deficiency in Aquaponic Systems with Aeroponic Cultivation. 2023. Available online: https://www.preprints.org/manuscript/202307.1912/v1 (accessed on 15 May 2025).
- Chiocchini, S. A New Method for Cultivating and Growing Plants and Relative Plant; World Intellectual Property Organization (WIPO): Geneva, Switzerland, 2019; n. WO 2021/059307 A1 2019; pp. 1–26. [Google Scholar]
- El-bashir, S.M.; Al-harbi, F.F.; Elburaih, H.; Al-fai, F.; Yahia, I.S. Red Photoluminescent PMMA Nanohybrid Fi Lms for Modifying the Spectral Distribution of Solar Radiation inside Greenhouses. Renew. Energy 2016, 85, 928–938. [Google Scholar] [CrossRef]
- Dong, R.; Li, Y.; Li, W.; Zhang, H.; Liu, Y.; Ma, L.; Wang, X.; Lei, B. Recent Developments in Luminescent Nanoparticles for Plant Imaging and Photosynthesis. J. Rare Earths 2019, 37, 903–915. [Google Scholar] [CrossRef]
- UbiGro. Redefining PAR: Far-Red Photons’ Role in Plant Lighting. Available online: https://ubigro.com/redefining-par-the-impact-of-far-red-photons-on-photosynthesis-and-horticultural-lighting/ (accessed on 16 May 2025).
- Pandey, G.; Parks, S.; Thomas, R.G. Polymer and Photo-Selective Covers on Plant and Fruit Development: A Review. Agron. J. 2023, 115, 3074–3091. [Google Scholar] [CrossRef]
- Gao, Y.; Li, G.; Cai, B.; Zhang, Z.; Li, N.; Liu, Y.; Li, Q. Effects of Rare-Earth Light Conversion Film on the Growth and Fruit Quality of Sweet Pepper in a Solar Greenhouse. Front. Plant Sci. 2022, 13, 989271. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, R.; Di Mola, I.; Conti, S.; Ottaiano, L.; Cozzolino, E.; Rippa, M.; Mormile, P.; Melchionna, G.; Testa, A.; Beltrame, L.; et al. Photosynthesis, Yield and Quality in Wild Rocket (Diplotaxis tenuifolia L.) under Photoluminescent Greenhouse Covers. Agronomy 2023, 13, 2372. [Google Scholar] [CrossRef]
- Shoji, S.; Saito, H.; Jitsuyama, Y.; Tomita, K.; Haoyang, Q.; Sakurai, Y.; Okazaki, Y.; Aikawa, K.; Konishi, Y.; Sasaki, K.; et al. Plant Growth Acceleration Using a Transparent Eu3+-Painted UV-to-Red Conversion Film. Sci. Rep. 2022, 12, 17155. [Google Scholar] [CrossRef]
- Yanykin, D.V.; Paskhin, M.O.; Simakin, A.V.; Burmistrov, D.E.; Pobedonostsev, R.V.; Vyatchinov, A.A.; Vedunova, M.V.; Kuznetsov, S.V.; Ermakova, J.A.; Alexandrov, A.A.; et al. Plant Photochemistry under Glass Coated with Upconversion Luminescent Film. Appl. Sci. 2022, 12, 7480. [Google Scholar] [CrossRef]
- Conti, S.; Di Mola, I.; Barták, M.; Cozzolino, E.; Melchionna, G.; Mormile, P.; Ottaiano, L.; Paradiso, R.; Rippa, M.; Testa, A.; et al. Crop Performance and Photochemical Processes Under a UV-to-Red Spectral Shifting Greenhouse: A Study on Aubergine and Strawberry. Agriculture 2025, 15, 569. [Google Scholar] [CrossRef]
- Magnani, G.; Filippi, F.; Borghesi, E.; Vitale, M. Impact of Sunlight Spectrum Modification on Yield and Quality of Ready-to-Use Lettuce and Rocket Salad Grown on Floating System. Acta Hortic. 2008, 801 Pt 1, 163–169. [Google Scholar] [CrossRef]
- Knight, S.L.; Mitchell, C.A. Enhancement of Lettuce Yield by Manipulation of Light and Nitrogen Nutrition. HortScience 1983, 108, 750–754. [Google Scholar] [CrossRef]
- Knight, S.L.; Mitchell, C.A. Stimulation of Lettuce Productivity by Manipulation of Diurnal Temperature and Light. HortScience 1983, 18, 462–463. [Google Scholar] [CrossRef]
- Rouchaud, J.; Moons, C.; Meyer, J.A. Effects of Fungicide Treatments on the Gustative Quality and the Biochemical Composition of Apples. HortScience 1986, 21, 1056–1058. [Google Scholar] [CrossRef]
- Farahanian, Z.; Zamindar, N.; Goksen, G.; Tucker, N.; Paidari, S.; Khosravi, E. Effects of Nano-Bentonite Polypropylene Nanocomposite Films and Modified Atmosphere Packaging on the Shelf Life of Fresh-Cut Iceberg Lettuce. Coatings 2023, 13, 349. [Google Scholar] [CrossRef]
- Flores, M.; Amorós, A.; Escalona, V.H. Changes in Agronomic, Antioxidant Compounds, and Morphology Parameters of Green and Red Lettuces (Lactuca sativa L.) by Successive Harvests and UV-B Supplementation. Horticulturae 2023, 9, 677. [Google Scholar] [CrossRef]
- Galieni, A.; Di Mattia, C.; De Gregorio, M.; Speca, S.; Mastrocola, D.; Pisante, M.; Stagnari, F. Effects of Nutrient Deficiency and Abiotic Environmental Stresses on Yield, Phenolic Compounds and Antiradical Activity in Lettuce (Lactuca sativa L.). Sci. Hortic. 2015, 187, 93–101. [Google Scholar] [CrossRef]
- De Bruno, A.; Gattuso, A.; Romeo, R.; Santacaterina, S.; Piscopo, A. Functional and Sustainable Application of Natural Antioxidant Extract Recovered from Olive Mill Wastewater on Shelf-Life Extension of “Basil Pesto ”. Appl. Sci. 2022, 12, 10965. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kowalczyk, K.; Sieczko, L.; Goltsev, V.; Kalaji, H.M.; Gajc-Wolska, J.; Gajewski, M.; Gontar, Ł.; Orliński, P.; Niedzińska, M.; Cetner, M.D. Relationship between Chlorophyll FLuorescence Parameters and Quality of the Fresh and Stored Lettuce (Lactuca sativa L.). Sci. Hortic. 2018, 235, 70–77. [Google Scholar] [CrossRef]
- Ghimire, B.; Timsina, D.; Nepal, J. Analysis of Chlorophyll Content and Its Correlation with Yield Attributing Traits on Early Varieties of Maize (Zea mays L.). J. Maize Res. Dev. 2015, 1, 134–145. [Google Scholar] [CrossRef]
- Lucero, L.; Lucero, D.; Ormeno-Mejia, E.; Collaguazo, G. Automated Aeroponics Vegetable Growing System. Case Study Lettuce. In Proceedings of the 2020 IEEE Andescon, Quito, Ecuador, 13–16 October 2020. [Google Scholar] [CrossRef]
- Ding, W.; Lin, R.; Zhou, W.; Zhou, K.; Lin, X. Comparison on the Difference of Growth and Nutritional Quality for Lettuce (Lactuca sativa L.) between Aeroponic and Hydroponic Cultivation Systems under Different Nitrogen Levels. J. Zhejiang Univ. (Agric. Life Sci.) 2016, 42, 703–712. [Google Scholar]
- El-Helaly, M.A.; Darwish, O.S. Effect of Culture System; Aerponic, Hydroponic and Sandy Substrate on Growth, Yield and Chemical Compositionsoflettuce. Plant Arch. 2019, 19, 2543–2550. [Google Scholar]
- Chowdhury, M.; Samarakoon, U.C.; Altland, J.E. Evaluation of Hydroponic Systems for Organic Lettuce Production in Controlled Environment. Front. Plant Sci. 2024, 15, 1401089. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Engeseth, N.J. Comparison of Growth Characteristics, Functional Qualities, and Texture of Hydroponically Grown and Soil-Grown Lettuce. LWT 2021, 150, 111931. [Google Scholar] [CrossRef]
- Ali, M.M.; Khater, E.G.; Ali, S.A.; El-Haddad, Z.A. Comparison Between Hydroponic and Aeroponic Systems for Lettuce. In Proceedings of the 20th Annual Conference of Misr Society of Agricultural Engineering, Banha, Egypt, 12 December 2015; pp. 1–24. [Google Scholar]
- Thomas, T.; Biradar, M.S.; Chimmad, V.P.; Janagoudar, B.S. Growth and Physiology of Lettuce (Lactuca sativa L.) Cultivars under Different Growing Systems. Plant Physiol. Reports 2021, 26, 526–534. [Google Scholar] [CrossRef]
- Kang, J.H.; Kim, D.; Yoon, H.I.; Son, J.E. Growth, Morphology, and Photosynthetic Activity of Chinese Cabbage and Lettuce Grown under Polyethylene and Spectrum Conversion Films. Hortic. Environ. Biotechnol. 2023, 64, 593–603. [Google Scholar] [CrossRef]
- Majeed, A.; Anwar-Ul-Haq, M.; Niaz, A.; Akhtar, J.; Ahmad, Z.; Muhmood, A.; Anjum, M.; Shah, S. Gas Exchange Traits and Biplot Analysis Method Can Be Used as Screening Criteria for Salt Tolerance in Maize (Zea mays L.) Cultivars. Am. J. Exp. Agric. 2016, 11, 23267. [Google Scholar] [CrossRef]
- Hu, H.J.; Xu, K.; He, L.C.; Wang, G.X. A Model for the Relationship between Plant Biomass and Photosynthetic Rate Based on Nutrient Effects. Ecosphere 2021, 12, e03678. [Google Scholar] [CrossRef]
- Poorter, H.; Anten, N.P.R.; Marcelis, L.F.M. Physiological Mechanisms in Plant Growth Models: Do We Need a Supra-Cellular Systems Biology Approach? Plant, Cell Environ. 2013, 36, 1673–1690. [Google Scholar] [CrossRef]
- Snowden, M.C.; Cope, K.R.; Bugbee, B. Sensitivity of Seven Diverse Species to Blue and Green Light: Interactions with Photon Flux. PLoS ONE 2016, 11, e0163121. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A Review of the Antioxidant Mechanisms of Polyphenol Compounds Related to Iron Binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Sutulienė, R.; Laužikė, K.; Pukas, T.; Samuolienė, G. Effect of Light Intensity on the Growth and Antioxidant Activity of Sweet Basil and Lettuce. Plants 2022, 11, 1709. [Google Scholar] [CrossRef] [PubMed]
- Amaro de Sales, R.; Chaves de Oliveira, E.; Buzatto, E.; Ferreira de Almeida, R.; Alves de Lima, M.J.; da Silva Berilli, S.; Lana Aguiar, R.; Lovo, M.; Prucoli Posse, R.; Casagrande dos Santos, J.; et al. Photo-Selective Shading Screens as a Cover for Production of Purple Lettuce. Sci. Rep. 2021, 11, 14972. [Google Scholar] [CrossRef]
- Choi, J.; An, J.; Lee, H.; Kim, W.J.; Lee, S. Comprehensive Analysis of Phenolic Compounds, Carotenoids, and Antioxidant Activities in Lactuca Sativa Var. Longifolia Cultivated in a Smart Farm System. Processes 2023, 11, 2993. [Google Scholar] [CrossRef]
- Ahmed, Z.F.R.; Alnuaimi, A.K.H.; Askri, A.; Tzortzakis, N. Evaluation of Lettuce (Lactuca sativa L.) Production under Hydroponic System: Nutrient Solution Derived from Fish Waste vs. Inorganic Nutrient Solution. Horticulturae 2021, 7, 292. [Google Scholar] [CrossRef]
- Di Mola, I.; Conti, S.; Bartak, M.; Cozzolino, E.; Ottaiano, L.; Giordano, D.; Melchionna, G.; Mormile, P.; Rippa, M.; Beltrame, L.; et al. Greenhouse Photoluminescent PMMA Panels Improve the Agronomical and Physiological Performances of Lettuce (Lactuca sativa L.). Horticulturae 2022, 8, 913. [Google Scholar] [CrossRef]
- Fu, W.; Li, P.; Wu, Y. Effects of Different Light Intensities on Chlorophyll Fluorescence Characteristics and Yield in Lettuce. Sci. Hortic. 2012, 135, 45–51. [Google Scholar] [CrossRef]
- Liu, Y.; Gui, Z.; Liu, J. Research Progress of Light Wavelength Conversion Materials and Their Applications in Functional Agricultural Films. Polymers 2022, 14, 851. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Fukumoto, Y.; Aruga, H.; Shimoi, Y. Growth and Developmental Characteristics of Vegetables Grown under Spectrum Conversion Film. Hortic. Environ. Biotechnol. 2009, 50, 416–421. [Google Scholar]
- Pinheiro, R.R.; Pinheiro, M.V.M.; Thiesen, L.A.; Diel, M.I.; Dos Santos, J.; Caron, B.O.; Schmidt, D. The Use of Photoselective Nets Affects the Leaf Characteristics of Lettuce Cultivars. Bulg. J. Agric. Sci. 2020, 26, 777–786. [Google Scholar]
- Masia, S.; Trabucco, A.; Spano, D.; Snyder, R.L.; Sušnik, J.; Marras, S. A Modelling Platform for Climate Change Impact on Local and Regional Crop Water Requirements. Agric. Water Manag. 2021, 255, 107005. [Google Scholar] [CrossRef]

| Cultivation System | Plants Height (cm) | Fresh Weight (g) | Dry Weight(g) | Dry Matter Content (%) | Number True Leaves | Leaf Width (cm) |
|---|---|---|---|---|---|---|
| A | 19.20 ± 1.00 ab | 52.70 ± 5.89 a | 3.19 ± 1.08 | 7.14 ± 1.88 a | 14.3 ± 0.54 b | 11.35 ± 0.40 a |
| PSO | 9.30 ± 0.37 c | 25.24 ± 1.29 c | 1.88 ± 0.09 | 7.54 ± 0.31 a | 10.1 ± 0.35 c | 7.90 ± 0.26 b |
| PSI | 20.90 ± 0.53 a | 40.28 ± 1.78 b | 1.42 ± 0.19 | 3.28 ± 0.51 b | 14.6 ± 0.28 b | 10.42 ± 0.20 a |
| HSN | 17.00 ± 0.39 b | 36.78 ± 2.59 b | 3.01 ± 0.54 | 8.53 ± 0.17 a | 16.8 ± 0.738 a | 10.17 ± 0.44 a |
| HSO | 8.17 ± 0.42 c | 11.19 ± 1.00 d | 0.93 ± 0.10 | 9.02 ± 0.36 a | 11.2 ± 0.16 c | 6.58 ± 0.38 b |
| Sign. | *** | *** | NS | * | * | ** |
| WUE (µmol CO2 µmol−1 H2O) | Transpiration Rate (µmol H2O m−2s−1) | Stomatal Conductance (mmol H2O m−2s−1) | Photosynthetic Rate (µmol CO2 m−2s−1) | |
|---|---|---|---|---|
| A | 6.28 ± 0.36 ab | 2.51 ± 0.30 a | 0.12 ± 0.02 a | 15.80 ± 1.77 ab |
| PSI | 5.10 ± 0.36 b | 2.01 ± 0.30 a | 0.09 ± 0.02 a | 10.01 ± 1.77 b |
| PSO | 5.76 ± 0.36 ab | 1.80 ± 0.30 a | 0.08 ± 0.02 a | 10.19 ± 1.77 b |
| HSN | 6.12 ± 0.36 ab | 2,74 ± 0.30 a | 0.13 ± 0.02 a | 16.80 ± 1.77 ab |
| HSO | 7.00 ± 0.44 a | 2.75 ± 0.37 a | 0.14 ± 0.02 a | 18.83 ± 2.17 a |
| Sign. | ** p < 0.05 | NS | NS | ** |
| (mg g−1) | Chl a | Chl b | Carotenoids | TPC (GAE mg g−1 DW) | DPPH (mm Trolox g−1 dw) | ABTS (mm Trolox g−1 dw) |
|---|---|---|---|---|---|---|
| A | 0.75 ± 0.03 a | 0.29 ± 0.01 a | 0.18 ± 0.02 a | 29.33 ± 1.34 c | 71.25 ± 3.64 c | 121.24 ± 7.32 d |
| PSI | 0.67 ± 0.03 a | 0.28 ± 0.02 a | 0.1 ± 0.01 b | 58.75 ± 3.39 b | 189.11 ± 13.39 b | 427.17 ± 18.70 b |
| PSO | 0.27 ± 0.03 c | 0.09 ± 0.01 c | 0.14 ± 0.01 ab | 193.5 ± 6.02 a | 1230.84 ± 39.31 a | 1113.34 ± 26.85 a |
| HSN | 0.48 ± 0.04 b | 0.19 ± 0.02 b | 0.13 ± 0.01 ab | 54.14 ± 3.37 b | 213.13 ± 12.64 b | 216.2 ± 14.14 c |
| HSO | 0.61 ± 0.06 b | 0.17 ± 0.02 b | 0.13 ± 0.01 ab | 60.62 ± 5.28 b | 124.85 ± 12.08 bc | 261.67 ± 34.74 c |
| Sign. | ** | ** | ** | ** | ** | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Impallomeni, G.; Lupini, A.; Sorgonà, A.; Gattuso, A.; Barreca, F. The Qualitative and Quantitative Relationship of Lettuce Grown in Soilless Systems in a Mediterranean Greenhouse. Int. J. Plant Biol. 2025, 16, 94. https://doi.org/10.3390/ijpb16030094
Impallomeni G, Lupini A, Sorgonà A, Gattuso A, Barreca F. The Qualitative and Quantitative Relationship of Lettuce Grown in Soilless Systems in a Mediterranean Greenhouse. International Journal of Plant Biology. 2025; 16(3):94. https://doi.org/10.3390/ijpb16030094
Chicago/Turabian StyleImpallomeni, Gabriella, Antonio Lupini, Agostino Sorgonà, Antonio Gattuso, and Francesco Barreca. 2025. "The Qualitative and Quantitative Relationship of Lettuce Grown in Soilless Systems in a Mediterranean Greenhouse" International Journal of Plant Biology 16, no. 3: 94. https://doi.org/10.3390/ijpb16030094
APA StyleImpallomeni, G., Lupini, A., Sorgonà, A., Gattuso, A., & Barreca, F. (2025). The Qualitative and Quantitative Relationship of Lettuce Grown in Soilless Systems in a Mediterranean Greenhouse. International Journal of Plant Biology, 16(3), 94. https://doi.org/10.3390/ijpb16030094








