Somatic Embryogenesis and Plant Regeneration of Farmer-Preferred Passion Fruit Varieties Grown in Kenya
Abstract
:1. Introduction
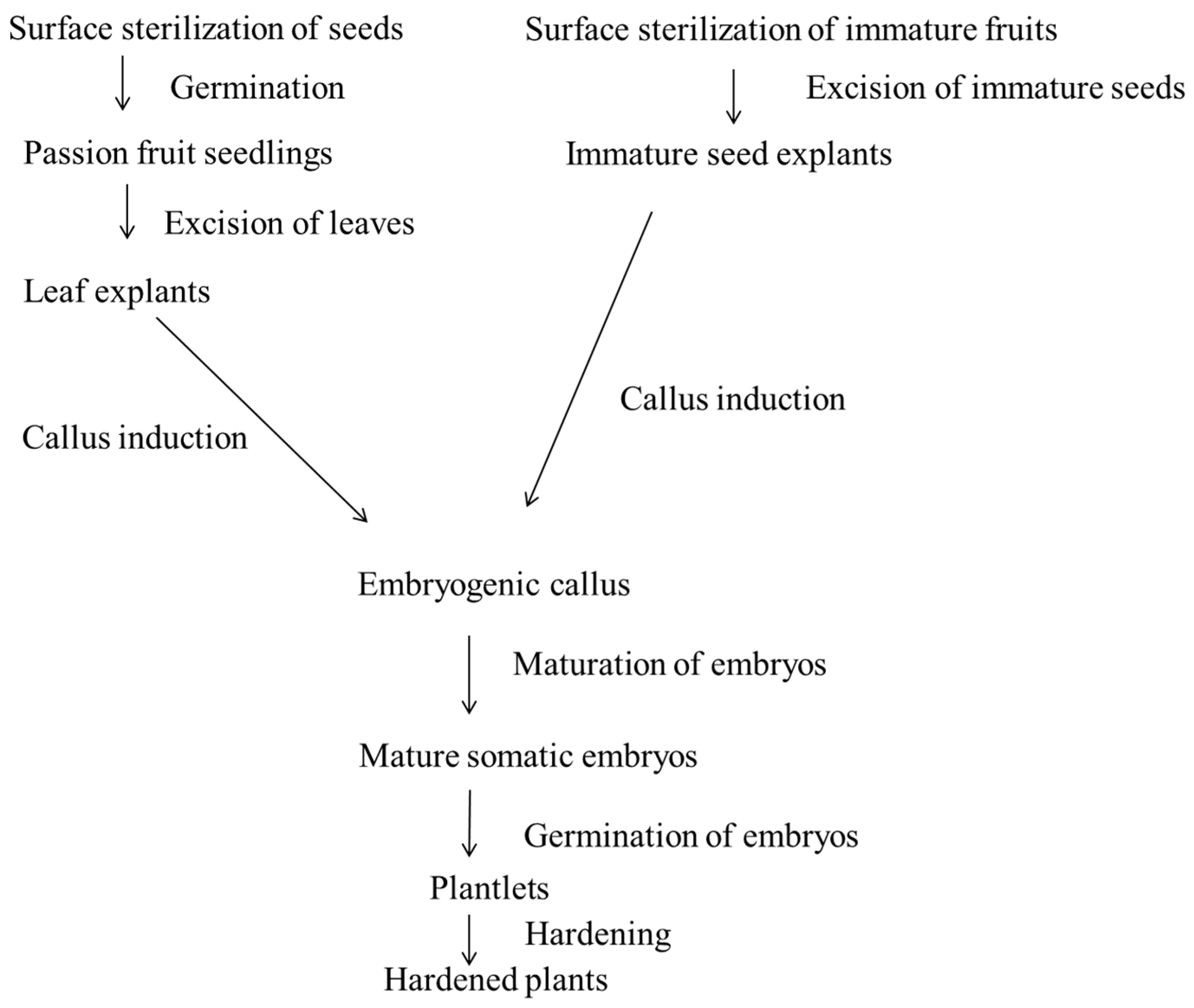
2. Materials and Methods
2.1. Source of Explants
2.2. Explant Preparation
2.3. Somatic Embryogenesis from Leaf Disc and Immature Seed Explants
2.4. Data Analysis
3. Results
3.1. Effect of 2,4-D on Callus Induction and Somatic Embryogenesis from Leaf Explants of Two Genotypes of Passion Fruits
3.2. Effect of 2,4-D and TDZ on Callus Induction and Somatic Embryogenesis from Immature Seeds of KPF4 and Purple Genotypes of Passion Fruit
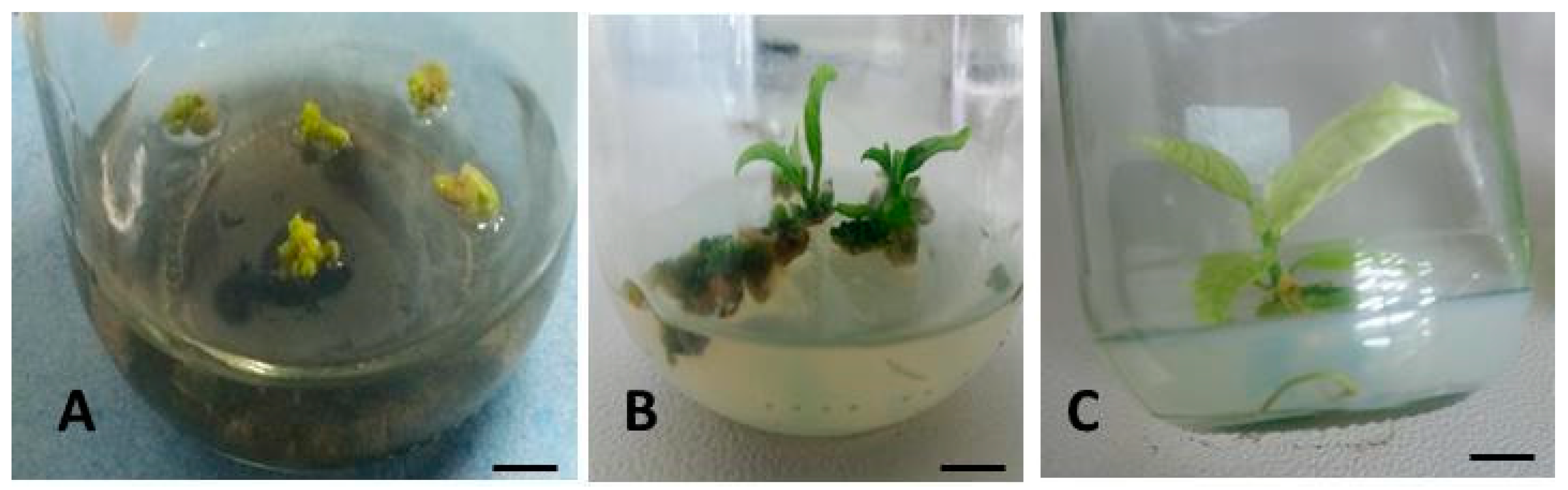
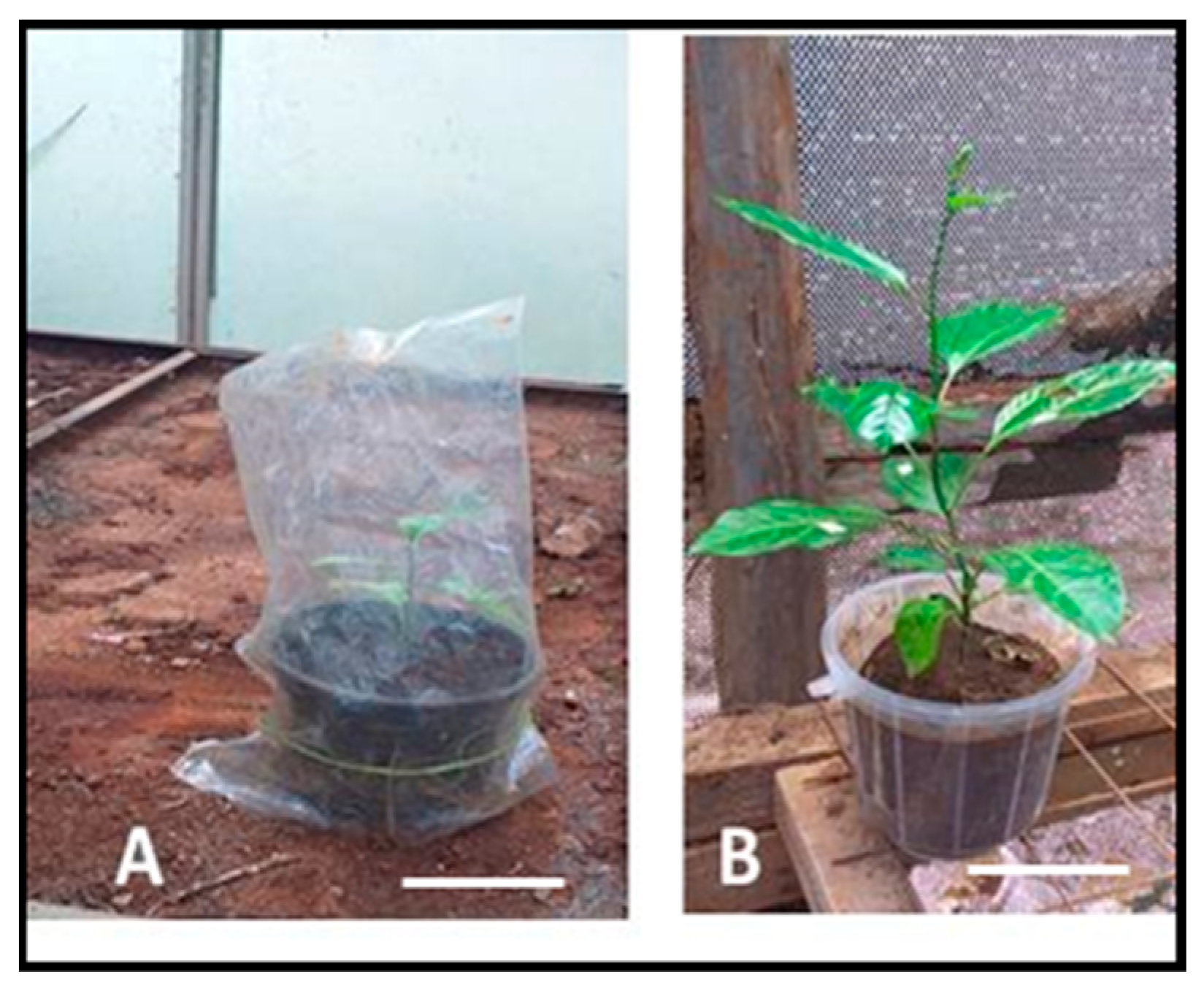
3.3. Conversion of Somatic Embryos to Form Plantlets
4. Discussion
4.1. Responses of Leaf Explants of KPF4 and Purple Passion Fruit Genotypes to 2,4-D Concentrations
4.2. Callus Induction and Somatic Embryogenesis Responses of Immature Seeds of KPF4 and Purple Passion Fruit Genotypes to 2,4-D Concentrations
4.3. Conversion of Somatic Embryos to Form Plantlets
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramaiya, S.D.; Bujang, J.S.; Zakaria, M.H.; King, W.S.; Shaffiq, S.M.A. Sugars, ascorbic acid, total phenolic content and total antioxidant activity in passion fruit (Passiflora) cultivars. J. Sci. Food Agric. 2013, 93, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Pereira, Z.C.; dos Anjos Cruz, J.M.; Corrêa, R.F.; Sanches, E.A.; Campelo, P.H.; de Araújo Bezerra, J. Passion fruit (Passiflora spp.) pulp: A review on bioactive properties, health benefits and technological potential. Food Res. Int. 2023, 166, 112626. [Google Scholar] [CrossRef] [PubMed]
- Zas, P.; John, S. Diabetes and medicinal benefits of Passiflora edulis. World J. Pharm. Res. 2016, 5, 453–465. [Google Scholar]
- Kormelinck, A.G.; Janssen, I. Business Case Passion Fruit Contract Farming Migori County, South West-Kenya; Centre for Development Innovation: Wageningen, The Netherlands, 2012. [Google Scholar]
- Faleiro, F.G.; Junqueira, N.T.V.; Junghans, T.G.; Jesus, O.N.D.; Miranda, D.; Otoni, W.C. Advances in passion fruit (Passiflora spp.) propagation. Rev. Bras. de Frutic. 2019, 41, e-155. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chang, C.; Lin, H.L. Topolins and red light improve the micropropagation efficiency of passion fruit (Passiflora edulis Sims)‘Tainung No. 1’. Hortscience 2020, 55, 1337–1344. [Google Scholar] [CrossRef]
- Huh, Y.S.; Lee, J.K.; Nam, S.Y. Effect of plant growth regulators and antioxidants on in vitro plant regeneration and callus induction from leaf explants of purple passion fruit (Passiflora edulis Sims). J. Plant Biotechnol. 2017, 44, 335–342. [Google Scholar] [CrossRef]
- Hieu, T.; Phong, T.H.; Khai, H.D.; Mai, N.T.N.; Cuong, D.M.; Luan, V.Q.; Tan, N.D. Efficient production of vigorous passion fruit rootstock for in vitro grafting. Plant Cell Tissue Organ Cult. 2022, 148, 635–648. [Google Scholar] [CrossRef]
- Ozarowski, M.; Thiem, B. Progress in micropropagation of Passiflora spp. to produce medicinal plants: A mini-review. Rev. Bras. de Farmacogn. 2013, 23, 937–947. [Google Scholar] [CrossRef]
- Chen, A.H.; Yang, J.L.; Niu, Y.D.; Yang, C.P.; Liu, G.F.; Yu, C.Y.; Li, C.H. High-frequency somatic embryogenesis from germinated zygotic embryos of Schisandra chinensis and evaluation of the effects of medium strength, sucrose, GA3, and BA on somatic embryo development. Plant Cell Tissue Organ Cult. 2010, 102, 357–364. [Google Scholar] [CrossRef]
- Asande, L.K.; Ombori, O.; Nyaboga, E.N.; Oduor, R.O. Efficient shoot organogenesis using leaf disc and nodal explants of passion fruit (Passiflora edulis Sims) and genetic fidelity assessment using sequence-related amplified polymorphism (SRAP) markers. Int. J. Agron. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Phong, T.H.; Hieu, T.; Tung, H.T.; Mai, N.T.N.; Khai, H.D.; Cuong, D.M.; Luan, V.Q.; Nam, N.B.; Nhut, D.T. Silver nanoparticles: A positive factor for in vitro flowering and fruiting of purple passion fruit (Passiflora edulis Sim f. edulis). Plant Cell Tissue Organ Cult. 2022, 151, 401–412. [Google Scholar] [CrossRef]
- Tung, H.T.; Hieu, T.; Phong, T.H.; Khai, H.D.; Hanh, N.T.M.; Van, K.T.T.; Nhut, D.T. The application of thin cell layer culture technique in plant regeneration and micropropagation: Latest achievements. In Plant Tissue Culture: New Techniques and Application in Horticultural Species of Tropical Region; Springer: Singapore, 2022; pp. 231–257. [Google Scholar]
- Raza, G.; Singh, M.B.; Bhalla, P.L. Somatic embryogenesis and plant regeneration from commercial soybean cultivars. Plants 2019, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Phong, T.H.; Hieu, T.; Tung, H.T.; Mai, N.T.N.; Khai, H.D.; Luan, V.Q.; Nam, N.B.; Nhut, D.T. Somatic embryogenesis as potential method for commercial propagation in Passiflora edulis Sims f. edulis-an important horticultural crop. Sci. Hortic. 2023, 316, 112020. [Google Scholar] [CrossRef]
- Pinto, D.L.P.; de Almeida, A.M.R.; Rêgo, M.M.; da Silva, M.L.; de Oliveira, E.J.; Otoni, W.C. Somatic embryogenesis from mature zygotic embryos of commercial passion fruit (Passiflora edulis Sims) genotypes. Plant Cell Tissue Organ Cult. 2011, 107, 521–530. [Google Scholar] [CrossRef]
- Silva, T.C.R.; Carvalho, C.R. Vertical heterogeneity of DNA ploidy level assessed by flow cytometry in calli of Passiflora Cincinnata. Vitr. Cell. Dev. Biol.-Plant 2014, 50, 158–165. [Google Scholar] [CrossRef]
- Rocha, D.I.; Pinto, D.L.P.; Vieira, L.M.; Tanaka, F.A.O.; Dornelas, M.C.; Otoni, W.C. Cellular and molecular changes associated with competence acquisition during passion fruit somatic embryogenesis: Ultrastructural characterization and analysis of SERK gene expression. Protoplasma 2016, 253, 595–609. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.D.; Souza, C.S.; Machado, M.; Reis, A.C.; de Sousa, S.M.; Matos, E.M.; Viccini, L.F.; Otoni, W.C.; de Carvalho, I.F.; Rocha, D.I.; et al. Novel avenues for passion fruit in vitro regeneration from endosperm culture, and morpho-agronomic and physiological traits of triploid Passiflora cincinnata Mast. emblings. Plant Cell Tissue Organ Cult. 2022, 150, 637–650. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Jiménez, V.; Thomas, C. Participation of Plant Hormones in determination and progression of somatic embryogenesis. Somat. Embryog. 2006, 2, 103–118. [Google Scholar]
- Pernisová, M.; Klíma, P.; Horák, J.; Válková, M.; Malbeck, J.; Souček, P.; Za, E. Cytokinins modulate auxin-induced organogenesis in plants via regulation of the auxin efflux. Proc. Natl. Acad. Sci. USA 2009, 106, 3609–3614. [Google Scholar] [CrossRef]
- Mohr, H.; Schopfer, P. Physiology of hormone action. In Plant Physiology; Springer: Berlin/Heidelberg, Germany, 1995; pp. 383–408. [Google Scholar]
- Braybrook, S.A.; Kuhlemeier, C. How a plant builds leaves. Plant Cell 2010, 22, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Amugune, N.O.; Gopalan, H.N.B.; Bytebier, B. Leaf disc regeneration of passion fruit. Afr. Crop Sci. J. 1993, 1, 99–104. [Google Scholar] [CrossRef]
- Mukasa, S.B.; Ssamula, A.; Asami, P.; Holton, T.A. In vitro propagation of three commercial passion fruit varieties in Uganda. Afr. Crop Sci. J. 2016, 24, 397–404. [Google Scholar] [CrossRef]
- Ferreira, D.A.T.; Sattler, M.C.; Carvalho, C.R.; Clarindo, W.R. Embryogenic potential of immature zygotic embryos of Passiflora: A new advance for in vitro propagation without plant growth regulators. Plant Cell Tissue Organ Cult. 2015, 122, 629–638. [Google Scholar] [CrossRef]
- Raghavan, V. Role of 2,4 dichlorophenoxyacetic acid (2,4-D) in somatic embryogenesis on cultured zygotic embryos of Arabidopsis: Cell expansion, cell cycling, and morphogenesis during continuous exposure of embryos to 2, 4 D. Am. J. Bot. 2004, 91, 1743–1756. [Google Scholar] [CrossRef] [PubMed]
- Fehér, A. Why somatic plant cells start to form embryos? In Somatic Embryogenesis; Mujib, A., Šamaj, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 85–101. [Google Scholar] [CrossRef]
- Feher, A.; Pasternak, T.P.; Dudits, D. Transition of somatic plant cells to an embryogenic state. Plant Cell Tissue Organ Cult. 2003, 74, 201–228. [Google Scholar] [CrossRef]
- Gaaj, M.D. Factors influencing somatic embryogenesis induction and plant regeneration with particular reference to Arabidopsis thaliana (L.) Heynh. Plant Growth Regul. 2004, 43, 27–47. [Google Scholar] [CrossRef]
- Cerqueira-Silva, C.B.M.; Santos, E.S.L.; Conceição, L.D.H.C.S.; Cardoso-Silva, C.B.; Pereira, A.S.; Oliveira, A.C.; Corrêa, R.X. Genetic variation in a wild population of the ‘sleep’passion fruit (Passiflora setacea) based on molecular markers. Genet. Mol. Res. 2012, 11, 731–738. [Google Scholar] [CrossRef]
- da Silva, M.L.; Pinto, D.L.P.; Guerra, M.P. A novel regeneration system for a wild passion fruit species (Passiflora cincinnata Mast.) based on somatic embryogenesis from mature zygotic embryos. Plant Cell Tissue Organ Cult. 2009, 99, 47–54. [Google Scholar] [CrossRef]


| PGR | KPF4 | Purple | ||
|---|---|---|---|---|
| 2,4-D (mg/L) | Explants with Embryogenic Callus (%) X | Callus with Somatic Embryos (%) X | Explants with Callus (%) X | Callus with Somatic Embryos (%) X |
| 0.0 | 24.4 ± 4.4 d | 0.0 ± 0.0 d | 0.0 ± 0.0 c | 0.0 ± 0.0 |
| 0.5 | 75.6 ± 5.6 c | 0.0 ± 0.0 d | 51.1 ± 5.6 b | 0.0 ± 0.0 |
| 1.5 | 81.1 ± 4.9 bc | 0.0 ± 0.0 d | 60.0 ± 4.5 b | 0.0 ± 0.0 |
| 2.5 | 97.8 ± 1.5 a | 0.0 ± 0.0 d | 86.7 ± 3.9 a | 0.0 ± 0.0 |
| 4.0 | 96.7 ± 1.8 a | 46.7 ± 3.6 b | 85.5 ± 3.8 a | 0.0 ± 0.0 |
| 6.0 | 97.8 ± 2.2 a | 53.3 ± 6.6 b | 93.3 ± 2.3 a | 0.0 ± 0.0 |
| 8.0 | 100.0 ± 0.0 a | 91.1 ± 4.3 a | 90 ± 0.7 a | 0.0 ± 0.0 |
| 10. | 92.2 ± 2.8 ab | 82.2 ± 4.5 a | 86.7 ± 2.7 a | 0.0 ± 0.0 |
| 12.0 | 93.3 ± 2.2 ab | 38.9 ± 6.3 bc | 90 ± 3.3 a | 0.0 ± 0.0 |
| 16.0 | 100.0 ± 0.0 a | 20.0 ± 3.6 c | 86.7 ± 3.9 a | 0.0 ± 0.0 |
| PGR | KPF4 | Purple | |||
|---|---|---|---|---|---|
| 2,4-D (mg/L) | TDZ (mg/L) | Explant with Callus (%) X | Callus with Somatic Embryos (%) X | Explants with Callus (%) X | Callus with Somatic Embryos (%) X |
| 0.0 | 0.0 | 80.0 ± 3.9 c | 0.0 ± 0.0 d | 56.6 ± 4.0 c | 0.0 ± 0 |
| 4.0 | 1.0 | 86.6 ± 3.6 b,c | 77.8 ± 3.9 c | 72.0 ± 2.9 b,c | 0.0 ± 0 |
| 8.0 | 1.0 | 95.5 ± 2.0 a | 91.1 ± 1.8 a | 93.3 ± 2.3 a | 0.0 ± 0 |
| 16.0 | 1.0 | 90.0 ± 3.7 b | 85.6 ± 2.9 a,b | 74.4 ± 4.2 b | 0.0 ± 0 |
| 32.0 | 1.0 | 92.2 ± 2.8 b | 75.5 ± 4.7 b,c | 73.3 ± 3.9 b | 0.0 ± 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asande, L.K.; Ombori, O.; Oduor, R.O.; Nyaboga, E.N. Somatic Embryogenesis and Plant Regeneration of Farmer-Preferred Passion Fruit Varieties Grown in Kenya. Int. J. Plant Biol. 2023, 14, 1180-1189. https://doi.org/10.3390/ijpb14040086
Asande LK, Ombori O, Oduor RO, Nyaboga EN. Somatic Embryogenesis and Plant Regeneration of Farmer-Preferred Passion Fruit Varieties Grown in Kenya. International Journal of Plant Biology. 2023; 14(4):1180-1189. https://doi.org/10.3390/ijpb14040086
Chicago/Turabian StyleAsande, Lydia K., Omwoyo Ombori, Richard O. Oduor, and Evans N. Nyaboga. 2023. "Somatic Embryogenesis and Plant Regeneration of Farmer-Preferred Passion Fruit Varieties Grown in Kenya" International Journal of Plant Biology 14, no. 4: 1180-1189. https://doi.org/10.3390/ijpb14040086
APA StyleAsande, L. K., Ombori, O., Oduor, R. O., & Nyaboga, E. N. (2023). Somatic Embryogenesis and Plant Regeneration of Farmer-Preferred Passion Fruit Varieties Grown in Kenya. International Journal of Plant Biology, 14(4), 1180-1189. https://doi.org/10.3390/ijpb14040086






