Frontline Warrior microRNA167: A Battle of Survival
Abstract
Author Contributions
Funding
Conflicts of Interest
References
- Redinbaugh, M.G.; Stewart, L.R. Maize lethal necrosis: An emerging, synergistic viral disease. Ann. Rev. Virol. 2018, 5, 301–322. [Google Scholar] [CrossRef] [PubMed]
- Mahuku, G.; Lockhart, B.E.; Wanjala, B.; Jones, M.W.; Kimunye, J.N.; Stewart, L.R.; Cassone, B.J.; Sevgan, S.; Nyasani, J.O.; Kusia, E.; et al. Maize Lethal Necrosis (MLN), an emerging threat to maize-based food security in sub-Saharan Africa. Phytopathology 2015, 105, 956–965. [Google Scholar] [CrossRef]
- Brewbaker, J.L.; Martin, I. Breeding tropical vegetable corns. Plant. Breed. Rev. 2015, 39, 125–198. [Google Scholar]
- Jones, M.W.; Penning, B.W.; Jamann, T.M.; Glaubitz, J.C.; Romay, C.; Buckler, E.S.; Redinbaugh, M.G. Diverse chromosomal locations of quantitative trait loci for tolerance to maize chlorotic mottle virus in five maize populations. Phytopathology 2018, 108, 748–758. [Google Scholar] [CrossRef]
- Jiao, Z.; Tian, Y.; Cao, Y.; Wang, J.; Zhan, B.; Zhao, Z.; Sun, B.; Guo, C.; Ma, W.; Liao, Z.; et al. A novel pathogenicity determinant hijacks maize catalase 1 to enhance viral multiplication and infection. New Phytol. 2021, 3, 1126–1141. [Google Scholar] [CrossRef]
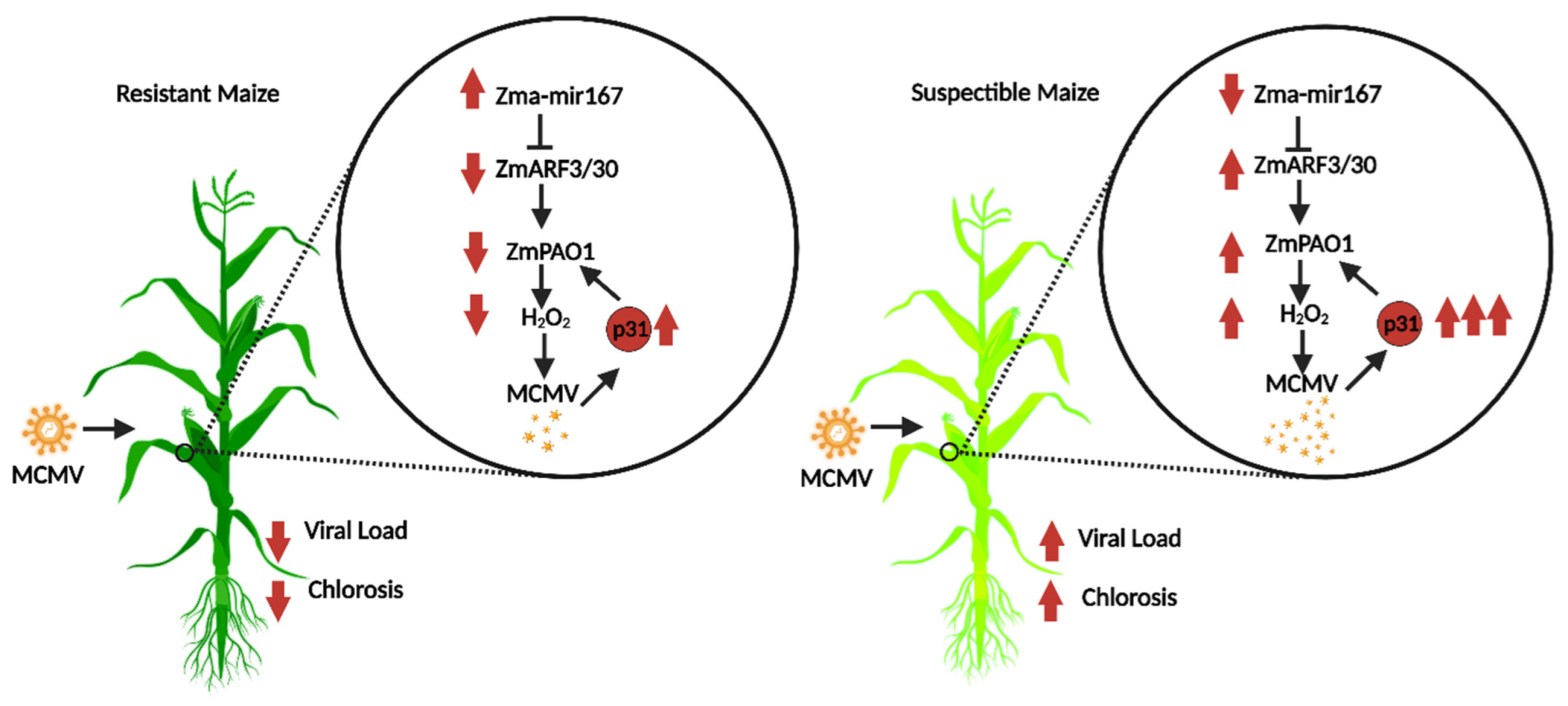
- Liu, X.; Liu, S.; Chen, X.; Prasanna, B.M.; Ni, Z.; Li, X.; He, Y.; Fan, Z.; Zhou, T. Maize miR167-ARF3/30-polyamine oxidase 1 module-regulated H2O2 production confers resistance to maize chlorotic mottle virus. Plant Physiol. 2022, 189, 1065–1082. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Bhatia, S. Expression profiling of miRNAs indicates crosstalk between phytohormonal response and rhizobial infection in chickpea. J. Plant Biochem. Biotechnol. 2020, 29, 380–394. [Google Scholar]
- Tiwari, M.; Singh, B.; Yadav, M.; Pandey, V.; Bhatia, S. High throughput identification of miRNAs reveal novel interacting targets regulating chickpea-rhizobia symbiosis. Environ. Exp. Bot. 2021, 186, 104469. [Google Scholar] [CrossRef]
- Tiwari, M.; Pandey, V.; Singh, B.; Bhatia, S. Dynamics of miRNA mediated regulation of legume symbiosis. Plant Cell Environ. 2021, 44, 1279–1291. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suri, G.S.; Tiwari, M. Frontline Warrior microRNA167: A Battle of Survival. Int. J. Plant Biol. 2022, 13, 598-600. https://doi.org/10.3390/ijpb13040047
Suri GS, Tiwari M. Frontline Warrior microRNA167: A Battle of Survival. International Journal of Plant Biology. 2022; 13(4):598-600. https://doi.org/10.3390/ijpb13040047
Chicago/Turabian StyleSuri, Gurparsad Singh, and Manish Tiwari. 2022. "Frontline Warrior microRNA167: A Battle of Survival" International Journal of Plant Biology 13, no. 4: 598-600. https://doi.org/10.3390/ijpb13040047
APA StyleSuri, G. S., & Tiwari, M. (2022). Frontline Warrior microRNA167: A Battle of Survival. International Journal of Plant Biology, 13(4), 598-600. https://doi.org/10.3390/ijpb13040047





