Abstract
Phytophthora infestans remains a major threat to global potato production. This study focused on characterizing and assessing the pathogenicity of P. infestans isolates on detached potato leaves and in greenhouse trials across four cultivars. Seven isolates were obtained from high potato-producing regions in the department of Nariño, Colombia. The isolates were analyzed using 12 microsatellite markers to determine genetic distances. Two genetically distinct isolates showed markedly different pathogenicity on detached leaves: isolate P00921 caused complete infection by day five, whereas P00321 showed no symptoms. These two isolates (P00921 and P00321) selected for having the greatest genetic distance and highest pathogenicity among the seven analyzed were further tested in a greenhouse setup on four potato cultivars using a randomized block design. Disease progression was monitored over nine days. The results indicated significant variations in pathogenicity linked to genetic diversity among isolates. Notably, Capiro and Margarita cultivars were more prone to severe disease than Suprema and Única. These findings highlight the complex nature of host–pathogen interactions and suggest the need for tailored approaches in disease management and cultivar selection.
1. Introduction
Phytophthora infestans (Mont.) de Bary represents a major challenge for small-scale potato farmers worldwide. Potatoes are the third most important food crop in the world and the second most consumed in Colombia [1]. Potato production is continually threatened by attacks from P. infestans, causing the disease commonly known as “late blight”, which can destroy the crop in less than two weeks or limit its production [2]. Late blight can cause losses ranging from 5 to 100% of yield, with an average economic loss of EUR 12 million per year in developing countries [3].
In Colombia, annual losses can reach 40%, and in severe epidemics, they can escalate to 100% due to the absence of resistant or tolerant varieties [4]. Blight results in production losses, and the costs of chemical control can reach up to USD 600 per hectare. The frequent application of agrochemicals, specifically azoxystrobin and difenoconazole, necessitates substantial quantities of water and gasoline, thereby exacerbating environmental impacts. Moreover, the excessive reliance on fungicides has led to a significant incidence of resistance among P. infestans populations [5]. This intensive focus on agrochemical use to combat late blight not only results in significant environmental impacts but also influences the genetic dynamics of P. infestans populations.
In Colombia, the P. infestans populations demonstrate genetic homogeneity, largely attributed to the A1 mating type [6,7], although an A2 type was once reported in 2009 in Physalis peruviana [8]. Low genetic diversity and clonal populations of P. infestans have been identified in the Northern Andean region, likely due to high migration and limited gene flow [7]. Few heterozygotes found in populations are believed to be polymorphisms inherited from a common ancestor rather than resulting from sexual reproduction [7,9]. Knowledge about the current status and evolution of P. infestans helps in developing effective disease-control strategies for late blight. SSR markers have been extensively used in population genetics studies for their co-dominance, polymorphic nature, reliability, and consistent results. These markers are effective in identifying polymorphisms within closely related isolates and are commonly employed to analyze the population structure of P. infestans [10,11].
Host plant resistance is one of the most effective and efficient methods for managing plant diseases. There are over 100 reported wild potato species with resistance to late blight [12], and more than 70 resistance genes have been mapped in 32 species of the Solanum genus [13]. Studies have demonstrated the successful introgressing of resistance genes into commercial potato cultivars [14,15,16]. In Nariño, the most widely planted potato varieties are Capiro, covering 38% of the planted area, Suprema with 20% of the area, Única with 16%, Superior with 4%, and Parda Pastusa also with 4% of the total planted area [17,18]. The most widely planted variety in Nariño is the most susceptible to attack by P. infestans among the cultivars mentioned, which is why it is typically used as the control for susceptibility [17,19]. The degree of resistance in the other cultivars remains a subject of debate. The Suprema and Superior cultivars were regarded as resistant to late blight [9], while the Única cultivar was considered moderately resistant [9,20,21,22]. However, some reports suggest that the pathogen has overcome the molecular resistance mechanisms in these cultivars, leading to yield reductions of up to 89% [17]. Therefore, further research is necessary to better understand the evolving interactions between P. infestans and these cultivars, as well as to develop high-throughput methods for evaluating resistance across many plants in a short period of time.
P. infestans exhibits significant genetic diversity, which enables it to develop a variety of molecular pathogenic mechanisms. This diversity must be carefully considered when selecting resistant plants and managing the pathogen in fields. Advanced and efficient pathogenicity assays in laboratory and greenhouse settings are essential for the rapid identification and screening of resistant potato plants. Detached leaf assays have been used for in vitro spore production [23] and to assess the foliar virulence on resistant and susceptible cultivars [24,25,26]. Pathogenicity tests should consider the pathogen’s geographic origin, as genetic diversity has been shown to vary accordingly [27]. This variation can influence both the screening of resistant potato genotypes to the disease and the overall management of P. infestans. This research aims to explore genetic variation and assess the pathogenicity of isolates of P. infestans from contrasting geographic areas on the four widely planted potato cultivars in the region.
2. Materials and Methods
2.1. Sampling and P. infestans Isolation
Potato leaf sampling expeditions targeting symptoms of P. infestans infection were carried out in the potato-producing corregimientos of the Pasto municipality, Nariño, Colombia. The sampling spanned a period between 2019 and 2021, at seven points, each featuring distinct geographical conditions (Table 1), which were characterized by being highly productive potato-growing regions. Leaves with wet-appearing necrotic spots and white mycelium on the underside were sampled (Supplementary Figure S1). Geographic data of potato leaf samples with symptoms of P. infestans are shown in Table 1.

Table 1.
Geographic data of the populations of P. infestans isolated from different localities in Pasto.
The isolation of the P. infestans samples was performed based on the protocol described by Berdúo-Sandoval et al. [28]. A 1 cm2 section of each sample, encompassing both necrotic and healthy tissue, was excised and disinfected using 70% alcohol, followed by 1.25% sodium hypochlorite and sterile distilled water. The disinfected samples were then cultured on tomato–pea agar medium supplemented with rifampicin (1 mL/L) and incubated for 8 days in darkness. Isolation was confirmed by macroscopic and microscopic observation, evidenced by the presence of sporangia (Supplementary Figure S2A–C) and amplification of a 946 bp band with primers ITS5 (5′-GGAAGTAAAAAAGTCGTAACAAGG) and ITS4 (5′-TCCTCCGCTTATTGATATATATATGC) (Supplementary Figure S3), according to the amplification conditions reported by [29]. Initial denaturation at 96 °C for 2 min was followed by 35 cycles of denaturation at 96 °C for 1 min, hybridization at 55 °C for 1 min, and extension at 72 °C for 2 min. Finally, a final extension at 72 °C for 10 min occurred.
2.2. Characterization of P. infestans
From 15-day-old tomato–pea agar plates, a portion of raised mycelium from each isolate was obtained with a sterile microbiological loop in Eppendorf tubes. The mycelium was macerated with liquid nitrogen and DNA extraction following the protocol proposed by Griffith and Shaw [30]. DNA quality and concentration were quantified by NanoDrop-One, while its integrity was verified by 1% agarose gel electrophoresis.
The isolates were genotyped using a set of 12 polymorphic microsatellite markers, including D13, G11, PI04, Pi4B, Pi63, Pi70, PinfSSR2, PinfSSR3, PinfSSR4, PinfSSR6, PinfSSR8, and PinfSSR11 [31,32,33] (Supplementary Table S1). The PCR conditions were as follows: 95 °C for 15 min, followed by 30 cycles of 95 °C for 20 s, 58 °C for 90 s, and 72 °C for 20 s, with a final extension at 72 °C for 20 min [33,34]. The PCR products were analyzed on 3% agarose gels alongside a 50 bp molecular weight marker. Band size measurement was conducted using ImageJ software v1.54 [35]. Genetic distances were calculated based on the microsatellite results using Bruvo’s genetic distance [36] and represented in a dendrogram with the Poppr package [37], according to the methodology described by Shakya et al. [38]. A correlation analysis was conducted between the genetic distance matrix and the geographical distance matrix. The Mantel test was performed to determine if there was a significant (p < 0.05) relationship between the correlation of these distance matrices [39]. All procedures were conducted using the R software [40].
2.3. Initial Assessment of Pathogenicity on Detached Leaves
The initial experiment was conducted in the laboratory of Genetics and Evolution of Tropical Organisms Group at the University of Nariño (01°13′58.65″ N and 77°17′35.554″ W). The conditions were room temperature with humidity of 80% or higher using humid chambers. The inoculum was prepared according to the methodology proposed by Padilla-Ramos et al. [41]. The mycelium grown for 15 days was scraped with sterile distilled water to achieve a concentration of 2.3 × 105 sporangia/mL. It was then kept at 4 °C for three hours to induce zoospore release. Inoculation was performed by placing drops of the solution on the abaxial surface on four detached leaves of Solanum tuberosum Phureja Group (susceptible to P. infestans) per isolate. After inoculation, the leaves were kept in a humid chamber and daily photographic records were taken from symptom onset for nine days. The percentage of infection was quantified as the ratio between the area of infection and the total leaf area using ImageJ software v1.54 [35]. The results were subjected to an analysis of variance (ANOVA) to determine if there were significant differences between the treatments, which correspond to the seven isolates. Regression models were developed to identify the most aggressive pathogen in terms of the symptom expansion rate on the detached leaf, which corresponds to the increase in the percentage of the leaf area affected by necrotic symptoms caused by P. infestans. All procedures were conducted using the R software v4.3.3 [40].
2.4. Location of the Second Experiment
The experiment took place in the research greenhouse of the Botana experimental center of the University of Nariño, located in Catambuco village (Nariño, Colombia), at coordinates 01°09′12″ N and 77°18′31″ W. It features an average temperature of 12 °C, 900 h of sunshine per year, relative humidity of 79% and annual precipitation of 820 mm, and an altitude of 2822 m above sea level [42].
2.5. Potato Cultivars
The experiment was conducted with four varieties of potato S. tuberosum, known as Suprema, Única, and Diacol Capiro, and an advanced cultivar for precocity and high yield currently known as “Margarita” kindly provided by Prof. Dr. Benjamin Sañudo Sotelo.
2.6. Plant Inoculation in the Greenhouse
The four S. tuberosum genotypes were inoculated following the methodology proposed by Ali et al. [43]. Five-week-old plants were placed in growth chambers with high relative humidity throughout the experiment using plastic bags. Subsequently, the plants were sprayed with a suspension of 2.3 × 105 sporangia/mL of P. infestans from each isolate until the leaf surfaces were completely saturated. A control group of plants was inoculated with water without the pathogen (mock). The plants were evaluated starting from the second day post-inoculation until they exhibited severe symptoms of the disease (by day 9).
2.7. Experimental Design
We investigate the interplay between potato genotypes, pathogen strains, and temporal factors using a randomized compete block design (RCBD). The first factor pertains to the two previously isolated and characterized strains, selected based on their highly contrasting levels of pathogenicity and their high genotypic distance values, with one being severe and the other less aggressive, in addition to a control solution (mock). The exposure of the four genotypes (Margarita, Única, Suprema, and Diacol Capiro) to the inoculum constitutes the second factor. To assess the progression of the disease, it was essential to conduct four measurements at intervals of 2, 4, 6, and 9 days post-inoculation. Each treatment was replicated four times, resulting in a total of 192 experimental units. The symptoms were quantitatively assessed by counting the number of affected leaflets, the number of affected leaves, and the presence of necrotic spots. The necrotic spots were measured using two distinct scales: small lesions of approximately 2 mm2 and larger lesions around 1 cm2. These measurements were taken consistently across all samples to ensure the precision and comparability of the data.
2.8. Statistical Analysis
An analysis of variance (ANOVA) was used to elucidate the main and interaction effects of genotype, strain, and temporal factors on the observed disease symptoms. When significant differences were determined in the ANOVA, Duncan’s multiple range test was employed for post hoc means comparison, adhering to a significance threshold of p < 0.05. In addition, facet line graphs were created to improve the interpretation of the results, using the ggplot2 package [44], among other native functions of the software R v4.3.3 [40].
3. Results
The results revealed that there were highly significant differences (p < 0.001) between the isolates regarding the progress of infection in detached potato leaves (Supplementary Table S2). The isolates P00921 and P002521 exhibited a rapid increase in infection percentage, with both approaching 100% by day 5 (Figure 1A). Infection growth patterns similar to those of isolates P02421 and P00119 were observed, both of which achieved complete infection by the sixth day (Figure 1A). The isolates P00519 and P00221 exhibited a moderate infection rate, reaching the maximum infection rate by the eighth day (Figure 1A). Isolate P00321 exhibited a lower rate of infection, achieving just over 25% by day 8 (Figure 1A). The characterization using 12 microsatellite markers resulted in the amplification of 3 to 15 alleles, averaging 5, which were grouped into a total of 7 multilocus genotypes corresponding to the 7 isolates of P. infestans obtained (Supplementary Figures S4–S15). This characterization revealed that the least aggressive P. infestans isolate (P00321) was also the most genetically distant from the others (Figure 1B). The results suggest a possible link between genetic distance and virulence in P00321 isolate. Isolates P00519 and P02521 as well as P00221 and P00119 were grouped together, reflecting a closer genetic relationship. In this case, the genetic divergence was not large enough to significantly influence the adaptive ability of P. infestans (Figure 1A,B). These results underscore that the genetic variability could affect the behavior of P. infestans, which could influence strategies for disease management in potato cultivation.
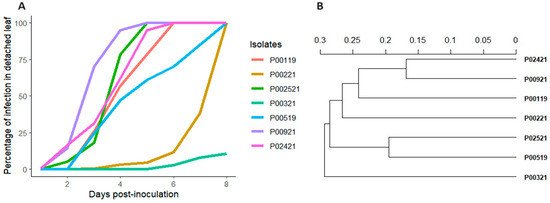
Figure 1.
Progression of infection and genetic relationships among seven P. infestans isolates. (A) Progression of infection over time in seven P. infestans isolates on detached leaves. The lines represent the average of four values. (B) Dendrogram representing the genetic distance among seven P. infestans isolates based on Bruvo distances for microsatellite markers. Isolate codes are displayed on the right, each representing a unique genetic profile collected for the study.
The two genetically most distant isolates (P00921 and P00321) exhibited the most divergent behavior both in detached leaf assays (Supplementary Figure S16) and in greenhouse conditions (Table 2). Significant differences (p < 0.05) were observed in the number of leaflets affected by the two isolates on the four potato genotypes in a period of nine days of plant growth in the greenhouse (Table 2). The progression of the disease, in terms of the number of affected leaves and necrotic spots (2 mm2 and 1 cm2), differs significantly (p < 0.05) for each cultivar over time, demonstrating that the spread of infection varies and is heavily dependent on the genetic makeup of the potato plants (Table 2). Among the simple effects, the isolates were the only treatment that did not present significant differences (Table 2). The overall findings suggest that disease progression is influenced by an interplay between the pathogen isolate and genetic makeup of the potato plant.

Table 2.
Mean square values from ANOVA for the influence of potato genotypes, strains, and days post-inoculation on disease severity in potato plants. The analysis evaluates the impact on four disease severity metrics: number of affected leaflets, number of affected leaves, number of necrotic spots sized 2 mm2, and number of necrotic spots sized 1 cm2.
The genotypes Capiro and Margarita exhibit a markedly greater vulnerability to infection by isolate P00921, in contrast to the Suprema and Única cultivars (Figure 2). The number of affected leaflets grew rapidly in the susceptible potato genotypes, increasing to approximately 75% by the ninth day (Figure 2). On the other hand, the number of leaflets affected by the P00321 isolate did not show statistically significant (p < 0.05) differences among the evaluated potato plants (Figure 2). Margarita cultivars display a modest increase in affected leaflets; however, the four potato genotypes exhibited comparable patterns of disease progression when inoculated with the P00321 isolate (Figure 2). As in the previous experiment, the comparative analysis of P. infestans isolates reveals varying degrees of pathogenicity, which depend on the host genome.
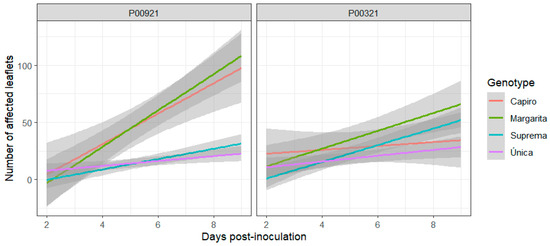
Figure 2.
Differential response of four potato genotypes to two distinct isolates of P. infestans isolates over time. The P00921 strain rapidly increases the number of affected leaflets, particularly in the Capiro and Margarita cultivars.
Potato plants demonstrate distinct reactions to P. infestans inoculation, suggesting the presence of diverse defense mechanisms (Figure 3A–C), although the molecular bases are completely unknown. Capiro and Margarita exhibited the highest values in terms of the number of affected leaves and symptomatic necrotic spots (2 mm2 and 1 cm2) compared to the other two materials (Figure 3A–C). Única demonstrates a gradual increase in the severity of the infection but the numbers remain much lower compared to the other cultivars (Figure 3A–C). The four potato genotypes display varying patterns of resistance to disease progression, suggesting distinct molecular defense mechanisms among the materials.
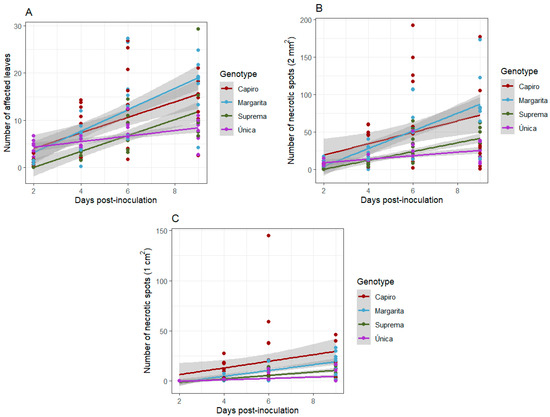
Figure 3.
Differential response of four potato cultivars to P. infestans infection. (A) Number of affected leaves, (B) number of necrotic spots (2 mm2), and (C) number of necrotic spots (1 cm2), measured at 2, 4, 6, and 9 days post-inoculation.
4. Discussion
These findings underscore the importance of considering both pathogen and host genetic variability when developing more effective disease management strategies and genetic improvement. Late blight in potatoes has worsened due to new, diverse genotypes that exhibit increased virulence and resistance to control methods, complicating management strategies. We identified at least two P. infestans isolates (P00921 and P00321) with distinct genetic profiles, each causing different initial symptoms in commercial potato cultivars. This suggests a potential link between genetic distance and virulence, although we recognize that genetic distance may also be influenced by other factors not directly related to virulence. Understanding these genetic variations could be crucial for breeding and managing disease resistance in potato crops. The initial pathogenic interactions could differ across the identified isolates, indicating that the molecular interaction between the potato cultivars and the P. infestans isolates may have a different downstream signaling pathway. This could be the result of differences in the effector arsenal among P. infestans genotypes characterized by SSR markers [45]. The effector genes exhibit a higher mutation rate than those in other gene families. Consequently, polymorphisms in effector sequences are frequently observed among species isolates, linked to their adaptability and virulence towards hosts [46,47,48]. Plants possess an expansive range of pattern-triggered immunity mechanisms, exerting evolutionary pressure on pathogen effector genes. For example, the Avr4 effector may show only 50.5% identity with its orthologous protein in species of the same class of Dothideomycetes [49].
No significant correlations were identified between geographical and genetic distances. The correlation between the Bruvo distance matrix (Supplementary Table S3) and the geographic distance matrix (Supplementary Table S4) was not significant, resulting in a correlation coefficient of r = −0.2451 (p-value = 0.857), based on the Mantel test. The spatial separation between P. infestans isolates may not adequately explain the observed genetic dissimilarities. Given the lack of sexual reproduction in P. infestans, there is no clear gradient of genetic diversity associated with geographical distance, as observed in its center of origin [38]. However, the use of genetic markers such as microsatellites allows for detecting genetic variability in asexual progeny [26,39]. On the other hand, long-distance dispersal of asexual reproduction structures is limited. The sporangia are susceptible to desiccation and radiation, limiting their migration to distances no greater than 10 km [50,51]. The observed genetic variability is largely a result of mutation rates and the intense selection pressure exerted by human activities.
The genome of P. infestans has factors that favor the mutational variability of the pathogen, such as the presence of a high number of transposons and dispersed regions of genes [40]. In Colombia, small-scale producers of potatoes often overuse anti-oomycete molecules, leading to alterations in the genetic structure of P. infestans populations [52]. This human-induced selection pressure accelerates genetic changes, potentially increasing the pathogen’s resistance to treatments and complicating control methods. Moreover, the frequent cultivation of potato varieties with similar genetic backgrounds can create a homogenous environment that may inadvertently foster specific adaptations in P. infestans.
Capiro and Margarita did not show significant differences (p < 0.05) in their initial symptoms when the plants were exposed to P. infestans. This outcome was anticipated, as Margarita is a cultivar derived from Capiro, sharing a highly similar genetic background (Dr. Benjamin Sañudo, personal communication). The two cultivars demonstrated greater initial susceptibility to isolate P00921 compared to the Suprema and Única varieties. The last two varieties have exhibited levels of late blight resistance higher than Capiro in the field, but this resistance is still insufficient to eliminate the need for fungicides [17]. The rapid evolution of pathogen effector genes through mechanisms such as insertion–deletion events, gene silencing, presence–absence variation, and point mutations hampers their interaction with host R genes, thereby limiting the effectiveness of resistance [41]. Capiro has been well received in the market and is highly palatable, which encourages its cultivation despite its well-known susceptibility. The absence of crop rotation and repeated planting of identical clonal material can enhance the virulence of P. infestans strains. The host preference is the primary evolutionary force shaping the genetic structure of P. infestans populations [53]. The adaptive capacity of P. infestans underscores the necessity for an integrated management strategy that incorporates genetic diversification among planted cultivars and improved resistance through breeding programs.
5. Conclusions
The results highlight the complex dynamics between P. infestans isolates and potato cultivars. Notably, the Capiro and Margarita varieties, which are closely related, exhibited the highest susceptibility to the pathogen. In contrast, Suprema and Única cultivars showed higher levels of resistance, although not sufficient to completely avoid the use of fungicides. On the other hand, isolate P00921 was highly aggressive, leading to full infection in a short time, while P00321 was much less aggressive, showing significantly lower infection levels over a longer period. These findings underscore the need for continuous breeding efforts and integrated management practices to develop more resilient potato cultivars and improve long-term disease management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijpb15040072/s1. Table S1: Primer sequences for P. infestans genotyping. Table S2: Analysis of variance (ANOVA) for the effects of strain and potato genotype on damage level late blight (%). Table S3: Bruvo’s distance for microsatellites: analyzing genetic diversity in P. infestans isolates from corregimientos of the Pasto (Nariño), Colombia. Table S4: Geographic distance matrix of potato P. infestans isolates from corregimientos of the Pasto (Nariño), Colombia. Figure S1: Necrotic spot on leaves of S. tuberosum with presence of sporulation on the underside of the leaf. Figure S2: Microscopic observation of mycelium and sporangia (400×) and macroscopic observation. Figure S3: Amplification with primers ITS4 and 5. Figures S4–S15: The characterization using 12 microsatellite markers resulted in the amplification of 3 to 15 alleles, averaging 5, which were grouped into a total of 7 multilocus genotypes corresponding to the 7 isolates of P. infestans obtained. Figure S16: Progression of disease caused by P. infestans isolates P00921 and P00321 in the detached leaf assay.
Author Contributions
Conceptualization, P.A.V.-V., C.B.G., and C.S.-G.; methodology, P.A.V.-V., C.B.G., and C.S.-G.; software, P.A.V.-V.; validation, R.Y.C.-O., J.D.P.U., and T.Y.H.D.; formal analysis, R.Y.C.-O., J.D.P.U., and T.Y.H.D.; investigation, P.A.V.-V., R.Y.C.-O., J.D.P.U., C.B.G., and C.S.-G.; resources, P.A.V.-V., C.B.G., and C.S.-G.; data curation, P.A.V.-V., C.B.G., and C.S.-G.; writing—original draft preparation P.A.V.-V.; writing—review and editing, P.A.V.-V.; visualization, P.A.V.-V., C.B.G., and C.S.-G.; supervision, C.B.G. and C.S.-G.; project administration, L.E.L.M., C.B.G., and C.S.-G.; funding acquisition, P.A.V.-V., L.E.L.M., C.B.G., and C.S.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “VICERRECTORÍA DE INVESTIGACIÓN E INTERACCIÓN SOCIAL de la UNIVERSIDAD DE NARIÑO, registrado con código 2684 Acuerdo No. 52”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available on Zenodo: Pedro Alexander Velasquez-Vasconez, Reyven Yair Chaves-Ordoñez, Juan David Pantoja Unigarro, Tharling Yadhannia Hernandez Diaz, Luz Estela Lagos Mora, Carlos Betancourth García, and Claudia Salazar-Gonzalez. (2024). Leaf necrosis of potato leaves infected with Phytophthora infestans [data set]. Zenodo. https://doi.org/10.5281/zenodo.11522489.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Franco-Lara, L.; Varela-Correa, C.A.; Guerrero-Carranza, G.P.; Quintero-Vargas, J.C. Association of phytoplasmas with a new disease of potato crops in Cundinamarca, Colombia. Crop Prot. 2023, 163, 106123. [Google Scholar] [CrossRef]
- Huang, X.; You, Z.; Luo, Y.; Yang, C.; Ren, J.; Liu, Y.; Wei, G.; Dong, P.; Ren, M. Antifungal activity of chitosan against Phytophthora infestans, the pathogen of potato late blight. Int. J. Biol. Macromol. 2021, 166, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Berhan, M. Review on epidemiology, sampling techniques, management strategies of late blight (Phytophthora infestans) of potato and its yield loss. Asian J. Adv. Res. 2021, 4, 199–207. [Google Scholar]
- Santa, J.D.; Berdugo-Cely, J.; Cely-Pardo, L.; Soto-Suárez, M.; Mosquera, T.; Galeano, M.; Galeano Mendoza, C.H. QTL. Analysis reveals quantitative resistant loci for Phytophthora infestans and tecia solanivora in tetraploid potato (Solanum tuberosum L.). PLoS ONE 2018, 13, e0199716. [Google Scholar] [CrossRef] [PubMed]
- Majeed, A.; Siyar, S.; Sami, S. Late blight of potato: From the great Irish potato famine to the genomic era—An overview. Hell. Plant Prot. J. 2022, 15, 1–9. [Google Scholar] [CrossRef]
- Ayala-Usma, D.A.; Cárdenas, M.; Guyot, R.; De Mares, M.C.; Bernal, A.; Muñoz, A.R.; Restrepo, S.A. Whole genome duplication drives the genome evolution of Phytophthora betacei, a closely related species to Phytophthora infestans. BMC Genom. 2021, 22, 795. [Google Scholar] [CrossRef]
- Cárdenas, M.; Grajales, A.; Sierra, R.; Rojas, A.; González-Almario, A.; Vargas, A.; Marín, M.; Fermín, G.; Lagos, L.E.; Grünwald, N.J.; et al. Genetic diversity of Phytophthora infestans in the Northern Andean region. BMC Genet. 2011, 12, 23. [Google Scholar] [CrossRef]
- Vargas, A.M.; Ocampo, L.M.Q.; Céspedes, M.C.; Carreño, N.; González, A.; Rojas, A.; Zuluaga, A.P.; Myers, K.; Fry, W.E.; Jiménez, P.; et al. Characterization of Phytophthora infestans populations in Colombia: First report of the A2 mating type. Phytopathology 2009, 99, 82–88. [Google Scholar] [CrossRef]
- Chaves, S.C.; Rodríguez, M.C.; Mideros, M.F.; Lucca, F.; Ñústez, C.E.; Restrepo, S. Determining whether geographic origin and potato genotypes shape the population structure of Phytophthora infestans in the central region of Colombia. Phytopathology 2019, 109, 145–154. [Google Scholar] [CrossRef]
- Zhang, J.; Hieno, A.; Otsubo, K.; Feng, W.; Kageyama, K. Population genetic analysis of Phytophthora colocasiae from taro in Japan using ssr markers. J. Fungi 2023, 9, 391. [Google Scholar] [CrossRef]
- Bukhari, T.; Rana, R.M.; Hassan, M.U.; Naz, F.; Sajjad, M. Genetic diversity and marker trait association for Phytophthora resistance in chilli. Mol. Biol. Rep. 2022, 49, 5717–5728. [Google Scholar] [CrossRef] [PubMed]
- Dufková, H.; Greplová, M.; Hampejsová, R.; Kuzmenko, M.; Hausvater, E.; Brzobohatý, B.; Černý, M. Secondary metabolites, other prospective substances, and alternative approaches that could promote resistance against Phytophthora infestans. Agronomy 2023, 13, 1822. [Google Scholar] [CrossRef]
- Blossei, J.; Gäbelein, R.; Hammann, T.; Uptmoor, R. Late blight resistance in wild potato species—Resources for future potato (Solanum tuberosum) breeding. Plant Breed. 2022, 141, 314–331. [Google Scholar] [CrossRef]
- Paluchowska, P.; Śliwka, J.; Yin, Z. Late blight resistance genes in potato breeding. Planta 2022, 255, 127. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Viquez-Zamora, M.; den Uil, D.; Sinnige, J.; Kruyt, H.; Vossen, J.; Lindhout, P.; van Heusden, S. Introgression of genes for resistance against Phytophthora infestans in diploid potato. Am. J. Potato Res. 2020, 97, 33–42. [Google Scholar] [CrossRef]
- Stefańczyk, E.; Plich, J.; Janiszewska, M.; Smyda-Dajmund, P.; Sobkowiak, S.; Śliwka, J. Marker-assisted pyramiding of potato late blight resistance genes Rpi-rzc1 and Rpi-phu1 on di- and tetraploid levels. Mol. Breed. 2020, 40, 89. [Google Scholar] [CrossRef]
- Rodriguez, D.; Uribe, P.; Benavides, C.A. Response of commercial potato genotypes Solanum tuberosum L. to Phytophthora infestans (Mont.) de Bary late blight attack. Rev. Cienc. Agrícolas 2023, 40, e1200. [Google Scholar] [CrossRef]
- Martínez Pachón, E.; Insuasty Cordoba, S.d.C.; Benavides Cardona, C.A.; Gómez Gil, L.F.; Uribe Mejía, P.; Marcillo Paguay, C.A.; Calvache Muñoz, D.A.; Mejía España, D.F.; Arana Chico, H.C.; Ramos Zambrano, H.S.; et al. Caracterización del Sistema Productivo de Papa en el Departamento de Nariño 2015–2020: Conocimiento para la Toma de Decisiones; Corporación Colombiana de Investigación Agropecuaria (AGROSAVIA): Mosquera, Colombia, 2021; ISBN 9789587404814. [Google Scholar]
- Reyes-Herrera, P.H.; Delgadillo-Duran, D.A.; Flores-Gonzalez, M.; Mueller, L.A.; Cristancho, M.A.; Barrero, L.S. Chromosome-scale genome assembly and annotation of the tetraploid potato cultivar Diacol Capiro adapted to the Andean region. G3 Genes Genomes Genet. 2024, 14, jkae139. [Google Scholar] [CrossRef]
- Sanabria, K.; Pérez, W.; Andrade-Piedra, J.L. Effectiveness of resistance inductors for potato late blight management in Peru. Crop Prot. 2020, 137, 105241. [Google Scholar] [CrossRef]
- Ñústez, C. Variedades Colombianas de Papa, 1st ed.; Universidad Nacional de Colombia, Ed.; Universidad Nacional de Colombia: Bogotá, Colombia, 2011. [Google Scholar]
- Barrientos, J.C.; Ñústez, C.E. Difusión de seis nuevas variedades de papa en Boyacá y Cundinamarca (Colombia) entre 2003 y 2010. Rev. Colomb. Cienc. Hortíc. 2014, 8, 126–147. [Google Scholar] [CrossRef]
- Céspedes, M.C.; Cárdenas, M.E.; Vargas, A.M.; Rojas, A.; Morales, J.G.; Jiménez, P.; Bernal, A.J.; Restrepo, S. Physiological and molecular characterization of Phytophthora infestans isolates from the central Colombian Andean Region. Rev. Iberoam. Micol. 2013, 30, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Revelo, E.; Dorado, G.; Lagos, L.E.; Burbano-Figueroa, O. Foliar virulence of isolates of Phytophthora infestans sensu lato on detached leaves of two Solanum betaceum cultivars. Trop. Plant Pathol. 2011, 36, 367–373. [Google Scholar] [CrossRef][Green Version]
- Karki, H.; Halterman, D. Phytophthora infestans (late blight) infection assay in a detached leaf of potato. Bio-Protocol 2021, 11, e3926. [Google Scholar] [CrossRef] [PubMed]
- Andriani, A.; Wouters, D.; Wolters, P.J.; Vleeshouwers, V.G.A.A. Quantifying the contribution to virulence of Phytophthora infestans effectors in potato. In Solanum tuberosum; Humana: New York, NY, USA, 2021; pp. 303–313. [Google Scholar]
- Chaves, S.C.; Guayazán, N.; Mideros, M.F.; Parra, M.; Lucca, F.; Restrepo, S. Two clonal species of Phytophthora associated to solanaceous crops coexist in central and southern Colombia. Phytopathology 2020, 110, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Berdúo-Sandoval, J.E.; Ruiz-Chután, J.A.; Sánchez-Pérez, A. Evaluación de la resistencia de genotipos de tomate frente a aislados de Phytophthora infestans provenientes de Guatemala. Cienc. Tecnol. Salud 2019, 6, 22–33. [Google Scholar] [CrossRef]
- Ristaino, J.B.; Madritch, M.; Trout, C.L.; Parra, G. PCR amplification of ribosomal DNA for species identification in the plant pathogen genus Phytophthora. Appl. Environ. Microbiol. 1998, 64, 948–954. [Google Scholar] [CrossRef]
- Griffith, G.W.; Shaw, D.S. Polymorphisms in Phytophthora infestans: Four mitochondrial haplotypes are detected after PCR amplification of DNA from pure cultures or from host lesions. Appl. Environ. Microbiol. 1998, 64, 4007–4014. [Google Scholar] [CrossRef]
- Knapova, G.; Gisi, U. Phenotypic and genotypic structure of Phytophthora infestans populations on potato and tomato in France and Switzerland. Plant Pathol. 2002, 51, 641–653. [Google Scholar] [CrossRef]
- Lees, A.K.; Wattier, R.; Shaw, D.S.; Sullivan, L.; Williams, N.A.; Cooke, D.E.L. Novel microsatellite markers for the analysis of Phytophthora infestans populations. Plant Pathol. 2006, 55, 311–319. [Google Scholar] [CrossRef]
- Li, Y.; Cooke, D.E.L.; Jacobsen, E.; van der Lee, T. Efficient multiplex simple sequence repeat genotyping of the oomycete plant pathogen Phytophthora infestans. J. Microbiol. Methods 2013, 92, 316–322. [Google Scholar] [CrossRef]
- Velasquez-Vasconez, P.A.; Hunt, B.J.; Dias, R.O.; Souza, T.P.; Bass, C.; Silva-Filho, M.C. Adaptation of Helicoverpa armigera to soybean peptidase inhibitors is associated with the transgenerational upregulation of serine peptidases. Int. J. Mol. Sci. 2022, 23, 14301. [Google Scholar] [CrossRef] [PubMed]
- Abramoff, M.; Magalhaes, P.; Ram, S. Biophotonics International. Image Processing with ImageJ; LAURIN: Pittsfield, MA, USA, 2004. [Google Scholar]
- Bruvo, R.; Michiels, N.K.; D’Souza, T.G.; Schulenburg, H. A simple method for the calculation of microsatellite genotype distances irrespective of ploidy level. Mol. Ecol. 2004, 13, 2101–2106. [Google Scholar] [CrossRef] [PubMed]
- Kamvar, Z.N.; Tabima, J.F.; Grünwald, N.J. Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2014, 2, e281. [Google Scholar] [CrossRef] [PubMed]
- Shakya, S.K.; Larsen, M.M.; Cuenca-Condoy, M.M.; Lozoya-Saldaña, H.; Grünwald, N.J. Variation in genetic diversity of Phytophthora infestans populations in Mexico from the center of origin outwards. Plant Dis. 2018, 102, 1534–1540. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Garduño, T.; Vázquez-Domínguez, E. Métodos de análisis genéticos, espaciales y de conectividad en genética del paisaje. Rev. Mex. Biodivers. 2013, 84, 1031–1054. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Padilla-Ramos, R.; Salas-Muñoz, S.; Velásquez-Valle, R.; Reveles-Torres, L.R. Un nuevo enfoque molecular en el estudio de la interacción parásito-hospedero. Rev. Mex. Fitopatol. Mex. J. Phytopathol. 2018, 37, 95–114. [Google Scholar] [CrossRef]
- Insuasty, S.E.G.; Jurado, G.H. Remolacha forrajera Beta vulgaris sembrada en microtúneles y su efecto en parámetros productivos del cuy. Biotecnol. Sect. Agropecu. Agroind. 2019, 18, 74–83. [Google Scholar] [CrossRef]
- Ali, A.; Alexandersson, E.; Sandin, M.; Resjö, S.; Lenman, M.; Hedley, P.; Levander, F.; Andreasson, E. Quantitative proteomics and transcriptomics of potato in response to Phytophthora infestans in compatible and incompatible interactions. BMC Genom. 2014, 15, 497. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2; Springer: New York, NY, USA, 2009; ISBN 978-0-387-98140-6. [Google Scholar]
- El-Ganainy, S.; Iqbal, Z.; Awad, H.; Sattar, M.; Tohamy, A.; Abbas, A.; Squires, J.; Cooke, D. Genotypic and Phenotypic Structure of the Population of Phytophthora Infestans in Egypt Revealed the Presence of European Genotypes. J. Fungi 2022, 8, 468. [Google Scholar] [CrossRef]
- Olave-Achury, A.; Cardenas, D.; Restrepo, S.; Lucca, F.; Fry, W.E.; Myers, K.L.; Danies, G.; Soto-Suarez, M. Phenotypic and genotypic characterization of Phytophthora infestans isolates associated with tomato and potato crops in Colombia. Phytopathology 2022, 112, 1783–1794. [Google Scholar] [CrossRef]
- Kanja, C.; Hammond-Kosack, K.E. Proteinaceous effector discovery and characterization in filamentous plant pathogens. Mol. Plant Pathol. 2020, 21, 1353–1376. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.N.A.; Carreón-Anguiano, K.G.; Couoh-Dzul, O.J.; de los Santos-Briones, C.; Canto-Canché, B. Effectors: Key Actors in Phytopathology. Rev. Mex. Fitopatol. Mex. J. Phytopathol. 2023, 41, 203–228. [Google Scholar] [CrossRef]
- Hurlburt, N.K.; Chen, L.-H.; Stergiopoulos, I.; Fisher, A.J. Structure of the Cladosporium Fulvum Avr4 effector in complex with (GlcNAc)6 reveals the ligand-binding mechanism and uncouples its intrinsic function from recognition by the Cf-4 resistance protein. PLoS Pathog. 2018, 14, e1007263. [Google Scholar] [CrossRef] [PubMed]
- Mizubuti, E.S.; Aylor, D.E.; Fry, W.E. Survival of Phytophthora Infestans Sporangia Exposed to Solar Radiation. Phytopathology 2000, 90, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Meno, L.; Abuley, I.K.; Escuredo, O.; Seijo, M.C. Factors influencing the airborne sporangia concentration of Phytophthora infestans and its relationship with potato disease severity. Sci. Hortic. 2023, 307, 111520. [Google Scholar] [CrossRef]
- Mboup, M.K.; Sweigard, J.W.; Carroll, A.; Jaworska, G.; Genet, J. Genetic mechanism, baseline sensitivity and risk of resistance to oxathiapiprolin in oomycetes. Pest Manag. Sci. 2022, 78, 905–913. [Google Scholar] [CrossRef]
- Mugao, L. Morphological and molecular variability of Alternaria solani and Phytophthora infestans causing tomato blights. Int. J. Microbiol. 2023, 2023, 8951351. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).