Abstract
Cercospora leaf spot (CLS) is the most destructive fungal disease, deteriorating the production and productivity of mungbean (Vigna radiata (L.) Wilczek). Mungbean is one of the most nutritionally and environmentally important legumes, with popularity currently increasing as a ‘future smart food crop’ due to its several health benefits. In recent years, there has been considerable research progress in improving disease resistance in legumes. However, only a limited number of studies have pinpointed potential genes and candidate genes associated with resistance traits to CLS in mungbeans. Identifying the potential resistant resources through combined omics approaches is an efficient strategy to screen the best Cercospora-resistant mungbean varieties for further molecular breeding and improvement. Potential omics approaches are important tools to predict disease management strategies, alleviate chemical overuse, and mitigate problems due to malnutrition. Sustainable breeding research efforts using potential combined omics, including automated phenotyping, to promote important resistant traits associated with CLS in mungbeans are still unexplored and a key issue that needs to be addressed. Omics-technology-based research findings on resistance genes, proteins, and metabolites against CLS in mungbean are recognised in this review. Due to a limitation of research findings specifically underscoring the use of omics tools for screening resistant mungbean against CLS, best related research outcomes on other crops are included in this review.
1. Introduction
Mungbean [Vigna radiata (L.) Wilczek] or greengram is a self-pollinated, fast-growing, and drought-tolerant legume crop having trifoliate leaves, diploid chromosome set, 2n = 2x = 22, with a genome size ranging from 494 to 579 megabases (Mbs) and mostly adapted to all the tropics and subtropic areas of the world [1,2,3,4,5,6]. Mungbean is the most important legume crop for the agricultural production system and a great source of high-quality protein, minerals, and vitamins [7,8]. The crop plays a valuable role in the agricultural crop rotation system as it has a short duration (60–75 days), is drought-tolerant, and possesses nitrogen fixation capabilities, which play a substantial role in increasing soil fertility [9,10,11]. As a legume food crop, mungbean is utilised in several ways. Seeds of mungbean are eaten as sources of starch, digestible proteins, minerals, vitamins, and amino acids and are used in industrial foods such as vermicelli and starch and cosmetics [12,13]. Nutritionally, mungbean is important as it contains protein up to 30%, carbohydrates (~60%), essential minerals such as iron (Fe), phosphorus (P), and calcium (Ca), small amounts of fats (~1.2%), and dietary fibre (about 6%) [10,13,14,15]. Moreover, a significant amount of micronutrients such as potassium, selenium, magnesium, copper, and zinc are reported in mungbean [2,16]. Mungbean sprouts and young pods are eaten as vegetables to maintain the microbial flora in the gut and reduce the risks of toxic substance absorption, hypercholesterolemia, coronary heart disease, and cancer [15,17]. The nutritional advantage of mungbean over the other legume crops is that it does not form any flatulence in the digestive system as it contains very low anti-nutritional factors, making it suitable for the preparation of ‘weaning food’ for babies [18]. Bunaka et al. [19] stated that mungbeans are considered helpful in defending against several chronic, age-related diseases, including heart disease, cancer, diabetes, and obesity. All these important properties of mungbean increase its popularity in the world and contribute to sustainable food and nutritional security in order to feed the alarmingly growing world population [9,19].
Despite the diverse importance of mungbean as food, animal feed, improving soil fertility, and foreign currency earnings, the crop has not received the same level of attention as cereals to increase its productivity [11,18,20]. It is threatened by a series of biotic stresses, especially diseases due to fungal pathogens such as Cercospora canescens Illis and Martin [21], Fusarium equiseti and Fusarium chlamydosporum [22], and Podosphaera xanthii [23], contributing to major yield losses in mungbean compared to all other crops [21,24]. Cercospora leaf spot (CLS) caused by the fungus Cercospora canescens Illis and Martin is a serious disease in mungbean [21,25,26,27,28]. CLS disease occurs in all the mungbean-growing areas of the world where its initial symptoms appear 30–40 days after sowing, depending upon the temperature and humidity [18,29,30,31]. Initially, the disease appears on leaves as water-soaked spots with greyish borders, but as the plant ages, the severity increases, causing the death of the tissues of infected leaves, petioles, stems, flowers, and pods [28,31]. According to Jackson and McKenzie [32], the brown round to angular spots on the infected leaves with greyish centres and darker margins, up to 1 cm in diameter, are in most cases used for the detection and inspection of lesions of CLS diseases. Sometimes the leaves may become unshaped and wrinkled, along with noticeable late maturity and immature seed formation due to CLS [27,33]. CLS increases in number and size during the flowering season of the mungbean plant, but the increase is most rapid in September during pod filling, causing a yield reduction of up to 96% [2,31,34].
The spread of the pathogen, Cercospora canescens, depends on temperature and humidity where warm wet climate conditions are favourable; 90–100% relative humidity and 20–30 °C temperatures are required for the successful production of conidia of the pathogen and to cause infection [33,35]. The disease is mostly foliar, and once it has developed, the pathogen can survive in the plant debris in the soil as dormant conidiophores or mycelium. As the conidia of the pathogen are contained in plant debris, most of the time the rain splashes play a major role in the dispersal factor [29,33]. As the fungal pathogen, Cercospora canescens has a wide range of hosts and dispersal ability across many geographic locations, and CLS is considered a major disease for many commercial crops such as soybean, common bean, and groundnut [27,36,37]. Das et al. [29] and Bhat et al. [38] stated that having wider host ranges enables the pathogen to have multiple sources of inoculum.
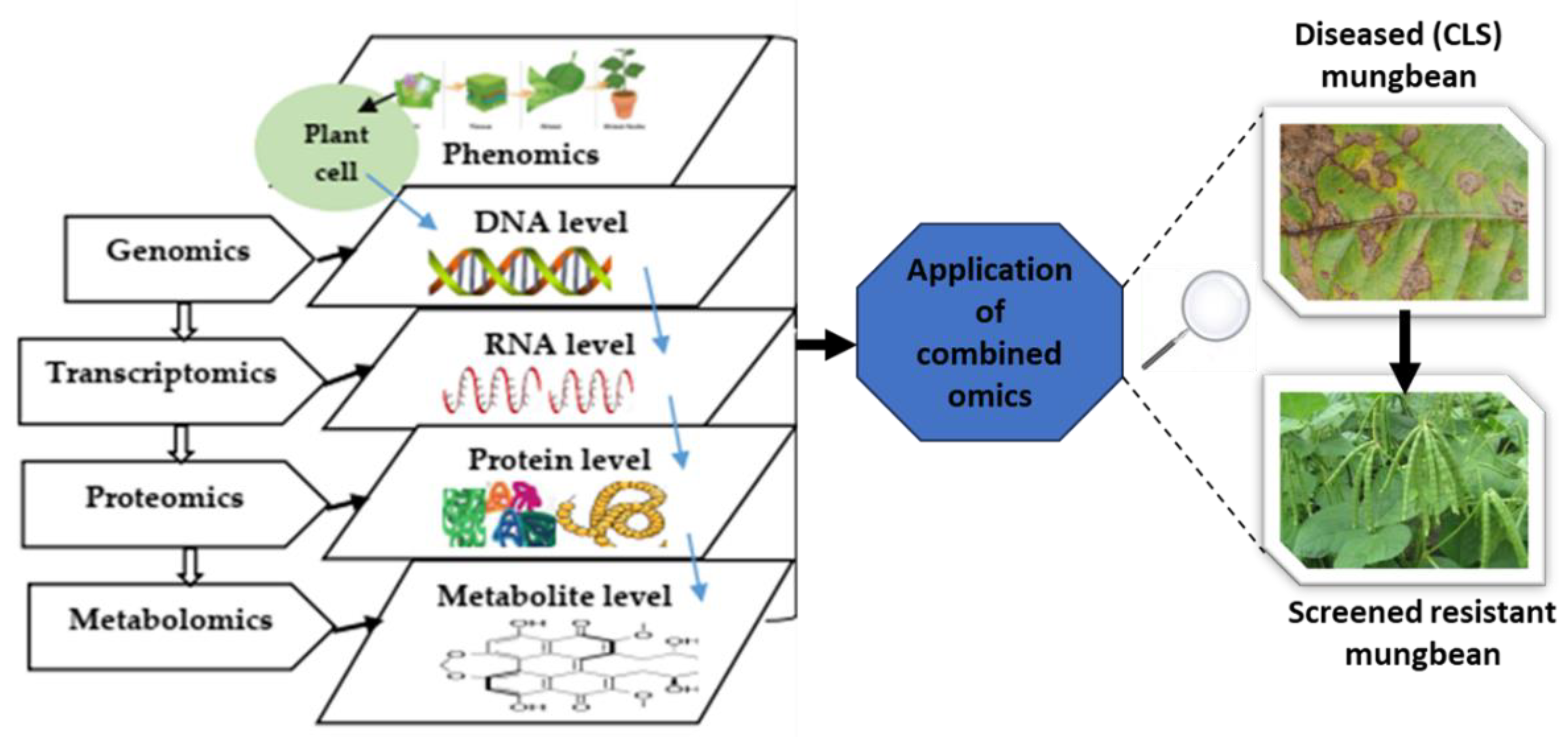
In an attempt to control CLS, several classical strategies of agricultural crop protection, such as fungicide applications, were evaluated mostly in field trials as seed treatments and/or foliar sprays [26,39,40]. Some of such investigations were carried out to evaluate the efficacies of fungicides in reducing disease incidence and/or severity and yield value. However, applications of modern high-throughput technologies and the genomic information for resistance breeding are inadequate in mungbean when compared to cereals and other legumes [41,42], which is a critical component to recommend for mungbean improvement. Furthermore, evaluations of fungal diseases, specifically mungbean’s CLS triggered by Cercospora canescens, have not been consolidated through the utilisation of combined omics such as genomics, transcriptomics, proteomics, metabolomics, and other advanced technologies. Therefore, this review manuscript was initiated to highlight the progressive achievements in recent years in mungbean resistance breeding using these combined omics-based approaches, particularly focusing on the works carried out for the development of robust varieties exhibiting strong and stable resistance against CLS. An overview of the use of potential combined omics approaches is shown in Figure 1. The use of innovative and progressive technologies like high-throughput phenotyping, genomics, proteomics, and metabolomics, as well as the genetic basis of the interactions of plants with pathogens and the environment, can be deciphered to develop effective strategies for plant improvement [43].
Figure 1.
A schematic representation of combined omics applications used in mungbean breeding and improvement.
2. Fundamental Overview of Omics Approaches
Fundamentally, ‘omic’ is the group of four letters that modern molecular breeders use in common as a suffix modifying basic words representing such biological molecules as genome, transcript (RNA), protein, and metabolites to formulate advanced terms like genomic, transcriptomic, proteomic, and metabolomic, respectively. The ‘omics’ approaches are, therefore, technologies that offer the possibility of obtaining new information via in-depth analysis either at the level of the genome, transcriptome, proteome, and metabolome, as well as at the structural and physiological level (phenome) using advanced techniques [2,43]. Genomics is the study of the structure and function of the entire genome of a living organism through mapping, sequencing, and characterising genomes [44]. Nair et al. [2] indicated that genomic markers provide a roadmap for breeders, and Solanke et al. [45] emphasise the use of genomics to realise the variability between genomes, which comes from a number of different sources. These sources include duplication events, transposons, and microsatellites, enabling them to adapt mungbean cultivars against both biotic and abiotic stresses. Qi et al. [46] described transcriptomics as the analysis of gene expression of an organism to provide information about the active genes and functional processes, whereas Kumar and Kirti [47] underlined that proteomics analysis reveals the translational products of gene expression of the plant under particular conditions. Metabolomics is the qualitative and quantitative analysis of the entire metabolome of a biological system [48,49].
Nowadays, researchers from molecular breeding and biotechnology are turning towards innovative combined omics approaches more than ever to identify genetic components implicated in plant resistance ability, growth promotion, secondary metabolite production, and beneficial microorganism habitat adaptation [46,50]. The advent of omics technologies has enabled researchers to directly and unbiasedly monitor the factors that influence growth, yield, metabolism, and biotic and abiotic stress responses [2,43]. Jha et al. [51] and Yang et al. [52] highlight the role of genomics-assisted approaches, such as RNA-seq, proteomics, and metabolomics, in improving resistance to diseases and biotic stresses in grain legumes and crop plants. Raza et al. [53] and Hasin et al. [54] underscore the potential of omics approaches—particularly integrated omics, genome editing and speed breeding—in developing temperature-resilient crops. However, the potential of an integrated omics approach in addressing biotic and abiotic constraints in mungbean production, including diseases and pests, has not been fully explored [2,41,55]. Jost et al. [56] indicated that as there are several complexities of genetic and molecular processes in the resistance mechanisms of host plants against pathogens, there is a need to conduct continuous and extensive research to save resistance genes from rapid breakdown due to constant pathogen evolution. The cultivation of potentially disease-resistant crop varieties could be a sustainable approach in integrated disease management, and host plant resistance can serve as a key strategy to control diseases such as CLS in mungbean [2,26,57]. Despite the lack of information and omics-based studies on the interaction between Cercospora canescens and the crop, it is essential to thoroughly review and consolidate the current research findings. This can demonstrate the significant progress and potential of omics approaches in modern plant breeding for resistance in mungbean to CLS.
3. Genomic-Assisted Approaches for Promoting Resistance in Mungbean to CLS
The establishment of genomics was directly related to the discovery of sequencing methods developed by Fred Sanger (dideoxy termination) and Maxam-Gilbert (chemical degradation) in 1977 [44]. Genomic research in mungbean began to contribute significantly to delivering improved productivity, reliability, and resilience of mungbean through developing and releasing varieties with superior yield, multiple disease resistance, and large green shiny grain for export markets [41]. Research on the genomics of CLS resistance in mungbeans has made significant progress over the past couple of decades, though only very few research reports are there. In this regard, quantitative trait loci (QTLs) mapping for CLS resistance in mungbean was first reported by Chankaew et al. [58] using 753 simple sequence repeat (SSR) markers from various legumes to assess polymorphism between the CLS-resistant mungbean (KPS1) and CLS-susceptible mungbean cultivar (V4718). According to the findings of Chankaew et al. [58], the resistant trait of mungbean to CLS is controlled by a single dominant gene, QTL (qCLS), on linkage group 3 in F2 populations. The authors confirmed the presence of a major QTL (qCLS) for CLS resistance, located between markers CEDG117 and VR393. The location or linkage map of the CLS resistance gene, qCLS, in chromosome 3 was validated in 2019 by Arsakit et al. [59]. Kang et al. [60] constructed a draft genome sequence of mungbean to facilitate genome research and added insights into evolution within Vigna species. This approach can help in mungbean breeding improvement in identifying resistance genes and QTLs and also enable understanding in plant–microbe interactions and develop predictive models for stress responses. Yundaeng et al. [61] from China reported fine mapping of qCLS for resistance to CLS disease in mungbean and identified LOC106765332 encoding TATA-binding-protein-associated factor 5 (TAF5) as the candidate gene for the resistance. The authors used the backcross method for F2 × BC1F1 and developed two mungbean populations from CLS-resistant cultivar (V4718) and susceptible cultivar (KPS1) and applied the In Del (inserts/deletions) single-nucleotide polymorphism (SNP) found in the TAF5 gene. According to this study, the TAF5 gene was observed to enable the mungbean plants to develop resistance against Cercospora canescens. In another study conducted by Sahoo et al. [62] on the assessment of genetic diversity for CLS resistance in mungbean using SSR markers, molecular mapping of pathogen resistance genes was identified in mungbean varieties.
Moreover, in recently conducted research by a team of experts, Babar et al. [42], the antifungal potential of MB-CLsRG gene-encoding protein was assessed to screen CLS-resistant mungbean varieties in genomic analysis that showed strong agreement with phenotypic selection. According to these authors, strong antifungal activity of recombinant protein encoded by the MB-CLsRG gene was observed as the protein inhibiting the growth of the mycelial mass of Cercospora canescens. Akhtar et al. [63] identified a single gene with complete dominance controlling CLS resistance in mungbean, suggesting the potential for single gene transfer methods in breeding for resistance. It was also reported that CLS resistance in mungbean has exhibited a high correlation between disease indicators and a potential oligogenic nature of inheritance in CLS resistance [64]. This oligogenic nature of inheritance was also observed by Yadav et al. [39], who identified sources of resistance in mungbean genotypes to CLS disease and its management. These findings provided a strong foundation for further research and breeding efforts to enhance mungbean resistance against CLS. This was further supported by Sahoo et al. [62], who carried out the assessment of genetic diversity, which found moderate molecular diversity and a wide range of alleles in mungbean genotypes. The importance of genetic factors in controlling CLS resistance was highlighted, with a focus on the disease resistance components of the area under the disease progress curve (AUDPC), incubation period (IP), latent period (LP), and degree of sporulation period [64]. The association and inter-relationship between yield and yield-contributing characters and CLS resistance in mungbean genotypes have been explored, with a significantly positive association between seed yield and several traits and a moderate tolerance to CLS in some mungbean genotypes [65]. Sharma et al. [66] reported successful inter-specific hybridisation between urdbean and moongbean, resulting in resistant reactions to the CLS. Mahapatra et al. [21] isolated and characterised new Cercospora canescens isolates from coastal regions of Odisha, India, contributing to the understanding and management of the disease. Liu et al. [67] provided whole genome scaffold sequences for a bruchid-resistant mungbean line and increased the annotation of mungbean genes as well as obtained a list of putative Br genes and candidates of molecular markers for selecting resistant lines. A near-complete genome sequence of the crop mungbean was constructed by Ha et al. [7] with a scaffold N50 value of 5.2 Mb and only a 0.4% gap, with a total scaffold size of 475 Mb. In 2022, high-quality genome assembly and pan-genome studies in mungbean were conducted by Liu et al. [67] to facilitate genetic discovery and its improvement through extracting genomic DNA from 217 mungbean accessions, sequenced on the Illumina NovaSeq platform.
Sahoo et al. [62] assessed the genetic diversity for CLS resistance in mungbean using SSR molecular markers and found a moderate molecular diversity, indicating the potential for marker-assisted selection (MAS). Koche and Chaudhary [68] investigated the role of defence gene expression in mungbean’s resistance to CLS, highlighting the potential use of biochemical markers in early screening for disease resistance. These studies collectively provide valuable insights into the potential markers and genetic factors for MAS in mungbean against CLS. Next-generation sequencing (NGS) played the greatest role in mungbean through whole genome sequencing, as explained by the work of Kang et al. [60], who constructed a draft of the chromosomal-level genome sequence of the elite mungbean breeding line known as ‘VC1973’ from WorldVeg using Illumina/Solexa and Roche 454 sequencing. Two years after the release of the draft genome sequence of ‘VC1973A’, the whole genome sequence of the mungbean breeding line ‘RIL59’ was reported by Liu et al. [67] through dealing with genomic and transcriptomic comparison of nucleotide variations for insights into bruchid resistance of mungbean. These studies collectively emphasise the capacity of high-throughput techniques to enhance the comprehension of disease resistance in mungbean. Bangar et al. [69] carried out the identification and characterisation of SNPs in released landrace and wild accessions of mungbean using Whole Genome Re-sequencing (WGRS). They identified 233,799 SNPs and 9544 insertions and deletions in coding and non-coding regions working on three mungbean accessions using the Ion Torrent Personal Genome MachineTM (PGMTM) platform, revealing great opportunity for future mungbean improvement using genomic-assisted breeding.
More importantly, to deal with modern genomics of mungbean, considering the Generation of Genomic Sequencing over the past three decades, starting from the Generation of Sanger Sequencing, is indispensable (Table 1). The first published scientific articles in mungbean genome linkage mapping were in 1992 [70,71], and consecutively, RFLP-based comparative genome analysis of mungbean—along with that of cowpea (Vigna unguinculata L.)—was tested for their genetic linkage map in 1993 [72]. During this era, the confirmation of the linkage map for mungbean was based merely on RFLP markers to identify QTLs controlling seed size [70], insect resistance [71], and disease resistance [73]. Table 1 highlights the progress in the use of genomics approaches to inform breeding strategies for the improvement of mungbean. The first report on SSR variation in mungbean was carried out in the late 1990s by Yu et al. [74], whereas polymorphic SSR markers were reported after the year 2000 [75,76]. The genetics of mungbean resistance to CLS disease, caused by Cercospora sp., is a complex trait involving multiple genes [64]. This is consistent with the findings in other plant species such as sugar beet (Beta vulgaris L.), where resistance to various diseases is often controlled by multiple genes [77]. The identification of resistance genes is crucial for the development of disease-resistant mungbean varieties. However, the specific genes and their mechanisms of action in mungbean resistance to CLS disease have not yet been indicated. Moreover, there is no adequate information regarding genomic research so far on the resistance of mungbean to CLS disease. Therefore, further genomic-assisted breeding of mungbean research is needed, which could potentially be used to develop mungbean varieties with enhanced resistance to CLS disease.
Furthermore, some of the very recently attempted genomic research efforts for mungbean breeding improvement are traced here, as in the case of Kohli et al. (2024), who conducted genome-wide association studies (GWAS) for earliness, mungbean yellow mosaic India virus (MYMIV) resistance, and other associated traits. In their study, Kohli et al. [6] used the genotyping by sequencing (GBS) approach to provide valuable insight for marker-assisted breeding aiming for the development of YMD-resistant and early-maturing mungbean varieties. Chiteri et al. [4] undertook a GWAS for dissecting the genetic architecture of leaf morphology traits in mungbean. Liu et al. [3] conducted high-quality genome assembly and pan-genome studies to facilitate genetic discovery in mungbean for its improvement. The same authors assembled a high-quality reference genome (Vrad_JL7) that was 479.35 Mb in size with a contig N50 length of 10.34 Mb and annotated a total of 40,125 protein-coding genes, representing 96.9% of the genetic region. According to the same authors, the overlapping or redundant region of DNA base sequences revealed during gel reading is said to be contig. Liu et al. [3] also sequenced this reference genome for 217 mungbean accessions, mainly for landraces and cultivars from China, and identified 2,229,343 high-quality SNPs. To our knowledge, though the use of gene editing via CRISPR in crop species was publicised 11 years ago by a team of authors [78,79], this molecular plant breeding technology (CRISPR/Cas9) has not yet been practised to provide better opportunities to develop CLS disease-resistant varieties. Thus far, CRISPR applications have been shown to be effective against bacterial, viral, and fungi-borne crop diseases, including powdery mildew in wheat and tomatoes, bacterial blight in rice, and bean yellow dwarf virus [80,81,82].

Table 1.
Generation of genomic sequencing in mungbean (Vigna radiata L.).
Table 1.
Generation of genomic sequencing in mungbean (Vigna radiata L.).
| Generation of Genomic Sequencing in Mungbean (Vigna radiata L.) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sanger Sequencing Generation (SSG) | Next Generation Sequencing (NGS) | Third Generation Sequencing (TGS) | ||||||
| Genomic approach executed | Generation Year | References | Genomic approach executed | Generation Year | References | Genomic approach executed | Generation Year | References |
| Gene mapping using RFLP markers | 1992 | Fatokun et al. [70]; Young et al. [71]; | SSR markers of NGS | 2009 | Gwang et al. [76] | Discovery of candidate genes for bruchid resistance | 2016 | Liu et al. [67] |
| QTL mapping for powdery mildew and CLS disease | 1993 | Young et al. [73] | Discovery of SNP | 2010 | Gwang et al. [76] | Study of Genome-Wide Association (GWA) | 2020 | Sokolkova et al. [83] |
| Enriching the genome library by 13 SSR markers | 2002 | Kumar et al. [75] | Resolution of SSR maps with 11 linkages | 2012 | Kajonphol et al. [84] | Candidate gene for powdery mildew was discovered | 2020 | Yundaeng et al. [85] |
| Draft genome sequence of VC1973A | 2014 | Kang et al. [60] | (1) The draft of genome sequence of VC1973A was improved (2) Candidate gene for CLS disease was discovered | 2021 | Liu et al. [7]; Papan et al. [86] | |||
| High-quality genome assembly and pan-genome studies to facilitate genetic discovery in mungbean and its improvement | 2022 | Liu et al. [3] | ||||||
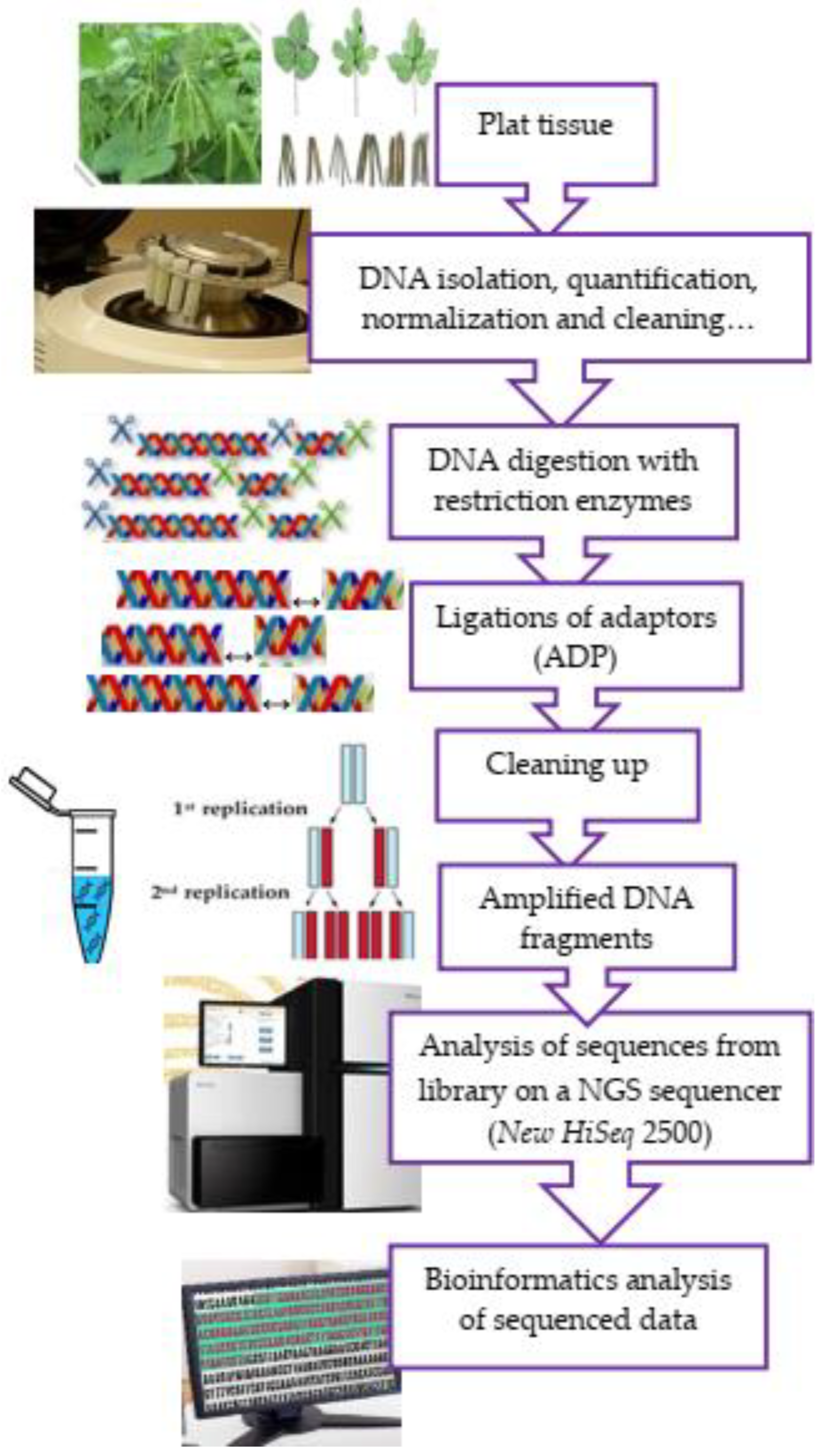
Talakayala et al. [87] worked on manipulating resistance to mungbean yellow mosaic virus (MYMV) in greengram (Vigna radiata L) through CRISPR/Cas9-mediated editing of the viral genome. In their experiment, these authors confirmed that manipulation of resistance to MYMV through the editing of the pathogen genome using the CRISPR/Cas9 tool can be a powerful approach to combat viruses and develop resistance in greengram. Lee et al. [88] experimented on the identification of a locus controlling compound raceme inflorescence in mungbean. Ha et al. [7]—from the Republic of Korea—reported a near-complete genome sequence of mungbean (Vigna radiata L.) to provide key insights into the modern breeding programme. All these studies inform possible genomic mechanisms that may be further investigated for molecular breeding improvement in mungbeans. Sandhu and Singh [89] characterised the Iowa mungbean for field adaptability traits in 2018 and 2019 and genotyped using genotype-by-sequencing (GBS) to conduct association mapping of important traits and identified genetic markers for both quantitative traits such as days to flowering, plant height, leaf drop at maturity, 100-seed weight, and Fusarium wilt score, and qualitative traits such as seed colour, seed coat texture, hypocotyl colour, and pod colour. The GBS method involves using restriction enzymes to break down genomic DNA, then ligating a barcode adaptor, amplifying the DNA pool using PCR, and sequencing the amplified DNA pool on a single flow cell lane. To analyse and interpret GBS datasets, bioinformatics pipelines are also required (Figure 2). GBS is a widely used method to generate abundant and cost-effective markers for genome-wide association studies. High-throughput sequencing technologies are intended to lower the cost of DNA sequencing beyond what is possible with standard dye-terminator methods, like in the case of the research conducted by Chen et al. [90], who generated a total of 36.18 GB of high-quality sequence data containing 183.34 M 100 bp paired-end reads after Specific Length Amplified Fragment Sequencing (SLAF) library construction and sequencing. Fatmawati et al. [91], from Indonesia, investigated the genetic variability of the F2 population of the inter-specific mungbean hybrids using retrotransposon-based markers—particularly InterRetrotransposon Amplified Polymorphism (IRAP) markers—and then identified retrotransposon from the mungbean genome and determined the Long Terminal Repeat (LTR) sequence using the LTR Finder, revealing high heterozygosity and moderate polymorphic information content.
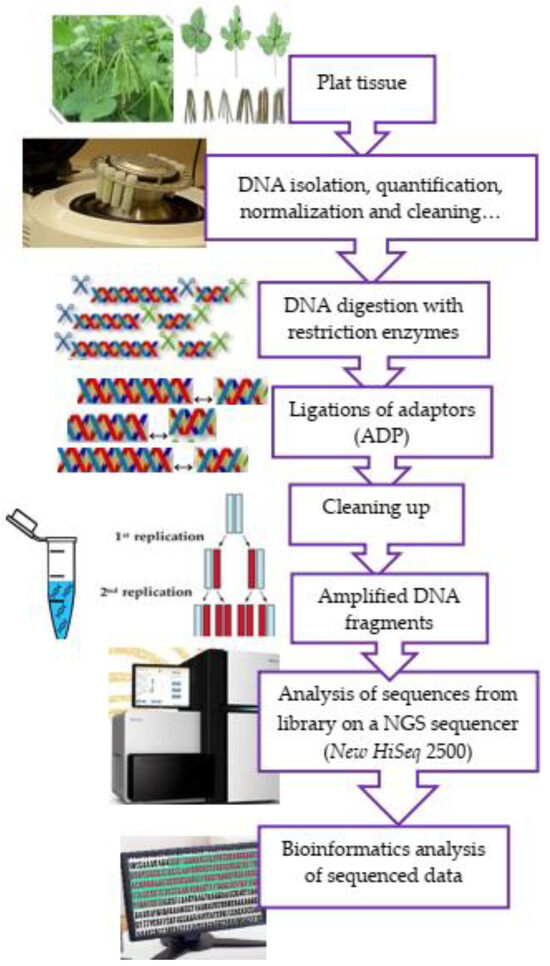
Figure 2.
A schematic flow diagram of genotype-by-sequencing (GBS).
A mungbean plant, like any other plant, possesses three protective layers as an integral component of its overall defence mechanism against a pathogen. These are namely the primary defence, which is related to pathogen-associated molecular pattern-triggered immunity (PTI), the secondary defence involves effector-triggered immunity (ETI), and the additional defence includes the exosome-mediated cross-kingdom RNA interference (CKRI) system [92]. One of the omics study approaches, a genomic study by Dubey et al. [93], is promoting the variation in the expression of various defence-related genes in susceptible and resistant mung bean varieties in response to biocontrol agent Trichoderma virens and R. solani interactions. The authors designed primers using sequences of defence-related genes, namely PR 10, epoxide hydrolase (EH), catalase, and calmodulin available in the NCBI database, and evaluated them against cDNA obtained from both susceptible and resistant mung bean plants at 1–4 days post-inoculation (dpi) with the test pathogen R. solani and biocontrol agent T. virens using conventional PCR and qPCR analyses. This can give an insight into the future breeding programme to identify resistant genes in mungbean against a particular pathogen, namely Cercospora canescens, which causes CLS, a fungal disease, making mungbean cultivation difficult worldwide.
Recent advances in omics, particularly genomics technologies, have made QTL mapping possible to identify the genes conferring resistance against CLS. Developing high-yielding varieties with enhanced resistance by tagging and mapping of loci/gene(s) governing resistance to the specific disease is a more effective and efficient approach [94]. Relatively, rapid progress has been made in mungbean genome research since 2010, and several ISSR- and SSR-based maps have been developed for QTL mapping of various traits, including agronomic and domestication-related traits, biotic and abiotic stress resistance, and chemical contents of seeds [58,95,96,97]. QTL mapping is structural genomics that includes QTL confirmation, QTL validation [98], and fine (or high-resolution) mapping of the genes [94]. The progress of QTL mapping for disease-resistant mungbean has advanced significantly in recent years, particularly starting from the study of Chankaew et al. [58] to the very recent findings where Chang et al. [99] constructed a genetic linkage map and identified QTLs for drought tolerance. Ha et al. [7] used an ultra-high-resolution linkage map constructed based on re-sequencing data and identified QTLs and the underlying candidate genes affecting synchronous pod maturity (SPM) in mungbean. QTL studies were carried out on different aspects of mungbean crops for agronomic traits [100,101], for bruchids [90], and for innovative study of CLS disease resistance [97]. The development of integrated consensus gene maps, which include map locations of resistance genes for CLS and phenotypic traits, has further enhanced the understanding of disease resistance in the mungbean. Moreover, these studies collectively stress the importance of QTL mapping complemented with sound and modern molecular markers in identifying genomic regions of interest that control CLS disease resistance in mungbean and the potential for this approach to inform breeding strategies for the improvement of the crop. Papan et al. [97] identified QTLs for CLS disease resistance (qCLSC72V18-1) in F2:9 and F2:10 recombinant inbred line (RIL) mungbean populations derived from ‘CN72’ × ‘V4718’ crosses over two years. In this regard, these authors used two flanking Indel markers—namely I16274 and VrTAF5 located on 34 and 35 cM—from the resistance gene and accounted for 60.38% and 41.56% of the phenotypic variation, respectively. To our knowledge, these are the only QTLs that have been detected for CLS disease in mungbean to date, next to the work of Chankaew et al. [58], who report the first time QTL mapping for CLS resistance in mungbean from Thailand. Furthermore, in different crops, a range of studies have identified QTL and candidate genes associated with resistance to several leaf spot diseases. Gu et al. [102] identified 6 loci and 55 genes associated with resistance to frogeye leaf spot (FLS) in soybean, while Sharma and Lightfoot [103] found QTLs near Satt319 and Satt632 on chromosomes 7 and 8, respectively, in the same species. Chen et al. [104] identified 11 QTLs for resistance to grey leaf spot (GLS) in maize. Though these studies collectively contribute to the understanding of the genetic basis of resistance to CLS diseases, there have been only a few studies reporting the genetics and genomics of resistance to CLS disease in mungbeans.
Of the mungbean genomics research conducted in different parts of the world to date, QTL studies have focused mainly on insect and fungal disease resistance, primarily because these problems pose a major threat to mungbean yield. In this regard, Mei et al. [105] and Wang et al. [106] from China worked on the bruchid resistance gene in mungbean, whereas Chankaew et al. [58] and Papan et al. [97] from Thailand studied CLSD. Mathivathana et al. [107] from India identified YMV, and Chankaew et al. [95] and Poolsawat et al. [108] from Thailand mapped out powdery mildew resistance genes based on QTLs analysis. In this regard, Chankaew et al. [95] detected QTL controlling powdery mildew disease resistance in two resistant mungbean accessions, V4718 and RUM5, during the years 2011 and 2012 and then identified three QTLs, namely qPMV4718-1, qPMV4718-2, and qPMV4718-3, in the accession V4718. According to this study, qPMV4718-2 on linkage group 4 (LG4) and qPMV4718-3 on LG9 were consistently identified in different years, whereas qPMV4718-3 explained a high proportion of the resistance to powdery mildew. Kitsanachandee et al. [109] reported QTLs for MYMIV resistance by evaluating 122 RIL mungbeans and their parents in infested fields of India and Pakistan. Accordingly, composite interval mapping with SSR markers was used, and then five total QTLs for MYMIV resistance were identified, which included three QTLs for India (qYMIV1, qYMIV2, and qYMIV3) and two QTLs for Pakistan (qYMIV4 and qYMIV5). Singh et al. [94] investigated a diverse panel of 127 mungbean genotypes against MYMIV and identified novel QTLs/associated markers for MYMIV resistance through an SSR-based genome-wide association mapping approach. Ye et al. [101] worked on a mungbean bi-parental cross between Sulu16-10 and Weilu11 and identified a total of 14 QTLs distributed on chromosomes 1, 3, 4, 6, 7, and 10 associated with several agronomic traits such as young stem colour (YSC), days to first flowering (DFF), days to maturity (DM), plant height (PH), and nodes on the main stem (NMS) during the study year 2019 in the Jiangsu Academy of Agricultural Sciences, Jiangsu, China. Evaluation of different mungbean plant populations using phenotypic traits such as PH [7] and leaf shape [100] and other agronomic traits relating to QTL mapping are valuable approaches for improving the yield, nutritional quality [96], and stress tolerance of the crop [110]. On the other hand, in order to identify consistent candidate QTLs for marker-assisted selection, genomic mapping should probably be conducted in several genetic backgrounds in which there is genetic variability for the important traits [111]. QTL mapping has been used in the mungbean to identify genomic regions associated with a range of traits such as drought tolerance [99,112], seed weight [113], and resistance to diseases [94]. Moreover, QTLs linked to seed coat colour in a panel of mungbeans were identified using genome-wide association mapping (GWAM) that included both cultivated and wild accessions [114].
Additionally, a team of researchers from Korea [7] reported mungbean homologs of two soybean flowering genes, namely E3 (phytochrome A) and J (early flowering 3) as the QTL candidate gene using ultra-high-resolution linkage mapping. According to this finding, the candidate genes for PH, node number, and SPM showed critical nucleotide substitutions between the reference cultivar and other genotypes. High-density genetic mapping, which is a high-resolution level, was carried out by Chen et al. [90], who identified resistant genes for bruchid (Callosobruchus spp.) in mungbean using 4180 SNP. Lin et al. [115] indicated that QTL with VrYSL3 is a strong candidate gene responsible for calcareous soil (iron deficiency) resistance in mungbean based on fine mapping. In the research conducted by Mathivathana et al. [107] using GBS, a linkage map was constructed, and five QTLs associated with MYMV resistance in an inter-specific population of mungbean and rice bean were identified. Likewise, QTLs linked to bruchid resistance in mungbean were identified by Schafleitner et al. [98] using GBS. Moreover, Jiao et al. [1] discussed the fine mapping locus of the lobed leaf margins (LMA) in mungbean and noted that the LMA locus on chromosome 3 in mungbean showed a syntenic region on chromosome 1 in common beans. Generally, these studies collectively stress the importance of QTL mapping complemented with sound and modern molecular markers in indicating ways to control CLS disease resistance in mungbean and the potential for this approach to inform breeding strategies for the improvement of the crop.
4. Transcriptomic Approach for Mungbean Breeding Improvement
To date, there is a lack of published transcriptomic research information targeting resistance in mungbean to CLS. However, it is important to undergo the review appraisal of some of the ideal mungbean transcriptomic-related research findings that can be a model for the modern high-throughput transcriptomic technologies so as to set future directions for further breeding improvement of the crop, mungbean. In this regard, nearly eight years ago Chen et al. [116] from China reported on the transcriptome sequencing of mungbean genes and the identification of EST-SSR (expressed sequence tag of simple sequence repeat) markers in which high-quality cDNA sequence reads were obtained from mungbean using Illumina paired end sequencing technology. As a result of this study, the average length of the assembled transcripts in mungbean genes was 1194 bp (N50 = 1936 bp), which was longer than the average length of the assembled unigenes (874 bp, N50 = 1563 bp), indicating the importance of EST-SSR markers for traits of interest in the molecular breeding of mungbean. Tian et al. [117] investigated the transcript profiling of mungbean seeds under different imbibition times for desiccation tolerance using RNA sequencing (RNA-seq). The authors identified key genes and pathways involved in desiccation tolerance, including those related to stress response, transcription regulation, and metabolism. Jiao et al. [1] reported on the gene, Vradi03g04470, encoding an A20/AN1 zinc finger domain transcription factor that was the probable lobed leaflet margin (LMA) gene within the margin of the (LMA) locus, having worked on genome re-sequencing of two accessions and fine mapping the locus of lobed leaflet margins in mungbean.
Wang et al. [118] integrated transcriptomic and metabolic frameworks for carbon metabolism and plant hormone regulation in mungbean during post-germination seedling growth, confirming that transcriptomic studies have made a significant contribution to the improvement of mungbean. Kumar et al. [119] conducted research on the screening of mungbean for drought tolerance and transcriptome profiling between drought-tolerant and susceptible genotypes in response to drought stress. According to these authors, a transcriptomic study between K-851 and PDM-139 revealed 22,882 differentially expressed genes (DEGs), which were identified under drought stress and were mainly mapped to phytohormone signal transduction, carbon metabolism, and flavonoid biosynthesis. Hu et al. [120] analysed the effect of Uniconazole (UNZ) application on the growth and transcriptomic profile of mungbean under chilling stress. Then Hu et al. [120] inferred that UNZ application effectively prevented the further downregulation of the gene expression of the KEGG (Kyoto Encyclopaedia of Genes and Genomes) pathways under chilling stress and increased mungbean tolerance to chilling stress. Sudha et al. [121] identified genes involved in MYMV resistance in the resistant mungbean genotype ‘VGGRU-1’, which is a derivative of the cross VRM (Gg) 1 × TNAU RED, by comparing the DEGs between VGGRU-1 and VRM (Gg) to candidates for further resistance investigation at a molecular level.
Moreover, Zhang et al. [122] studied the transcriptional profiles during seed development and subcellular localisation of two genes, VrPGIP1 and VrPGIP2, located in the Br locus, from bruchid-resistant mungbean cultivar V2802. Their findings inferred that the two genes play an important role in defence against stored insect bruchids, and PGIP proteins are important in the selective breeding of resistant mungbeans to Callosobruchus maculatus. Lim et al. [123] informed on transcriptomic changes in mungbean sprouts through RNA sequencing, where 728 DEGs were identified for several metabolite biosynthetic pathways. In the recently conducted study by Guo et al. [5] on transcriptomic and biochemical analyses of drought response mechanism in mungbean leaves, RNA sequencing-based DEGs were analysed to clarify the molecular mechanism of mungbean in response to drought stress. In this study, the relationship between molecular and physiological changes was analysed to screen candidate gene responses to drought stress in mungbean, which provides a vital foundation for future studies assessing drought resistance in mungbean. Generally, these studies collectively highlight the potential of high-throughput transcriptomics in improving the molecular breeding of mungbeans.
5. Proteomics Approach in Mungbean Retrospective Investigations
Modern molecular approaches like proteomics, along with other combined omics and genetic engineering, are powerful tools for promoting resistance against plant pathogens through the proteins expressed by an organism [46,55]. The amino acid, serine, can be changed to theorine in the mungbean resistance gene to CLS disease by the functional SNPs known as Indel in exon 8 located in the gene [61]. Antimicrobial peptides and other compounds derived from plants can suppress pathogenicity by direct detoxification or through inhibition of the activity of virulence factors [42]. Proteomics can be described as the study of the interactions, function, composition, and structures of proteins and their cellular activities where the automated, high-throughput functional and structural proteomics technologies use advanced materials such as multidimensional nuclear magnetic resonance spectroscopy and X-ray crystallography [124]. Chunkao et al. [125] used anion-exchange chromatography separation and a continuous enzymatic membrane reactor (cEMR) for the production of mungbean protein hydrolysate (MBPH). From the other previously conducted research by Hu et al. [120], Ca2+-dependent protein kinases (CDPKs), mitogen-activated protein kinase cascade transcription factors (TFs), and reactive oxygen species (ROS) were found to be upregulated from transcriptomic profiling of mungbean under chilling stress by the expression of important Ca2+ signalling genes. Zaker [126] described plant antifungal proteins as strong agents for sustainable regulation of biotic and abiotic stresses, as they are nontoxic to humans.
As mungbean is a protein-rich leguminous food crop, Wang et al. [100] tested for various metabolic and physiological changes during mungbean post-germination seedling growth leading to the improvement of its nutritional value. During this experiment, most amino acids kept increasing and accumulated in six-day germinated sprouts. From the study conducted on metabolic and regulatory pathways in the desiccation tolerance of mungbean seeds, in embryogenesis abundant proteins and genes encoding methyltransferase and histone were differentially expressed [117]. Having intensively worked on mungbean proteins and peptides, Yi-Shen et al. [127] from Ireland concluded that mungbean proteins and their hydrolysates hold great sources of compounds with significant nutritional, functional, and bioactive potential uses in foods, pharmaceuticals, and other products and processes. Correspondingly, amino acid sequences of peptides present in the reverse-phase HPLC fraction with the strongest iron-binding capacity were identified from mungbean seeds by Chunkao et al. [125] using mass spectrometry and 10 peptides of 5–8 amino acids synthesised for antioxidant characterisation. The mechanism of action of proteins to inhibit the mycelia of fungal pathogen growth includes damage to cellular ribosomes, inhibition of DNA synthesis and cell cycle, as well as the degradation of cell walls. For example, Maurya et al. [128] identified enzymes like catalase and superoxide dismutase that are involved in detoxifying cercosporin (a toxic substance secreted by the pathogen that destroys the host plant membrane) in mungbean resistance against the fungal pathogen known as Cercospora canescens. Zhang et al. [122] expressed two polygalacturonase inhibiting proteins (PGIP) regulated by two family genes, VrPGIP1 and VrPGIP2, located in the Br locus from mungbean responsible for bruchid resistance in mungbean. They then prokaryotically expressed and purified the two VrPGIP proteins and demonstrated that VrPGIP1 and VrPGIP2 play a critical role in bruchid resistance, probably through inhibiting polygalacturonase enzyme activity. Moreover, Babar et al. [42] tested the antifungal activity of the purified protein assessed through the modified radial diffusion method, and then the purified protein (~15.4 kDa) exhibited a strong inhibitory effect on the mycelial growth of Cercospora canescens.
6. Metabolomic Approaches to Mungbean
Tugizimana et al. [48] elucidated that metabolomics is progressing beyond biomarkers towards mechanisms as applied in the plant sciences with the concern of chemometrics and metabolite network analysis pathways detected by comparison of metabolite profiles with subsequent quantification of discriminatory biomarkers. A series of studies have shed light on the transcriptomic and metabolic processes in mungbean (Vigna radiata L.). A team of researchers (Lim et al.) [123] from Gangneung-Wonju National University of South Korea conducted an innovative experiment on mungbean sprouts after treating them with NaCl (salinity stress) to check for metabolomic and transcriptomic changes. They clearly qualified and quantified those polyphenolic metabolites—such as catechin, chlorogenic acid, isovitexin, p-coumaric pcoumaric acid, syringic acid, ferulic acid, and vitexin—significantly increased and identified the key enzymes involved in the biosynthetic pathways of these compounds. Wang et al. [118] studied the regulatory mechanisms of primary metabolites during post-germination seedling growth, highlighting the roles of starch and sucrose metabolism, glycolysis, and plant hormones. Inayati et al. [129] investigated the growth performance and metabolic changes in mungbean during interactions with pathogens and beneficial microorganisms, revealing significant alterations in metabolic pathways. Wang et al. [118] further explored transcriptomic and metabolomic analyses of mungbean samples from 6 h, 3 days, and 6 days after imbibition to characterise the regulatory mechanism of the primary metabolites during the post-germination seedling growth. According to this study, rapid changes were observed at a transcript level, including metabolites such as starch and sucrose metabolism, glycolysis, citrate cycle, amino acid synthesis, and plant hormone regulation. Metabolites such as carbohydrates and amino acids exhibited an increment in 6-day germinated sprouts. This finding of the study conducted by Wang et al. [118] is in line with the findings of the previously conducted research by Tian et al. [117], who also identified key metabolic and regulatory pathways, including starch and sucrose metabolism, glycolysis, and plant hormone regulation, as well as the roles of TFs, heat shock proteins, and late embryogenesis abundant proteins. Dynamic changes in phytochemical composition and antioxidant capacity in mungbean sprouts were tested by Gan et al. [130], and then metabolites such as caffeic acid, ferulic acid, gallic acid, p-coumaric acid, catechin, and rutin were identified. These metabolite compounds were also confirmed as important to maintain human health and prevent chronic diseases [131,132].
Salicylic acid, a signalling metabolite, was identified as the most potent antifungal drug by activating mungbean defence mechanisms for the treatment of CLS disease, according to a new study by Malik et al. [31]. The study looked at the induction of resistance to CLS disease in mungbeans through the use of plant defence activators such as K2H2HPO4, salicylic acid, carboxylic acid, citric acid, and benzoic acid. This finding indicates that these plant defence activator compounds are kinds of various signalling metabolites involved in a plant defence pathway system. Malik et al. [31], in their discussions, clearly described that the fungal pathogen for CLS disease, Cercospora canescens, produces a toxin known as perylenequinone-based cercosporin. This cercosporin significantly contributes to the severity of the disease and affects plants through creating ROS in plant tissues that destroy the host plant’s membrane and provides nutrients to support the growth of this intercellular pathogen [133]. The other latest report, where Rafiq et al. [134] conducted an experiment on biofabrication of zinc oxide nanoparticles to rescue mungbean against CLS disease, shows that green synthesised ZnO NPs can control Cercospora canescens in mungbean, pointing to their use in plant disease control and growth enhancement. Generally, all these studies collectively highlight the potential of metabolomics in elucidating the molecular mechanisms of mungbean plant resistance to CLS.
7. Phenomics Achievements in Instances for Mungbean Breeding
Phenomics is described as the high-throughput analysis of phenotype through the evaluation of the morphological, physiological, and biochemical traits of an organism and the correlation of genetic, epigenetic, and environmental factors [135,136,137,138]. Since the advent of molecular breeding technologies, genotyping has been evolving from labour-intensive to highly automated methods, whereas most on-farm phenotyping is still being carried out in a simplistic, manual, time-consuming, and traditional way [139]. This is an obstacle for breeders and reduced breeding efforts [140]. However, the phenotyping systems should operate in a field setting or in a controlled environment, where plants are automatically tested and analysed in a machine-augmented manner [141]. Nair et al. [2] highlighted the importance of genetic improvement in addressing biotic and abiotic constraints in mungbean production, suggesting that advanced technologies such as genomics and proteomics could be valuable in this effort. A very recently published review article by Ngugi et al. [142] on revolutionising crop disease detection with computational deep learning stated that the adoption of deep learning (DL) algorithms—such as digital image processing—has prompted the development of cutting-edge techniques for early detection and diagnosis of crop diseases, leveraging tools such as convolutional neural networks (CNN), K-nearest neighbour (KNN), support vector machines (SVM), and artificial neural networks (ANN). Automated and high-throughput on-farm phenotyping is very important during data gathering so as to make it more reliable over the conventional ones, like in the case of previously conducted research by Azam et al. [143] on the genetic analyses of mungbean breeding traits for selecting superior genotype(s). In this regard, these authors used a handheld infrared thermometer (model AG-42, Tela Temp Corporation, Fullerton, CA, USA) to calculate canopy temperature (°C) from each plant on clean and vivid sunny days between 12:00 and 14:00. Liu et al. [144] developed a real-time and visual loop-mediated isothermal amplification (LAMP) assay based on the cytochrome b (cytb) gene to detect Cercospora canescens in mungbean using SYBR Green I dye. The LAMP primer set designed in this study was shown to enable reliable discrimination of C. canescens, confirming its specificity. SYBR Green I is a commonly used fluorescent dye or stain that binds double-stranded DNA molecules by intercalating between the DNA bases in an automated manner. Crop disease detection using the random forest model algorithm (RFM) and the histogram of an oriented gradient (HOG) was developed by [145]. Ramesh et al. [145] used HOG to extract features from a labelled image dataset consisting of 160 images of papaya leaves. The extracted features are used to build the RF model. The model produced a classification accuracy of 70.14%.
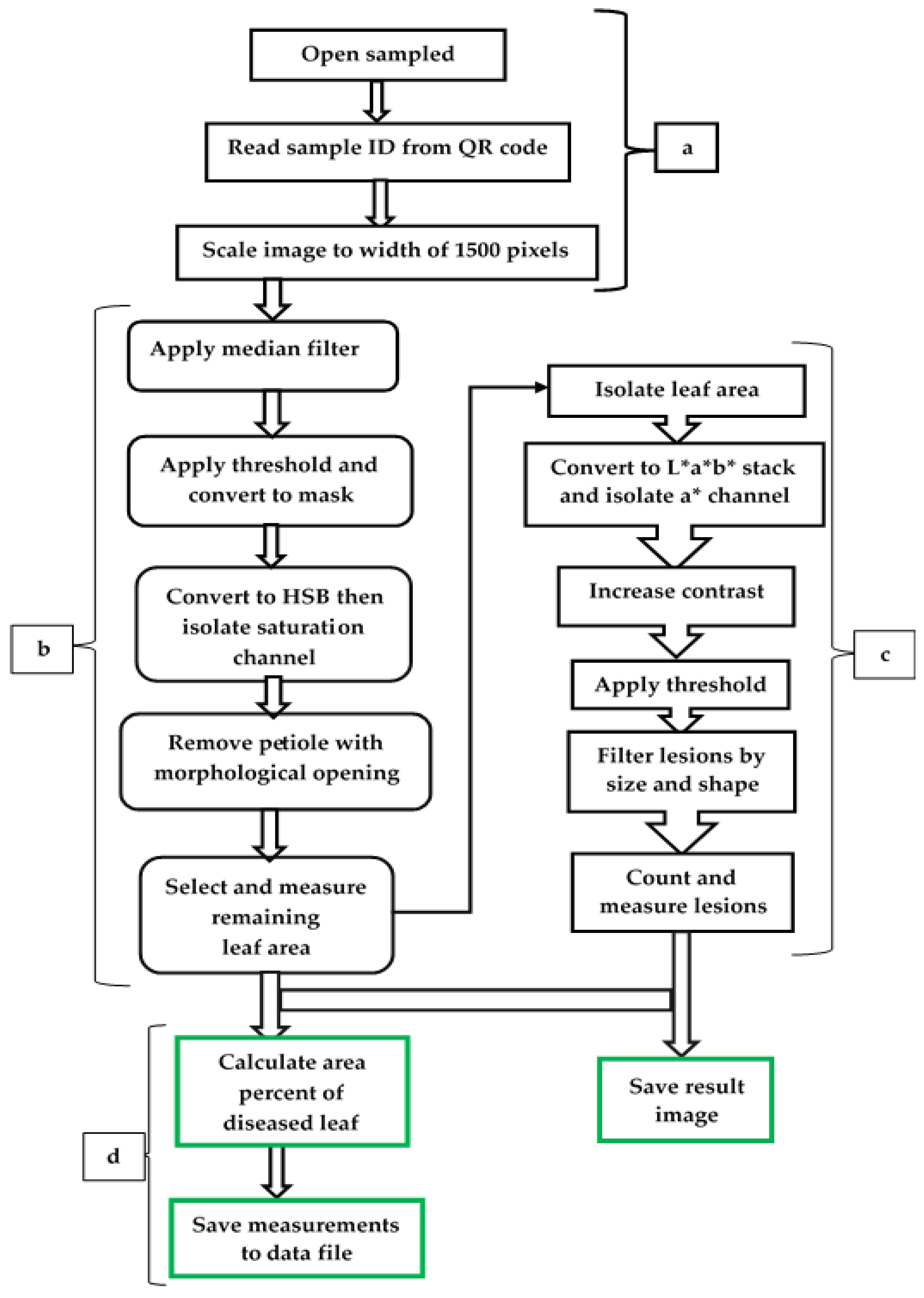
Automated phenotypic data are more reliable and can help recognise which gene variants affect a phenotype, pleiotropic effects, and infer the causes of health, crop yields, disease, and evolutionary fitness [146,147]. A hands-free, automated ResNet9 model was described by Anim-Ayeko et al. [148] to be used to detect the blight disease of both potato and tomato leaf images for farmers to leverage, considering leaf shape, its diseased area, and general green areas for its predictions. The model achieved 99.25% accuracy, 99.67% precision, 99.33% recall, and an F1-score of 99.33%. Abdu et al. [149] undertook digital phenotypic characterisation of seedling growth in mungbean and identified the external morphology of its seeds using image analysis and the morphological characteristics associated with high physiological potential. In their study, they confirmed that in the green mungbean, there was a direct relationship between seed area and perimeter, length, width, and colour; however, the best seedling performance was found in the smaller seeds, inferring that the use of image analysis makes it possible to identify seeds of greater physiological potential, which allows more vigorous batches of mungbean seeds to be selected. Experimental research was conducted by McDonald et al. [150] to develop an automated image analysis phenotyping pipeline to measure and count frogeye leaf spot (FLS) lesions, which were caused by Cercospora sojina, on soybean leaves with high precision and resolution while ensuring data integrity. This team of researchers [150] used colour space transformations and pixel value thresholding to segment key features from the image of the leaf and FLS lesions. Given that FLS and CLS, both diseases, share nearly identical traits in the development of leaf lesions and impact comparable legume species, despite being caused by distinct fungal species within the same genus, it is also feasible to suggest an automated phenotyping model (Figure 3) for CLS in mungbean. This enables the identification of new sources of crop genetic resistance to CLS in mungbean, which demands an advanced method that produces accurate and detailed phenotype information.
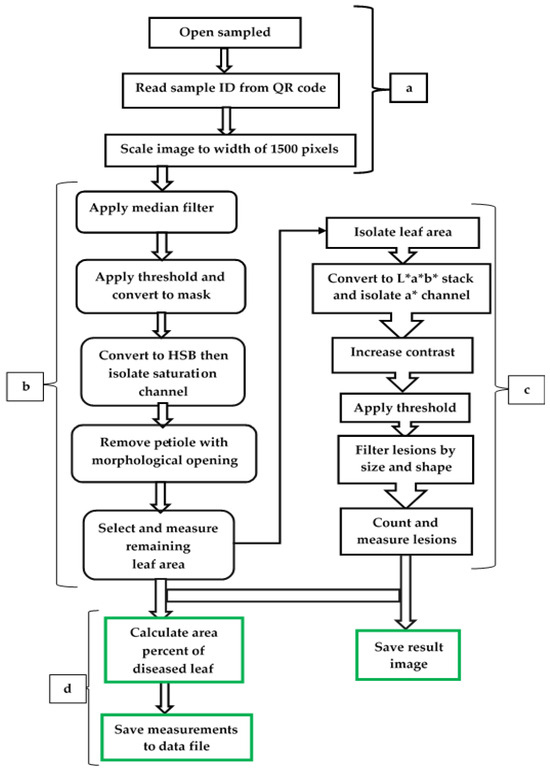
Figure 3.
A proposed image-based automated phenotyping model for CLS, modified from a method for measuring and counting frogeye leaf spot lesions on soybean leaves [148]. There are four integrated main steps as image preprocessing (a), leaf segmentation (b), lesion segmentation (c), and calculation and data reporting (d).
The recent breeding developments have paved the way for high-throughput phenotyping by inventing mechanised tools such as infrared thermography, spectroscopic techniques, fluorescence imaging, and integrated imaging techniques that are being used by the developed breeding programmes [138,139,151]. Automated digital cameras have been effectively used in screening for stress tolerance in mungbean, whereby the integrated use of software for high-quality imaging of complex phenotypic parameters—such as leaf area and chlorophyll fluorescence imaging—helps to dissect the genetics of photosynthesis at the different levels of both plant physiology and development [141]. Wang et al. [152] studied automatic image-based apple black rot disease severity estimation using DL and inferred that vast disease data at different disease stages can be collected with versatile sensors, like infrared cameras and multispectral cameras. Based on the findings of Wang et al. [152], they advised that a DL model can be associated with treatment recommendation, yield prediction, and so on. Maurya et al. [128] tested on the interspecific cross of Vigna radiata (Kopergaon susceptible) × Vigna mungo (Pant Urd 31 resistant) by using an automated method in which CLS was induced by the photoactivated toxin cercosporin and confirmed that antioxidants can elevate the resistance ability of Cercospora canescens. The study identified few RIL-resistant mungbean cultivars to CLS infection in response to utilisation of the antioxidants such as superoxide dismutase (SOD) and catalase (CAT) enzymes scavenging corcosporin of the pathogen after CLS infection.
Jangra et al. [153] stated that high-throughput phenotyping technology has revolutionised phenotyping, which is rapidly growing and relies on automated trait analysis to generate phenotypic data, which involves automated sensing, data acquisition, and data analysis. In this regard, a real-time, automated phenotyping workflow integrating imaging, data analytics, and machine learning was developed by Naik et al. [146] to determine the severity level of iron deficiency chlorosis (IDC), which causes interveinal chlorosis and necrosis in soybean. Rane et al. [154] unveiled perhaps the first report stating the use of a phenomics method as a promising tool for assessing growth rates and the productive use of water in mungbean crops based on three purposes, i.e., the imposition of suboptimal soil moisture stress, acquisition of different parameters of images of plants from the top view and side view, and identification of the suitable biomass. The application of ultrasound technology has also been explored with promising results in improving mungbean hydration and germination [155]. Parmar and Patel [156] identified several mungbean varieties—including Vishal, Samrat, and GM-3—as resistant to the storage pest Callosobruchus chinensis, and Fang et al. [157] demonstrated the potential of the PR-1 gene, MuPR1, in enhancing disease resistance in mulberry, suggesting a similar approach can be addressed in the investigation of mungbean resistance to CLS in the future.
The methods of molecular techniques that are commonly employed, usually concentrating on just one type of omics at a time, are not fully providing the required information about cellular molecules to accurately predict the amount and quality of the results. One of the potential ways could involve the use of technologies that can process large amounts of data in a continuous manner to represent various interconnected and combined omics such as genomics, transcriptomics, proteomics, metabolomics, and phenomics. This allows for the reduction of difficulties related to variations in data cleansing, standardisation, identification of biomolecules, reduction of data dimensions, statistical confirmation, storage and management of data, dissemination, and long-term preservation of data. The ultimate aim is to achieve a comprehensive understanding of the biological challenges through a ‘system biology’ approach. Therefore, using methodologies for blending the combined omics data is crucial to integrating the many insights from each omics component.
8. Conclusions and Future Directions
Disease is one of the biotic factors affecting crop production and productivity, of which fungal diseases, specifically Cercospora leaf spot (CLS), caused by Cercospora canescens, are the most important disease causing severe yield loss in mungbean. Mungbean is one of the world’s most important leguminous crops that is expected to play an important role in the future of dry land agriculture to alleviate global food stress and malnutrition. As a disease has complicated characteristics and its incidence and severity fluctuate along with the changing global climate, nowadays, the crop mungbean is becoming more vulnerable to CLS. Therefore, there is a need to improve the mungbean breeding programme for resistance traits related to CLS through developing climate-resilient and climate-resistant mungbean cultivars using potential combined omics technologies. Developing and enhancing crop varieties that are resistant to diseases is the most effective method for managing diseases while ensuring the environment remains safe. Because of the complex nature of pathogens, particularly that of C. canescens, it requires the integration of potential high-throughput combined omics approaches to increase breeding efficiency and genomic selection for rapid genetic gains to develop CLS-resistant mungbeans. Few advancements have been made in the last couple of decades to promote resistance in legumes against diseases through third-generation sequencing coupled with omics approaches, in addition to classical and next-generation breeding. The concern of mapping genes of interest and QTLs, which can be used as markers for introgression resistance from donors to recipient mungbean germplasm, has not yet been addressed, where only two articles were included in this review. The QTL studies targeting important genes in mungbean that help withstand environmental conditions and resist pathogenic factors are ultimately important to improve mungbean’s quality and productivity. There must be systematic efforts towards studying the entire profile of genes, proteins, and metabolites informing resistance in mungbean against CLS. High-throughput omics approaches will help to save time with minimum labour to obtain more reliable and accurate data during the evaluation and identification of disease resistance, which further enable to predict disease management systems and crop loss. Genomic editing technologies through CRISPR/Cas have not yet been practised for mungbean, including its resistance investigations against CLS. Therefore, future studies must pay more attention to utilising high-throughput tools to promote the resistance in mungbean against CLS. Furthermore, automated CLS disease detection in mungbean plants using deep learning methods is needed to provide reliable information at both on-farm phenotyping and in vitro conditions. An automated phenotyping model, such as image-based disease characterisation for phenotyping resistance to mungbean CLS, is important to ensure data integrity with high precision and accuracy. This further enables us to consistently predict the status of future food security for the alarmingly increasing global human population.
Author Contributions
T.B.G.: Collection of materials and literature survey on combined omics approaches, writing the contents; S.F.: planning, drafting, guiding and editing the manuscript; G.K.: references arrangement. All authors have read and agreed to the published version of the manuscript.
Funding
This work was based on generous financial support from the University of South Africa.
Acknowledgments
The authors would like to appreciate the language editor Jane Franz. The authors also express their gratitude to the authors whose valuable work has been utilized in this review, and extend their apologies to those authors whose exceptional work could not be referenced due to space constraints.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jiao, K.; Li, X.; Guo, W.; Yuan, X.; Cui, X.; Chen, X. Genome Re-Sequencing of Two Accessions and Fine Mapping the Locus of Lobed Leaflet Margins in Mungbean. Mol. Breed. 2016, 36, 1–12. [Google Scholar] [CrossRef]
- Nair, R.M.; Pandey, A.K.; War, A.R.; Hanumantharao, B.; Shwe, T.; Alam, A.K.M.M.; Pratap, A.; Malik, S.R.; Karimi, R.; Mbeyagala, E.K. Biotic and Abiotic Constraints in Mungbean Production—Progress in Genetic Improvement. Front. Plant Sci. 2019, 10, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, Y.; Peng, J.; Fan, B.; Xu, D.; Wu, J.; Cao, Z.; Gao, Y.; Wang, X.; Li, S. High-Quality Genome Assembly and Pan-Genome Studies Facilitate Genetic Discovery in Mung Bean and Its Improvement. Plant Commun. 2022, 3, 100352. [Google Scholar] [CrossRef] [PubMed]
- Chiteri, K.O.; Chiranjeevi, S.; Jubery, T.Z.; Rairdin, A.; Dutta, S.; Ganapathysubramanian, B.; Singh, A. Dissecting the Genetic Architecture of Leaf Morphology Traits in Mungbean [Vigna radiata (L.) Wizcek] Using Genome-Wide Association Study. Plant Phenome J. 2023, 6, 1–22. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, S.; Ai, J.; Zhang, P.; Yao, H.; Liu, Y.; Zhang, X. Transcriptomic and Biochemical Analyses of Drought Response Mechanism in Mung Bean [Vigna radiata (L.) Wilczek] leaves. Plant Genome. 2023, 18, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kohli, M.; Bansal, H.; Mishra, G.P.; Dikshit, H.K.; Reddappa, S.B.; Roy, A.; Sinha, S.K.; Shivaprasad, K.M.; Kumari, N.; Kumar, A. Genome-Wide Association Studies for Earliness, MYMIV Resistance, and Other Associated Traits in Mungbean [Vigna radiata (L). Wilczek] Using Genotyping by Sequencing Approach. PeerJ. 2024, 12, e16653. [Google Scholar] [CrossRef]
- Ha, J.; Satyawan, D.; Jeong, H.; Lee, E.; Cho, K.; Kim, M.Y.; Lee, S. A Near-Complete Genome Sequence of Mungbean (Vigna radiata L.) provides key insights into the Modern Breeding Program. Plant Genome. 2021, 14, 1–16. [Google Scholar] [CrossRef]
- Li, C.; Gao, Z.; Hu, W.; Zhu, X.; Li, Y. Integration of Comparative Transcriptomics and WGCNA Characterizes the Regulation of Anthocyanin Biosynthesis in Mung Bean (Vigna radiata L.). Front. Plant Sci. 2023, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Nair, R.M.; Lee, J.; Lee, S.H. Genomic Resources in Mungbean for Future Breeding Programs. Front. Plant Sci. 2015, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.M.; Yang, R.Y.; Easdown, W.J.; Thavarajah, D.; Thavarajah, P.; Hughes, J.d.A.; Keatinge, J.D.H. Biofortification of Mungbean (Vigna radiata L.) as a Whole Food to Enhance Human Health. J. Sci. Food Agric. 2013, 93, 1805–1813. [Google Scholar] [CrossRef] [PubMed]
- Besfat, B.T.; Merkuz, A.A.; Gezahegne, G.D. Assessment and Characterization of Mung Bean (Vigna radiata L.) Bacterial Brown Spot in Eastern Amhara, Ethiopia. Afr. J. Agric. Res. 2020, 16, 606–621. [Google Scholar] [CrossRef]
- Uppalwar, S.V.; Garg, V.; Dutt, R. Seeds of Mung Bean [Vigna radiata (L.) Wilczek]: Taxonomy, Phytochemistry, Medicinal Uses and Pharmacology. Current Bioactive Compounds. 2020, 17, 220–233. [Google Scholar] [CrossRef]
- Dahiya, P.K.; Linnemann, A.R.; Van Boekel, M.A.J.S.; Khetarpaul, N.; Grewal, R.B.; Nout, M.J.R. Mung Bean: Technological and Nutritional Potential. Crit. Rev. Food Sci. Nutr. 2015, 55, 670–688. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Yousaf, L.; Xue, Y.; Hu, J.; Wu, J.; Hu, X.; Feng, N.; Shen, Q. Mung Bean (Vigna radiata L.): Bioactive Polyphenols, Polysaccharides, Peptides, and Health Benefits. Nutrients. 2019, 11, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Elobuike, C.S.; Idowu, M.A.; Adeola, A.A.; Bakare, H.A. Nutritional and Functional Attributes of Mungbean (Vigna radiata [L] Wilczek) Flour as Affected by Sprouting Time. Legum. Sci. 2021, 3, 1–11. [Google Scholar] [CrossRef]
- Ali, A.; Altaf, M.T.; Nadeem, M.A.; Karaköy, T.; Shah, A.N.; Azeem, H.; Baloch, F.S.; Baran, N.; Hussain, T.; Duangpan, S. Recent Advancement in OMICS Approaches to Enhance Abiotic Stress Tolerance in Legumes. Front. Plant Sci. 2022, 13, 952759. [Google Scholar] [CrossRef] [PubMed]
- Shafique, S.; Attia, U.; Shafique, S.; Tabassum, B.; Akhtar, N.; Naeem, A.; Abbas, Q. Management of Mung Bean Leaf Spot Disease Caused by Phoma Herbarum through Penicillium Janczewskii Metabolites Mediated by MAPK Signaling Cascade. Sci. Rep. 2023, 13, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yoseph, T. Performance Evaluation of Mung Bean [Vigna radiata (L.) Wilczek] Varieties in Pastoral Areas of South Omo Zone, Southern Ethiopia. Int. J. Agric. Res. Innov. Technol. 2022, 12, 141–144. [Google Scholar] [CrossRef]
- Bunaka, A.; Bibiso, M.; Lelago, A. Determination of Levels of Essential and Non-Essential Metals and Nutritional Value in Mung Bean Seed (Vigna radiate L.) Cultivated in Wolaita Zone, Southern Ethiopia. Indian J. Agric. Res. 2023, 57, 198–202. [Google Scholar] [CrossRef]
- Ademe, B.E. Mung Bean (Vigna radiata L.) Production Vis-Avis Market Potential in Ethiopia: A Review. Asian J. Biol. Sci. 2023, 16, 614–622. [Google Scholar] [CrossRef]
- Mahapatra, S.S.; Beura, S.K.; Swain, D.; Jadhao, K.R.; Rout, G.R. New Report of Cercospora Canescens Isolates from Coastal Regions of Odisha, India Causing Cercospora Leaf Spot (CLS) Disease in Mung Bean (Vigna radiata L.). Legum. Res. 2023, 46, 1247–1252. [Google Scholar] [CrossRef]
- Buttar, H.S.; Singh, A.; Sirari, A.; Anupam; Kaur, K.; Kumar, A.; Lal, M.K.; Tiwari, R.K.; Kumar, R. Investigating the Impact of Fungicides and Mungbean Genotypes on the Management of Pod Rot Disease Caused by Fusarium equiseti and Fusarium chlamydosporum. Front. Plant Sci. 2023, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kelly, L.A.; Vaghefi, N.; Bransgrove, K.; Fechner, N.A.; Stuart, K.; Pandey, A.K.; Sharma, M.; Nemeth, M.Z.; Liu, S.Y.; Tang, S.R. One Crop Disease, How Many Pathogens? Podosphaera xanthii and Erysiphe vignae Sp. Nov. Identified as the Two Species That Cause Powdery Mildew of Mungbean (Vigna radiata) and Black Gram (V. mungo) in Australia. Phytopathology 2021, 111, 1193–1206. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Meena, A.K.; Kumar, V.; Nathawat, B.D.S.; Yadav, A.L. Management of Cercospora Leaf Spot [Cercospora canescens Ellis and Martin] of Mothbean [Vigna aconitifolia (Jacq.) Marechal] through Fungicides. Legum. Res.-Int. J. 2023, I, 1–5. [Google Scholar] [CrossRef]
- Songwattana, P.; Boonchuen, P.; Piromyou, P.; Wongdee, J.; Greetatorn, T.; Inthaisong, S.; Tantasawat, P.A.; Teamtisong, K.; Tittabutr, P.; Boonkerd, N. Insights into Antifungal Mechanisms of Bacillus Velezensis S141 against Cercospora Leaf Spot in Mungbean (V. radiata). Microbes Environ. 2023, 38, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.; Iqbal, M.A.; Kamran, M.; Shahbaz, M.U.; Kamber, H.U.; Javed, N.; Junaid, M.; Abbas, H.; Haq, M.E. Evaluation of Advanced Mung Bean Germplasm against Cercospora Leaf Spot and Its In-Vitro Management by Different Fungicides. Pak. J. Agric. Res. 2020, 33, 872–877. [Google Scholar] [CrossRef]
- Shahzady, M.; Ahmad, T.; Moosa, A.; Khan, W.; Naeem, I.; Nasir, M.; Aslam Khan, M.; Hin, P. A General Review of Cercospora Leaf Spot Disease of Mungbean and Its Management. Int. J. Sci. Res. 2017, 5, 1–84. [Google Scholar] [CrossRef]
- Chand, R.; Singh, V.; Pal, C.; Kumar, P.; Kumar, M. First Report of a New Pathogenic Variant of Cercospora canescens on Mungbean (Vigna radiata L.) from India. New Dis. Reports. 2012, 26, 6. [Google Scholar] [CrossRef]
- Das, A.; Gupta, S.; Parihar, A.K.; Singh, D.; Chand, R.; Pratap, A.; Dayamoy, K.; Pati, K.; Kushwaha, S. Delineating Genotype × Environment Interactions towards Durable Resistance in Mungbean against Cercospora Leaf Spot (Cercospora Canescens) Using GGE Biplot. Plant Breed. 2019, 00, 1–12. [Google Scholar] [CrossRef]
- Rehman, A.U.; Khan, M.E.; Akhtar, K.P.; Kaukab, S.; Saeed, S.; Asghar, M.J.; Salim, J. Identification of Sources of Disease Resistance To Mungbean Yellow Mosaic Virus (Mymv) and Cercospora Leaf Spot Disease (Cls) in Mungbean (Vigna radiata L.). Pak. J. Phytopathol. 2021, 33, 335–347. [Google Scholar] [CrossRef]
- Malik, L.; Atiq, M.; Rajput, N.A.; Usman, M.; Akram, A.; Jabbar, A.; Zahid, M.N.; Ahmad, W.; Qasim, M.W. Induction of Resistance in Mungbean Against Cercospora Leaf Spot Through Plant Defense Activators. Agric. Sci. J. 2023, 5, 28–34. [Google Scholar] [CrossRef]
- Jackson, G.; McKenzie, E. Pacific Pests, Pathogens, Weeds & Pesticides—Online Edition Yam Mosaic Virus Disease. 2022. No. 526. Available online: https://apps.lucidcentral.org/pppw (accessed on 22 March 2024).
- Kumar, N.; Kumar, S.; Satyadev, P.; Shivam, M. Cercospora Leaf Spot Disease of Green Gram and Its Management: A Review. J. Pharmacogn. Phytochem. 2020, 9, 1574–1576. [Google Scholar]
- Ilyas, S.; Ali, S.; Habib, A.; Ali, M.; Zeshan, M.A.; Iftikhar, Y.; Ghani, M.U.; Umair, M. Unveiling the factors affecting leaf spot disease in mungbean and its management. Pak. J. Agric. Research. 2023, 36, 147–154. [Google Scholar] [CrossRef]
- Braun, U.; Nakashima, C.; Crous, P.W. Cercosporoid fungi (Mycosphaerellaceae) 1. Species on other fungi, Pteridophyta and Gymnospermae. Ima Fungus. 2013, 4, 265–345. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Souframanien, J.; Chand, R.; Pawar, S.E. Genetic Diversity Study of Cercospora Canescens (Ellis & Martin) Isolates, the Pathogen of Cercospora Leaf Spot in Legumes. Curr. Sci. 2006, 90, 564–568. [Google Scholar]
- Batzer, J.C.; Singh, A.; Rairdin, A.; Chiteri, K.; Mueller, D.S. Mungbean: A Preview of Disease Management Challenges for an Alternative U.S. Cash Crop. J. Integr. Pest Manag. 2022, 13, 1–21. [Google Scholar] [CrossRef]
- Bhat, N.A.; Maheshwari, S.K.; Ahmad, S.; Beig, B.A.; Masoodi, S.D. Field evaluation of mung bean accessions against Cercospora leaf spot. Ann. Biol. 2008, 24, 1–4. [Google Scholar]
- Yadav, L.D.; Pandey, R.N.; Jaisani, P.; Gohel, N.M. Sources of Resistance in Mungbean Genotypes to Cercospora Leaf Spot Disease and Its Management. Afr. J. Agric. Res. 2014, 9, 3111–3114. [Google Scholar] [CrossRef][Green Version]
- Akhtar, J.; Lal, H.C.; Kumar, Y.; Singh, P.K.; Ghosh, J.; Khan, Z.; Gautam, N.K. Multiple disease resistance in greengram and blackgram germplasm and management through chemicals under rain-fed conditions. Legume Res. 2014, 37, 101–109. [Google Scholar] [CrossRef]
- Nair, R.M.; Mahbubul Alam, A.; Douglas, C.; Gowda, A.; Pratap, A.; Mar Win, M.; Karimi, R.; Emmanuel, M.K.; Binagwa, P.; Boddepalli, V. Establishing the International Mungbean Improvement Network-Final Report (FR2021-086); Australian Centre for International Agricultural Research (ACIAR): Canberra, ATC, Australia, 2022; pp. 1–77.
- Babar, M.; Ijaz, S.; Haq, I.U.; Khan, M.S. Development of Molecular Marker Linked with Cercospora Leaf Spot (CLS) Disease Resistance in Vigna radiata, Cloning, and Expression for Evaluating Antifungal Activity against Cercospora Canescens. Phyton-Int. J. Exp. Bot. 2023, 92, 1289–1300. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, D.; Chauhan, S.; Dhillon, H.K.; Kumar, S.; Kumar, V.; Kapoor, R. Role of Omics Approaches in Vegetable Breeding for Insect Pest Resistance. SN Appl. Sci. 2023, 5, 1–8. [Google Scholar] [CrossRef]
- Khedher, M.B.; Ghedira, K.; Rolain, J.M.; Ruimy, R.; Croce, O. Application and Challenge of 3rd Generation Sequencing for Clinical Bacterial Studies. Int. J. Mol. Sci. 2022, 23, 1395. [Google Scholar] [CrossRef] [PubMed]
- Solanke, A.; Tribhuvan, K. Genomics: An Integrative Approach for Molecular Biology. Biotechnol. Prospect. 2015, 234–270. [Google Scholar]
- Qi, S.; Wang, J.; Zhang, Y.; Naz, M.; Afzal, M.R.; Du, D.; Dai, Z. Omics Approaches in Invasion Biology: Understanding Mechanisms and Impacts on Ecological Health. Plants 2023, 12, 1860. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Kirti, P.B. Transcriptomic and Proteomic Analyses of Resistant Host Responses in Arachis diogoi Challenged with Late Leaf Spot Pathogen, Phaeoisariopsis personata. PLoS ONE. 2015, 10, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Tugizimana, F.; Mhlongo, M.I.; Piater, L.A.; Dubery, I.A. Metabolomics in Plant Priming Research: The Way Forward? Int. J. Mol. Sci. 2018, 19, 1759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, L.; Liu, Z.; Zhao, Z.; Zhao, J.; Wang, Z.; Zhou, G.; Liu, P.; Liu, M. Transcriptome and Metabolome Profiling Unveil the Mechanisms of Ziziphus Jujuba Mill. Peel Coloration. Food Chem. 2020, 312, 125903. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.K.; Shen, Y.; Wang, J.; Sheng, X.; Zhao, Z.; Yu, H.; Gu, H. Advances in Multi-Omics Approaches for Molecular Breeding of Black Rot Resistance in Brassica oleracea L. Front. Plant Sci. 2021, 12, 2553. [Google Scholar] [CrossRef] [PubMed]
- Jha, U.C.; Nayyar, H.; Parida, S.K.; Bakır, M.; von Wettberg, E.J.B.; Siddique, K.H.M. Progress of Genomics-Driven Approaches for Sustaining Underutilized Legume Crops in the Post-Genomic Era. Front. Genet. 2022, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Saand, M.A.; Huang, L.; Abdelaal, W.B.; Zhang, J.; Wu, Y.; Li, J.; Sirohi, M.H.; Wang, F. Applications of Multi-Omics Technologies for Crop Improvement. Front. Plant Sci. 2021, 12, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Tabassum, J.; Kudapa, H.; Varshney, R.K. Can Omics Deliver Temperature Resilient Ready-to-Grow Crops? Crit. Rev. Biotechnol. 2021, 41, 1209–1232. [Google Scholar] [CrossRef] [PubMed]
- Hasin, Y.; Seldin, M.; Lusis, A. Multi-Omics Approaches to Disease. Genome Biol. 2017, 18, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Scossa, F.; Alseekh, S.; Fernie, A.R. Integrating Multi-Omics Data for Crop Improvement. J. Plant Physiol. 2021, 257, 153352. [Google Scholar] [CrossRef] [PubMed]
- Jost, M.; Outram, M.A.; Dibley, K.; Zhang, J.; Luo, M.; Ayliffe, M. Plant and Pathogen Genomics: Essential Approaches for Stem Rust Resistance Gene Stacks in Wheat. Front. Plant Sci. 2023, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Edet, I.A.; Afolabi, C.G.; Popoola, A.R.; Arogundade, O.; Akinbode, O.A. Identification and Molecular Characterisation of Cercospora Leaf Spot Disease Pathogen on Cowpea (Vigna unguiculata L. Walp). Arch. Phytopathol. Plant Prot. 2022, 55, 109–120. [Google Scholar] [CrossRef]
- Chankaew, S.; Somta, P.; Sorajjapinun, W.; Srinives, P. Quantitative Trait Loci Mapping of Cercospora Leaf Spot Resistance in Mungbean, Vigna radiata (L.) Wilczek. Mol. Breed. 2011, 28, 255–264. [Google Scholar] [CrossRef]
- Arsakit, K.; Banno, K. ISSR and SSR Markers Linked to Powdery Mildew and Cercospora Leaf Spot Resistance In Mungbean. Master’s Thesis, School of Crop Production Technology Institute of Agricultural Technology Suranaree University of Thailand, Nakhon Ratchasima, Thailand, 2019; pp. 1–61. [Google Scholar]
- Kang, Y.J.; Kim, S.K.; Kim, M.Y.; Lestari, P.; Kim, K.H.; Ha, B.K.; Jun, T.H.; Hwang, W.J.; Lee, T.; Lee, J. Genome Sequence of Mungbean and Insights into Evolution within Vigna Species. Nat. Commun. 2014, 5, 5443. [Google Scholar] [CrossRef] [PubMed]
- Yundaeng, C.; Somta, P.; Chen, J.; Yuan, X.; Chankaew, S.; Chen, X. Fine Mapping of QTL Conferring Cercospora Leaf Spot Disease Resistance in Mungbean Revealed TAF5 as Candidate Gene for the Resistance. Theor. Appl. Genet. 2020, 134, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, J.P.; Samal, K.C.; Lenka, D.; Beura, S.K.; Behera, L.; Khamari, B.; Sawant, S.B. Assessment of Genetic Diversity for Cercospora Leaf Spot (CLS) Resistance in Mung Bean [Vigna radiata (L.) Wilczek] Using SSR Markers. Legum. Res. Int. J. 2022, 1–6. [Google Scholar] [CrossRef]
- Akhtar, M.A.; Aslam, M.; Schafleitner, R.; Atif, R.M.; Murtaza, G. Genetics of Cercospora Leaf Spot Resistance in Mung Bean. Sabrao J. Breed. Genet. 2023, 55, 1109–1122. [Google Scholar] [CrossRef]
- Choudhary, P.; Chand, R.; Singh, A.K. Genetics of Cercospora Leaf Spot Resistance in Mungbean [Vigna radiata (L.) Wilczek] through Generation Mean Analysis. Legum. Res. 2023, 46, 1526–1533. [Google Scholar] [CrossRef]
- Ahmad, A.; Razvi, S.M.; Rather, M.A.; Gulzafar; Dar, M.A.; Ganie, S.A. Association and Inter-Relationship among Yield and Yield Contributing Characters and Screening against Cercospora Leaf Spot in Mung Bean (Vigna radiata L.). Sci. Res. Essays. 2013, 8, 2008–2014. [Google Scholar] [CrossRef]
- Sharma, S.; Mittal, R.K.; Chaudhary, H.K.; Sood, V.K. Introgression of Disease Resistance in Urdbean (Vigna mungo) by Involving Its Related Species. Indian J. Agric. Sci. 2022, 92, 1348–1352. [Google Scholar] [CrossRef]
- Liu, M.; Sen; Kuo, T.C.Y.; Ko, C.Y.; Wu, D.C.; Li, K.Y.; Lin, W.J.; Lin, C.P.; Wang, Y.W.; Schafleitner, R.; et al. Genomic and Transcriptomic Comparison of Nucleotide Variations for Insights into Bruchid Resistance of Mungbean (Vigna radiata [L.] R. Wilczek). BMC Plant Biol. 2016, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Koche, D.; Chaudhary, A.D. Defense Gene Expression of Vigna radiata (L.) Wilczek., against Cercospora Leaf Spots (CLS). Asian J. Res. Crop. Sci. 2019, 3, 1–8. [Google Scholar] [CrossRef][Green Version]
- Bangar, P.; Tyagi, N.; Tiwari, B.; Kumar, S.; Barman, P.; Kumari, R.; Gaikwad, A.; Bhat, K.V.; Chaudhury, A. Identification and Characterization of SNPs in Released, Landrace and Wild Accessions of Mungbean (Vigna radiata (L.) Wilczek) Using Whole Genome Re-Sequencing. J. Crop. Sci. Biotechnol. 2021, 24, 153–165. [Google Scholar] [CrossRef]
- Fatokun, C.A.; Menancio-hautea, D.I.; Danesh, D.; Young, N.D. Evidence for OrthologousSeed Weight Genes in Cowpea and Mung Bean Based on RFLP Mapping. Genetics 1992, 132, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Young, N.D.; Kumar, L.; Menancio-Hautea, D.; Danesh, D.; Talekar, N.S.; Shanmugasundarum, S.; Kim, D.H. RFLP Mapping of a Major Bruchid Resistance Gene in Mungbean (Vigna radiata, L. Wilczek). Theor. Appl. Genet. 1992, 84, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Menancio-Hautea, D.; Fatokun, C.A.; Kumar, L.; Danesh, D.; Young, N.D. Comparative Genome Analysis of Mungbean (Vigna radiata L. Wilczek) and Cowpea (V. unguiculata L. Walpers) Using RFLP Mapping Data. Theor. Appl. Genet. 1993, 86, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Young, N.D.; Danesh, D.; Menancio-Hautea, D.; Kumar, L. Mapping Oligogenic Resistance to Powdery Mildew in Mungbean with RFLPs. Theor. Appl. Genet. Int. J. Plant Breed. Res. 1993, 87, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Park, S.J.; Poysa, V.; Gepts, P. Integration of Simple Sequence Repeat (SSR) Markers Into a Molecular Linkage Map of Common Bean (Phaseolus vulgaris L.). J. Hered. 2000, 91, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.V.; Tan, S.G.; Quah, S.C.; Yusoff, K. Isolation of Microsatellite Markers in Mungbean, Vigna radiata (L.). Mol. Ecol. Notes 2002, 2, 96–98. [Google Scholar] [CrossRef]
- Gwag, J.G.; Dixit, A.; Park, Y.J.; Ma, K.H.; Kwon, S.J.; Cho, G.T.; Lee, G.A.; Lee, S.Y.; Kang, H.K.; Lee, S.H. Assessment of Genetic Diversity and Population Structure in Mungbean. Genes Genom. 2010, 32, 299–308. [Google Scholar] [CrossRef]
- Taguchi, K.; Kubo, T.; Takahashi, H.; Abe, H. Identi Fi Cation and Precise Mapping of Resistant QTLs of Cercospora Leaf Spot Resistance in Sugar Beet (Beta vulgaris L.). G3 Genes|Genomes|Genetics 2011, 1, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Zhang, B.; Ding, W.; Liu, X.; Yang, D.L.; Wei, P.; Cao, F.; Zhu, S.; Zhang, F.; Mao, Y. Efficient Genome Editing in Plants Using a CRISPR/Cas System. Cell Res. 2013, 23, 1229–1232. [Google Scholar] [CrossRef]
- Shan, Q.; Wang, Y.; Li, J.; Zhang, Y.; Chen, K.; Liang, Z.; Zhang, K.; Liu, J.; Xi, J.J.; Qiu, J.L.; et al. Targeted Genome Modification of Crop Plants Using a CRISPR-Cas System. Nat. Biotechnol. 2013, 31, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Ma, L. Recent Advances in CRISPR/Cas9 and Applications for Wheat Functional Genomics and Breeding. aBIOTECH 2021, 2, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Bonagiri, G.; Gurjar, D.; Kuri, A.; Kumar Yella, V.; Author Ganesh Bonagiri, C. CRISPR/Cas9 Gene Editing Tool for Diseases Resistant Varieties. Pharma Innov. J. 2022, 11, 2731–2738. [Google Scholar]
- Elsharawy, H.; Refat, M. CRISPR/Cas9 Genome Editing in Wheat: Enhancing Quality and Productivity for Global Food Security—A Review. Funct. Integr. Genom. 2023, 23. [Google Scholar] [CrossRef] [PubMed]
- Sokolkova, A.; Burlyaeva, M.; Valiannikova, T.; Vishnyakova, M.; Schafleitner, R.; Lee, C.R.; Ting, C.T.; Nair, R.M.; Nuzhdin, S.; Samsonova, M.; et al. Genome-wide association study in accessions of the mini-core collection of mungbean (Vigna radiata) from the World Vegetable Gene Bank (Taiwan). BMC Plant Biol. 2020, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kajonphol, T.; Sangsiri, C.; Somta, P.; Toojinda, T.; Srinives, P. SSR map construction and quantitative trait loci (QTL) identification of major agronomic traits in mungbean (Vigna radiata (L.) Wilczek). Sabrao J. Breed. Genet. 2012, 44, 71–86. [Google Scholar]
- Yundaeng, C.; Somta, P.; Chen, J.; Yuan, X.; Chankaew, S.; Srinives, P.; Chen, X. Candidate gene mapping reveals VrMLO12 (MLO Clade II) is associated with powdery mildew resistance in mungbean (Vigna radiata [L.] Wilczek). Plant Sci. 2020, 298, 110594. [Google Scholar] [CrossRef] [PubMed]
- Papan, P.; Chueakhunthod, W.; Jinagool, W.A.; Tharapreuksapong, A.; Masari, C.; Kaewkasi, S.; Ngampongsai, T.; Girdthaj, P.A. Improvement of Cercospora leaf spot and powdery mildew resistance of mungbean variety KING through marker-assisted selection. J. Agric. Sci. 2021, 159, 9–10, 676–687. [Google Scholar] [CrossRef]
- Talakayala, A.; Mekala, G.K.; Reddy, M.K.; Ankanagari, S.; Garladinne, M. Manipulating Resistance to Mungbean Yellow Mosaic Virus in Greengram (Vigna radiata L): Through CRISPR/Cas9 Mediated Editing of the Viral Genome. Front. Sustain. Food Syst. 2022, 6. [Google Scholar] [CrossRef]
- Lee, E.; Yang, X.; Ha, J.; Kim, M.Y.; Park, K.Y.; Lee, S.H. Identification of a Locus Controlling Compound Raceme Inflorescence in Mungbean [Vigna radiata (L.) R. Wilczek]. Front. Genet. 2021, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, K.; Singh, A. Strategies for the utilization of the USDA mung bean germplasm collection for breeding outcomes. Crop. Sci. 2021, 1, 422–442. [Google Scholar] [CrossRef]
- Chen; Hu, L.; Wang, S.; Wang, L.; Cheng, X.; Chen, H. Construction of High-Density Genetic Map and Identification of a Bruchid Resistance Locus in Mung Bean (Vigna radiata L.). Front. Genet. 2022, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fatmawati, Y.; Setiawan, A.B.; Purwantoro, A.; Respatie, D.W.; Teo, C.H. Analysis of Genetic Variability in F2 Interspecific Hybrids of Mung Bean (Vigna radiata L.) Using Inter-Retrotransposon Amplified Polymorphism Marker System. Biodiversitas. 2021, 22, 4880–4889. [Google Scholar] [CrossRef]
- Zehra, A.; Raytekar, N.A.; Meena, M.; Swapnil, P. Efficiency of microbial bio-agents as elicitors in plant defense mechanism under biotic stress: A review. Curr. Res. Microb. Sci. 2021, 2, 100054. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.C.; Tripathi, A.; Tak, R. Expression of defense-related genes in mung bean varieties in response to Trichoderma virens alone and in the presence of Rhizoctonia solani infection. 3 Biotech 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.M.; Pratap, A.; Gupta, S.; Biradar, R.S.; Singh, N.P. Association Mapping for Mungbean Yellow Mosaic India Virus Resistance in Mungbean (Vigna radiata L. Wilczek). Biotech. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chankaew, S.; Somta, P.; Isemura, T.; Tomooka, N.; Kaga, A.; Vaughan, D.A.; Srinives, P. Quantitative Trait Locus Mapping Reveals Conservation of Major and Minor Loci for Powdery Mildew Resistance in Four Sources of Resistance in Mungbean [Vigna radiata (L.) Wilczek]. Mol. Breed. 2013, 32, 121–130. [Google Scholar] [CrossRef]
- Sompong, U.; Somta, P.; Raboy, V.; Srinives, P. Mapping of Quantitative Trait Loci for Phytic Acid and Phosphorus Contents in Seed and Seedling of Mungbean (Vigna radiata (L.) Wilczek). Breed. Sci. 2012, 62, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Papan, P.; Chueakhunthod, W.; Poolsawat, O.; Arsakit, K.; Tharapreuksapong, A.; Tantasawat, P.A. Validation of Molecular Markers Linked to Cercospora Leaf Spot Disease Resistance in Mungbean (Vigna radiata [L.] WILCZEK). Sabrao J. Breed. Genet. 2021, 53, 749–757. [Google Scholar] [CrossRef]
- Schafleitner, R.; Huang, S.M.; Chu, S.H.; Yen, J.Y.; Lin, C.Y.; Yan, M.R.; Krishnan, B.; Liu, M.S.; Lo, H.F.; Chen, C.Y. Identification of Single Nucleotide Polymorphism Markers Associated with Resistance to Bruchids (Callosobruchus spp.) in Wild Mungbean (Vigna radiata Var. Sublobata) and Cultivated V. Radiata through Genotyping by Sequencing and Quantitative Trait Locus A. BMC Plant Biol. 2016, 16, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Peng, L.; Ji, L.; Wang, S.; Wang, L.; Wu, J. Genome-Wise Association Study Identified Genomic Regions Associated with Drought Tolerance in Mungbean (Vigna radiata (L.) R. Wilczek). Theor. Appl. Genet. 2023, 136, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang; Li, J.; Liu, Z.; Yuan, X.; Wang, S.; Chen, H.; Chen, X.; Cheng, X.; Wang, L. Construction of a High-Density Genetic Map and Its Application for QTL Mapping of Leaflet Shapes in Mung Bean (Vigna radiata L.). Front. Genet. 2020, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Yang, Y.; Wang, P.; Zhang, Y.; Zhang, L.; Tian, D.; Zhang, L.; Zhang, L.; Zhou, B. InDel Marker Development and QTL Analysis of Agronomic Traits in Mung Bean [Vigna radiata (L.) Wilczek]. Mol. Breed. 2021, 41, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Huang, S.; Zhu, Z.; Ma, Y.; Yang, X.; Yao, L.; Gao, X.; Zhang, M.; Liu, W.; Qiu, L.; et al. Genome-Wide Association of Single Nucleotide Polymorphism Loci and Candidate Genes for Frogeye Leaf Spot (Cercospora Sojina) Resistance in Soybean. BMC Plant Biol. 2021, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Lightfoot, D.A. Quantitative Trait Loci Underlying Partial Resistance to Cercospora Sojina Race 2 Detected in Soybean Seedlings in Greenhouse Assays. Atlas J. Biol. 2014, 3, 175–182. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Li, Z.; Zhang, Y.; Kang, M.S.; Wang, Y.; Fan, X. High-Density Mapping for Gray Leaf Spot Resistance Using Two Related Tropical Maize Recombinant Inbred Line Populations. Mol. Biol. Rep. 2021, 48, 3379–3392. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Cheng, X.Z.; Wang, S.H.; Wang, L.X.; Liu, C.Y.; Sun, L.; Xu, N.; Humphry, M.E.; Lambrides, C.J.; Li, H.B. Relationship between Bruchid Resistance and Seed Mass in Mungbean Based on QTL Analysis. Genome 2009, 52, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, C.; Zhong, M.; Zhao, D.; Mei, L.; Chen, H.; Wang, S.; Liu, C.; Cheng, X. Construction of an Integrated Map and Location of a Bruchid Resistance Gene in Mung Bean. Crop. J. 2016, 4, 360–366. [Google Scholar] [CrossRef]
- Mathivathana, M.K.; Murukarthick, J.; Karthikeyan, A.; Jang, W.; Dhasarathan, M.; Jagadeeshselvam, N.; Sudha, M.; Vanniarajan, C.; Karthikeyan, G.; Yang, T.J. Detection of QTLs Associated with Mungbean Yellow Mosaic Virus (MYMV) Resistance Using the Interspecific Cross of Vigna radiata × Vigna umbellata. J. Appl. Genet. 2019, 60, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Poolsawat, O.; Kativat, C.; Arsakit, K.; Tantasawat, P.A. Identification of Quantitative Trait Loci Associated with Powdery Mildew Resistance in Mungbean Using ISSR and ISSR-RGA Markers. Mol. Breed. 2017, 37, 1–12. [Google Scholar] [CrossRef]
- Kitsanachandee, R.; Somta, P.; Chatchawankanphanich, O.; Akhtar, K.P.; Shah, T.M.; Nair, R.M.; Bains, T.S.; Sirari, A.; Kaur, L.; Srinives, P. Detection of Quantitative Trait Loci for Mungbean Yellow Mosaic India Virus (MYMIV) Resistance in Mungbean (Vigna radiata (L.) Wilczek) in India and Pakistan. Breed. Sci. 2013, 63, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Breria, C.M.; Hsieh, C.H.; Yen, T.B.; Yen, J.Y.; Noble, T.J.; Schafleitner, R. A SNP-Based Genome-Wide Association Study to Mine Genetic Loci Associated to Salinity Tolerance in Mungbean (Vigna radiata L.). Genes 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Mittal, S.; Wankhede, D.P.; Parida, S.K.; Chattopadhyay, D.; Prasad, G.; Mishra, D.C.; Joshi, D.C.; Singh, M.; Singh, K. Transcriptome Analysis Reveals Key Pathways and Candidate Genes Controlling Seed Development and Size in Ricebean (Vigna umbellata). Front. Genet. 2022, 12, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Wang, L.; Fan, B.; Cao, Z.; Su, Q.; Zhang, Z.; Wang, Y.; Tian, J.; Wang, S. Quantitative Trait Locus Mapping under Irrigated and Drought Treatments Based on a Novel Genetic Linkage Map in Mungbean (Vigna radiata L.). Theor. Appl. Genet. 2017, 130, 2375–2393. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.K.M.; Somta, P.; Muktadir, M.A.; Srinives, P. Quantitative Trait Loci Associated with Seed Weight in Mungbean (Vigna radiata (L.) Wilczek. Kasetsart J. -Nat. Sci. 2014, 48, 197–204. [Google Scholar]
- Noble, T.J.; Tao, Y.; Mace, E.S.; Williams, B.; Jordan, D.R.; Douglas, C.A.; Mundree, S.G. Characterization of Linkage Disequilibrium and Population Structure in a Mungbean Diversity Panel. Front. Plant Sci. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Amkul, K.; Laosatit, K.; Liu, J.; Yimram, T.; Chen, J.; Yuan, X.; Chen, X.; Somta, P. Fine mapping of QTL conferring resistance to calcareous soil in mungbean reveals VrYSL3 as candidate gene for the resistance. Plant Sci. 2023, 332, 111698. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, L.; Wang, S.; Liu, C.; Blair, M.W.; Cheng, X. Transcriptome Sequencing of Mung Bean (Vigna radiate L.) Genes and the Identification of EST-SSR Markers. PLoS ONE. 2015, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Li, S.; Liu, Y.; Liu, X. Transcriptomic Profiling Reveals Metabolic and Regulatory Pathways in the Desiccation Tolerance of Mungbean (Vigna radiata [L.] R. Wilczek). Front. Plant Sci. 2016, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, X.; Li, Q.; Lu, Y.; Huang, W.; Zhang, F.; Chen, L.; Liu, R.H.; Yan, S. Integrated Transcriptomic and Metabolic Framework for Carbon Metabolism and Plant Hormones Regulation in Vigna radiata during Post-Germination Seedling Growth. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kumar Seema, R.; Kushwaha, A.; Rashid, M.H.; Tarannum, N.; Chakraborty, S. Mungbean (Vigna radiata (L.) Wilczek): Progress in Breeding and Future Challenges. Int. J. Plant Soil Sci. 2020, 34, 50–59. [Google Scholar] [CrossRef]
- Hu, H.; Feng, N.; Shen, X.; Zhao, L.; Zheng, D. Transcriptomic Analysis of Vigna radiata in Response to Chilling Stress and Uniconazole Application. BMC Genom. 2022, 23, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sudha, M.; Karthikeyan, A.; Madhumitha, B.; Veera Ranjani, R.; Kanimoli Mathivathana, M.; Dhasarathan, M.; Murukarthick, J.; Samu Shihabdeen, M.N.; Eraivan Arutkani Aiyanathan, K.; Pandiyan, M. Dynamic Transcriptome Profiling of Mungbean Genotypes Unveil the Genes Respond to the Infection of Mungbean Yellow Mosaic Virus. Pathogens 2022, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yan, Q.; Yuan, X.; Lin, Y.; Chen, J.; Wu, R.; Xue, C.; Zhu, Y.; Chen, X. Two Polygalacturonase-Inhibiting Proteins (VrPGIP) of Vigna radiata Confer Resistance to Bruchids (Callosobruchus spp.). J. Plant Physiol. 2021, 258–259, 153376. [Google Scholar] [CrossRef]
- Lim, I.; Kang, M.; Kim, B.C.; Ha, J. Metabolomic and Transcriptomic Changes in Mungbean (Vigna radiata (L.) Wilczek) Sprouts under Salinity Stress. Front. Plant Sci. 2022, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Al-Amrani, S.; Al-Jabri, Z.; Al-Zaabi, A.; Alshekaili, J.; Al-Khabori, M. Proteomics: Concepts and Applications in Human Medicine. World J. Biol. Chem. 2021, 12, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Chunkao, S.; Youravong, W.; Yupanqui, C.T.; Alashi, A.M.; Aluko, R.E. Structure and Function of Mung Bean Protein- Derived Iron-Binding Antioxidant Peptides. Foods 2020, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zaker, M. Natural Plant Products as Eco-Friendly Fungicides for Plant Diseases Control—A Review. Agriculturists 2016, 14, 134–141. [Google Scholar] [CrossRef]
- Yi-Shen, Z.; Shuai, S.; Fitzgerald, R. Mung Bean Proteins and Peptides: Nutritional, Functional and Bioactive Properties. Food Nutr. Res. 2018, 62, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.K.; Navathe, S.; Mohapatra, C.; Chand, R. Antioxidants Elevates the Resistance to Cercospora canescens in Interspecific Cross of Vigna radiata (Kopergaon) × Vigna mungo (Pant Urd 31). Indian Phytopathol. 2018, 71, 519–528. [Google Scholar] [CrossRef]
- Inayati, A.; Aini, L.Q.; Yusnawan, E. Growth Performance and Metabolic Changes in Susceptible Mung Bean [Vigna radiata (L.) Wilczek] during Interaction with Rhizoctonia Solani and Trichoderma Virens. Legum. Res. 2023, 46, 228–232. [Google Scholar] [CrossRef]
- Gan, R.; Wang, M.; Lui, W.; Wu, K.; Corke, H. Original Article Dynamic Changes in Phytochemical Composition and Antioxidant Capacity in Green and Black Mung Bean (Vigna radiata) Sprouts. Int. J. Food Sci. Technol. 2016, 51, 2090–2098. [Google Scholar] [CrossRef]
- Tiwari, U.; Servan, A.; Nigam, D. Comparative Study on Antioxidant Activity, Phytochemical Analysis and Mineral Composition of the Mung Bean (Vigna radiata L.) and Its Sprouts. J. Pharmacogn. Phytochem. 2017, 6, 336–340. [Google Scholar]
- Kabré, J.d.W.; Dah-Nouvlessounon, D.; Hama-Ba, F.; Agonkoun, A.; Guinin, F.; Sina, H.; Kohonou, A.N.; Tchogou, P.; Senou, M.; Savadogo, A.; et al. Mung Bean (Vigna radiata (L.) R. Wilczek) from Burkina Faso Used as Antidiabetic, Antioxidant and Antimicrobial Agent. Plants 2022, 11, 3556. [Google Scholar] [CrossRef] [PubMed]
- Świderska-Burek, U.; Daub, M.E.; Thomas, E.; Jaszek, M.; Pawlik, A.; Janusz, G. Phytopathogenic Cercosporoid Fungi—From Taxonomy to Modern Biochemistry and Molecular Biology. Int. J. Mol. Sci. 2020, 21, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, H.; Aftab, Z.E.H.; Anjum, T.; Ali, B.; Akram, W.; Bashir, U.; Mirza, F.S.; Aftab, M.; Ali, M.D.; Li, G. Bio-Fabrication of Zinc Oxide Nanoparticles to Rescue Mung Bean against Cercospora Leaf Spot Disease. Front. Plant Sci. 2022, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chitayat, S.; Rudan, J.F. Phenome Centers and Global Harmonization; Elsevier Inc.: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Bhavyasri, T.; Delvadiya, I.R.; Ginoya, A.V. Breeding of Physiological Traits and Improving the Productivity of Green Gram (Vigna radiata L. Wilczek). Pharma J. 2022, 11, 2591–2601. [Google Scholar]
- Ninomiya, S. High-Throughput Field Crop Phenotyping: Current Status and Challenges. Breed. Sci. 2022, 72, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Jamie, C.S.M.; Spicer, J.I.; Ibbini, Z.; Tills, O. Phenomics as an Approach to Comparative Developmental Physiology. Front. Physiol. 2023, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Wang, X.; Kumar, U.; Gao, L.; Noor, M.; Imtiaz, M.; Singh, R.P.; Poland, J. High-Throughput Phenotyping Enabled Genetic Dissection of Crop Lodging in Wheat. Front. Plant Sci. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, C.; Hong, S.; Kim, S.L.; Baek, J.; Kim, K.H. High-Throughput Phenotyping Methods for Breeding Drought-Tolerant Crops. Int. J. Mol. Sci. 2021, 22, 8266. [Google Scholar] [CrossRef] [PubMed]
- Pratap, A.; Gupta, S.; Nair, R.M.; Gupta, S.K.; Schafleitner, R.; Basu, P.S.; Singh, C.M.; Prajapati, U.; Gupta, A.K.; Nayyar, H.; et al. Using Plant Phenomics to Exploit the Gains of Genomics. Agronomy 2019, 9, 126. [Google Scholar] [CrossRef]
- Ngugi, H.N.; Ezugwu, A.E.; Akinyelu, A.A.; Abualigah, L. Revolutionizing Crop Disease Detection with Computational Deep Learning: A Comprehensive Review. Environ. Monit. Assess. 2024, 196, 302. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.G.; Hossain, M.A.; Sarker, U.; Alam, A.K.M.; Nair, R.M.; Roychowdhury, R.; Ercisli, S.; Golokhvast, K.S. Genetic Analyses of Mungbean [Vigna radiata (L.) Wilczek] Breeding Traits for Selecting Superior Genotype(s) Using Multivariate and Multi-Traits Indexing Approaches. Plants 2023, 12, 1984. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, X.; Chen, D.; Hou, J.; Liu, T. Real-Time and Visual Detection of Cercospora canescens Based on the Cytb Gene Using Loop-Mediated Isothermal Amplification (LAMP). Can. J. Plant Pathol. 2021, 43, 551–558. [Google Scholar] [CrossRef]
- Ramesh, S.M.; Vinod, P.V.; Niveditha, M.; Pooja, R.; Prasad Bhat, N.; Shashank, N.; Hebbar, R. Plant Disease Detection Using Machine Learning. In Proceedings of the 2018 International Conference on Design Innovations for 3Cs Compute Communicate Control (ICDI3C), Bangalore, India, 25–28 April 2018; pp. 41–45. [Google Scholar] [CrossRef]
- Naik, H.S.; Zhang, J.; Lofquist, A.; Assefa, T.; Sarkar, S.; Ackerman, D.; Singh, A.; Singh, A.K.; Ganapathysubramanian, B. A Real-Time Phenotyping Framework Using Machine Learning for Plant Stress Severity Rating in Soybean. Plant Methods 2017, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Van Haeften, S.; Dudley, C.; Kang, Y.; Smith, D.; Nair, R.M.; Douglas, C.A.; Potgieter, A.; Robinson, H.; Hickey, L.T.; Smith, M.R. Building a Better Mungbean: Breeding for Reproductive Resilience in a Changing Climate. Food Energy Secur. 2023, 12, 1–18. [Google Scholar] [CrossRef]
- Anim-Ayeko, A.O.; Schillaci, C.; Lipani, A. Automatic Blight Disease Detection in Potato (Solanum tuberosum L.) and Tomato (Solanum lycopersicum, L. 1753) Plants Using Deep Learning. Smart Agric. Technol. 2023, 4, 100178. [Google Scholar] [CrossRef]
- Abud, H.F.; de Souza Mesquita, C.M.; Sarmento, E.C.S.; de Sá Melo, R.; de Lima, K.A.P.; da Silva, A.K.F. Image Analysis of the Seeds and Seedlings of Vigna radiata L. Rev. Cienc. Agron. 2022, 53, 1–9. [Google Scholar] [CrossRef]
- McDonald, S.C.; Buck, J.; Li, Z. Automated, image-based disease measurement for phenotyping resistance to soybean frogeye leaf spot. Plant Methods. 2022, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Prashant, K. A Review on High Throughput Phenotyping for Vegetable Crops. J. Bot. Res. 2023, 6, 170–175. [Google Scholar] [CrossRef]
- Wang, G.; Sun, Y.; Wang, J. Automatic Image-Based Plant Disease Severity Estimation Using Deep Learning. Comput. Intell. Neurosci. 2017, 2017, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jangra, S.; Chaudhary, V.; Yadav, R.C.; Yadav, N.R. High-Throughput Phenotyping: A Platform to Accelerate Crop Improvement. Phenomics 2021, 1, 31–53. [Google Scholar] [CrossRef] [PubMed]
- Rane, J.; Raina, S.K.; Govindasamy, V.; Bindumadhava, H.; Hanjagi, P.; Giri, R.; Jangid, K.K.; Kumar, M.; Nair, R.M. Use of Phenomics for Differentiation of Mungbean (Vigna radiata L. Wilczek) Genotypes Varying in Growth Rates Per Unit of Water. Front. Plant Sci. 2021, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Miano, A.C.; Pereira, J.D.C.; Castanha, N.; Júnior, M.D.D.M.; Augusto, P.E.D. Enhancing Mung Bean Hydration Using the Ultrasound Technology: Description of Mechanisms and Impact on Its Germination and Main Components. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Parmar, V.R.; Patel, B.H. Susceptibility of Mung Bean Varieties to Callosobruchus Chinensis under Storage Conditions. Legum. Res. 2016, 39, 637–642. [Google Scholar] [CrossRef]
- Fang, L.J.; Qin, R.L.; Liu, Z.; Liu, C.R.; Gai, Y.P.; Ji, X.L. Expression and Functional Analysis of a PR-1 Gene, MuPR1, Involved in Disease Resistance Response in Mulberry (Morus multicaulis). J. Plant Interact. 2019, 14, 376–385. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).