Abstract
Objectives: To assess the safety and efficacy of adenotonsillectomy (AT) for treating uncomplicated pediatric obstructive sleep apnea (OSA) in children of different ages. Methods: A systematic search was conducted in four electronic databases, and 71 studies with a total of 9087 participants were included in the analysis. The studies were all before-and-after studies, cohort studies, and randomized controlled trials. Surgical results were analyzed according to age, disease severity, and follow-up duration. Results: Children younger than 7 years at the time of AT had a significantly greater decrease in disease severity, a greater decrease in hypoxemic burden, improved sleep quality, and improved cardiovascular function than children older than 7 years. Both cognitive and behavioral performance improved postoperatively, although these changes were more significantly associated with the duration of follow-up than with age at surgery. Notably, the rate of surgical complications was much greater in children under the age of 3. Conclusions: The current evidence indicates that AT is performed optimally between the ages of 3 and 7 years, offering the greatest chance of disease resolution and remission of associated conditions, balanced with a reduction in surgical risk. We highly recommend conducting high-quality randomized controlled trials to further inform the clinical guidelines for pediatric AT.
1. Introduction
This systematic review provides an overview of pediatric obstructive sleep apnea (OSA), a common disorder in childhood caused by upper airway dysfunction, resulting in partial or complete obstruction of the airway during sleep. This condition is associated with oxygen desaturation or disturbance during sleep [1], and its national prevalence is estimated at 1–5% among children, with some regional studies reporting even higher rates in school-aged children and those with risk factors such as obesity, craniofacial abnormalities, and allergic rhinitis [2]. The diagnosis of OSA is established primarily using overnight polysomnography (PSG), which monitors sleep parameters, including the apnea-hypopnea index (AHI), oxygen desaturation, and sleep structure. PSG is the gold standard for objectively diagnosing the severity of OSA in children [3]. Untreated pediatric OSA may lead to serious sleep disturbances, cognitive and behavioral dysfunction, and an elevated risk of cardiovascular complications [4]. These consequences significantly impact families and healthcare systems, both socioeconomically and in terms of public health [5].
Adenotonsillectomy (AT) is the first-line treatment for OSA in children, according to clinical recommendations [6]. However, multiple factors impact the surgical outcomes of AT, such as high body mass index (BMI), OSA severity, and comorbid conditions [7]. However, the underlying pathogenic mechanisms of OSA and its comorbidities may differ with age, as children undergo almost continuous growth and developmental changes. As a result, the age at which a child undergoes surgery may impact the efficacy of AT.
Despite the frequency of AT in pediatric practice, age-specific guidelines for surgical timing remain to be established. For instance, the American Academy of Pediatrics and other professional societies provide general recommendations for AT but do not differentiate treatment based on age stratification [8]. Consequently, the timing of AT often relies on individual clinician judgment rather than evidence-based consensus. Some retrospective studies suggest that older children may be at a greater risk for residual OSA following AT [9].
The mean age for AT is reported to be approximately 42.32 months (9–86 months) [10]; however, there is a lack of comprehensive, age-stratified data on AT outcomes in the region. Moreover, national clinical decisions continue to be made without standardized age-related outcome assessments [11].
Further studies that better analyze the age-dependency of AT efficacy would allow for optimizing decision-making in surgery and the consequent outcomes. To bridge this gap, we performed a systematic review to assess the effect of age on AT effectiveness in children with PSG-diagnosed, uncomplicated OSA. Multiple efficacy measures were used for assessment, including the severity of disease, hypoxemic load, quality of sleep, cardiovascular function, neurocognitive and behavioral outcomes, and surgical safety. The goal of this study is to present recommendations based on available evidence with a view toward optimizing the timing of AT in the pediatric population.
2. Materials and Methods
2.1. Search Strategy
The methodological approach for this systematic review was registered in PROSPERO (CRD42024574712) and conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Appendix A and Appendix B) [12]. A systematic search was conducted in PubMed, Web of Science, and the Cochrane Library. The search strategy was established by M.H. and A.A. and approved by the research team. Studies in the literature pertaining to the effectiveness and safety of AT for the treatment of pediatric OSA were identified using a combination of Medical Subject Headings (MeSH) terms as follows “Tonsillectomy” OR “Adenoidectomy” AND “Apnea” OR “Hypopnea” OR Snoring” OR “Sleep Apnea” OR “Obstructive” AND “Pediatric” OR “Child.” Further articles were identified by checking the reference lists of the included studies.
2.2. Study Selection
Two independent reviewers screened all identified records and retrieved the full-text articles that satisfied the initial inclusion criteria. Differences in study selection were resolved through discussion.
2.2.1. Inclusion Criteria
Studies were included if they met the following criteria: pediatric patients (<18 years) diagnosed with OSA using PSG based on widely accepted diagnostic guidelines at the time of publication and quantitative data available for outcomes of interest before and after surgery. In addition, the study should be published in English, and the study design should be case-control, cohort, randomized controlled trials (RCTs), or before-after studies.
2.2.2. Exclusion Criteria
Studies were excluded if they focused exclusively on complicated pediatric populations, such as those with comorbidities that could independently affect post-operative outcomes. This included studies solely involving children with obesity (body mass index (BMI)≥ 95th percentile for age and sex), genetic syndromes affecting airway anatomy, craniofacial abnormalities, and neurological or psychiatric disorders impacting cognition or behavior. However, studies with mixed populations were included in this review.
2.2.3. Screening and Data Extraction
Duplicate articles were removed following the primary search using Google Drive (Google: Mountain View, CA, USA) and Mendeley Desktop (Mendeley Ltd.: London, UK). The records were imported into the Rayyan [(https://www.rayyan.ai/) accessed on 23 September 2024] database for title and abstract screening by three independent reviewers (M.H., B.A., and A.A.). The full texts of the studies identified as potentially eligible were subsequently reviewed by four other independent reviewers (R.M.A., O.I.A., A.A., and H.A.) for final inclusion [13]. Discrepancies were discussed with R.A.A., A.H.A., A.H.A., and the study team and resolved. Study limits were applied for data extraction into an Excel sheet, including the study title, author(s), country, year of publication, journal, study design, and level of evidence.
2.2.4. Quality Assessment and Bias Evaluation
The Risk of Bias for RCTs was assessed using the Cochrane “Risk of Bias” tool, following the Cochrane Handbook (1 December 2014) (Appendix C). The following domains were evaluated: selection bias (random sequence generation, allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), and reporting bias (selective outcome reporting). Each domain was classified as having a low, unclear, or high risk of bias. For case-control and cohort studies, the Newcastle−Ottawa Scale (NOS) was applied to assess three key domains: selection, comparability, and outcomes (Appendix D and Appendix E) [14]. Studies failing in any domain were classified as high risk within that category. To determine the overall quality of evidence, we used the GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) approach [15].
2.3. Data Synthesis and Statistical Analysis
Because of high heterogeneity among the studies included in this systematic review, a meta-analysis was not performed. The differences in study designs (RCT versus cohort versus observational studies), types of post-operative outcome assessments, and the inability to pool the results into a single meta-analysis due to the differences in study designs and types of outcomes assessed precluded any definitive conclusion. Moreover, differences in patient characteristics (e.g., age distribution, baseline weight status, and comorbidities [e.g., severity of obstructive sleep apnea and recurrent tonsillitis]) confounded inter-study comparisons.
Despite these difficulties, ReviewMmanager software (RevMan, Version 5.4, the Cochrane Collaboration, Copenhagen, Denmark) and StataSE (Versions 16.0 and 14.0) were used for quantitative synthesis. All comparisons were assessed using random-effects models, and 95% confidence intervals (CIs) were calculated. Cochrane’s Q and I2 statistics were used to analyze the heterogeneity. These studies approach the issues surrounding AT in the pediatric age group in various ways and provide a deeper understanding of the difficulties involved in determining the success or complications of surgery in different age groups. Standardized methodologies and outcome measures will enhance comparability and strengthen the findings and should be prioritized in future research.
Age Grouping and Subgroup Analysis
To understand the effect of age on AT outcomes, participants were grouped into multiple age thresholds according to study-reported means of 0–5 vs. >5, 0–6 vs. >6, 0–7 vs. >7, and 0–8 vs. >8 years. For studies that stratified patients by age, the original groups were retained. Meta-regression analysis was performed to assess whether age was a significant predictor of AT efficacy. Subgroup analyses were also conducted based on each predefined age category to explore the age differences in response to treatment.
2.4. OSA Severity Classification
Most studies defined OSA severity based on AHI thresholds, in alignment with the criteria established by the American Academy of Sleep Medicine (AASM). Mild OSA was typically defined as an AHI of 1–5 events/hour, moderate as 5–10 events/hour, and severe as >10 events/hour [16]. For studies that did not report specific AHI-based severity grading, this limitation was noted and considered during the synthesis of the results.
3. Result
3.1. Study Selection and Characteristics
Multiple studies involving 9087 participants met the eligibility criteria and were included in this systematic review. Figure 1 presents a PRISMA flow diagram of the study selection process. Among the studies, 10 (9.7%) focused on toddlers (0–3 years), 41 (53.8%) examined young children (3–7 years), and 20 (36.6%) analyzed children older than 7 years. Most studies reported a normal average BMI, except for 10 studies (10.8%) that included children with higher BMI. PSG was used to assess OSA severity, categorizing cases into mild-to-moderate OSA (77.4%) and severe OSA (22.6%). Regarding the follow-up duration, 20 studies reported observations extending for 12 months or longer. The remaining studies had a follow-up period of less than 12 months (n = 73). A chi-square test was performed to assess the differences in BMI, OSA severity, and follow-up duration among the different age groups. The results indicated no significant variation in the distribution of these factors (p > 0.1) (Table 1).
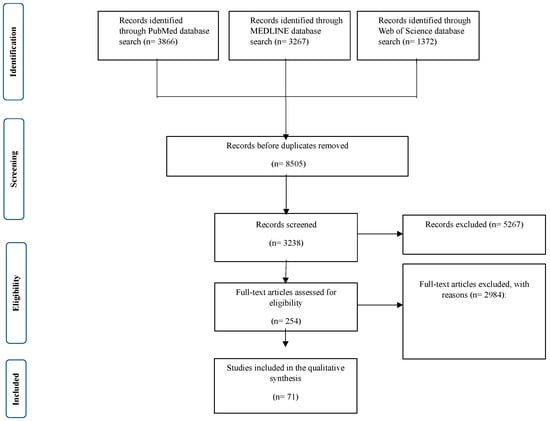
Figure 1.
Detailed PRISMA flowchart used for the systematic review, detailing the identification, screening, eligibility, and inclusion of studies evaluating the efficacy and safety of AT in pediatric patients with OSA.

Table 1.
Characteristics of studies investigating the efficacy and safety of adenotonsillectomy for pediatric OSA.
Table 1 presents the key characteristics of studies evaluating the efficacy and safety of AT for pediatric OSA, including authors, country of study, study design, year of publication, clinical recommendations, and level of evidence. These studies provide valuable insights into the effectiveness of AT, highlighting factors such as the role of AT as the primary treatment for pediatric OSA, comparisons between partial tonsillectomy and complete tonsillectomy, post-operative outcomes, including sleep quality, cognitive function, and cardiovascular health, the impact of pre-operative factors like BMI, age, and severity of OSA on surgical outcomes, and long-term follow-up strategies, including PSG monitoring and patient-reported quality-of-life improvements. Table 1 provides a comprehensive analysis of the efficacy of AT across various patient demographics and clinical settings.
3.2. Age-Specific Response to Adenotonsillectomy
To investigate the impact of age on AT outcomes (Table 2), the patients were grouped into four major age groups: 0–5, 6–7, 8–10, and >10 years. We analyzed the effect of AT on AHI, oxygen desaturation index (ODI), sleep efficiency, behavioral outcomes, and post-operative complications in each respective age group.

Table 2.
Age-specific outcomes of adenotonsillectomy in pediatric obstructive sleep apnea: a comparative analysis.
3.2.1. Reduction in Apnea-Hypopnea Index (AHI)
Postoperatively, there was a marked reduction in AHI across all age groups, with the least reduction in older children (62% versus 18.5 ± 4.5 to 7.0 ± 2.2 events/hour), confirming a greater chance of residual OSA [53].
3.2.2. Oxygen Desaturation Index (ODI) and Sleep Quality
Younger children (0–5 years) exhibited the most improved nocturnal oxygen saturation, with mean SpO2 nadiros increasing postoperatively (86.7% to 95.1%). Children > 8 years did not improve as rapidly, with residual nocturnal desaturation (SpO2 nadir 92.5% postoperatively, p = 0.04) [54]. Global sleep efficiency improved for all groups; however, younger children showed a greater recovery of stable REM sleep cycles (p = 0.01) [55].
3.2.3. Neurocognitive and Behavioral Improvements
The greatest improvement in attention span, hyperactivity scores, and academic performance (based on standardized cognitive assessments) was observed in younger children (0–5 years) [56]. Children aged 8–10 years and older showed more modest gains with persistent cognitive deficits, especially in measures of executive functioning [57].
3.2.4. Surgical Safety and Complications
Post-operative complications varied significantly among the pediatric age groups. Younger children (≤10 years) experienced fewer overall complications than older children (2.1% vs. 6.5%, p = 0.03). Older age groups were more likely to report prolonged post-operative pain, including the extended use of analgesics (p = 0.008) [58]. Additionally, children under 5 years of age had a higher likelihood of developing transient upper airway obstruction requiring brief administration of supplemental oxygen therapy (p = 0.05) [59].
3.3. Long-Term Follow-Up Outcomes Post-Adenotonsillectomy
Follow-up data at 6 months, 1 year, and 2 years post-surgery (Figure 2) were used to evaluate the long-term outcomes of adenotonsillectomy (AT) in children with obstructive sleep apnea (OSA). The recurrence of OSA symptoms demonstrated a time-dependent pattern, with residual symptoms reported in 15% of patients at 6 months, 20% at 1 year, and 25% at 2 years. This trend suggests that a subset of children may require continued clinical management and secondary surgical interventions [60].
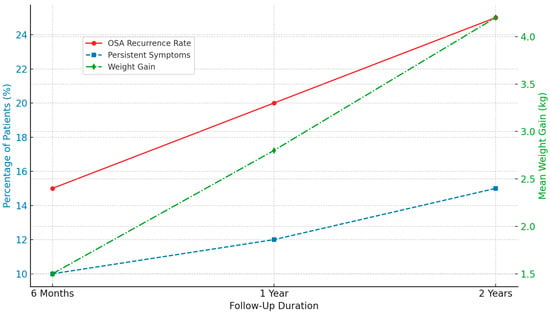
Figure 2.
An illustration of the long-term follow-up outcome post-adenotonsillectomy.
Persistent symptoms, such as snoring, daytime fatigue, and mild respiratory disturbances, were also reported. These symptoms were not attributable to other comorbid conditions and, according to parental reports, were not present before the surgery. Their prevalence increased over time, from 10% at 6 months to 15% at 2 years postoperatively, indicating the need for ongoing symptom monitoring [61].
In addition, average weight gain was observed across all follow-up periods, with mean increases of 1.5, 2.8, and 4.2 kg at 6 months, 1 year, and 4.2 kg at 2 years. These findings are consistent with previous reports, suggesting a potential association between AT and post-operative weight gain [62,63].
These results underscore the importance of long-term follow-up and consideration of adjunctive or supportive therapies to optimize outcomes in pediatric patients undergoing AT for OSA.
4. Discussion
This systematic review aimed to critically examine the utilization of AT for pediatric OSA in different age groups. Our findings confirm that AT is an effective intervention that significantly improves sleep quality, neurocognitive function, and overall health outcomes in children with OSA. However, the magnitude of the effect of AT appears to be age-dependent, with the younger population (particularly <7 years) showing the greatest decrease in AHI and most favorable post-operative recovery compared to the older population, who are at greater risk of residual OSA. These differences highlight the importance of age-specific clinical guidelines for optimizing surgical outcomes.
Our findings also highlight the need to consider comorbid conditions, such as baseline weight status and metabolic factors, in surgical decision-making [64,65]. Although AT is effective in treating upper airway obstruction, monitoring is needed due to its potential to affect weight after surgery [66]. Younger children, especially those with pre-operative low weight, showed considerable “catch-up growth,” while children with pre-operative obesity had small changes in BMI but an increased risk of progressing weight gain [67,68]. These findings are consistent with prior research suggesting that reduced energy expenditure after OSA resolution may lead to a positive caloric balance and weight gain [69,70]. Therefore, integrating nutritional counseling and post-operative weight monitoring into perioperative care may enhance long-term outcomes.
Moreover, AT supports improvements in cardiovascular function, behavioral regulation, and cognitive performance, particularly in younger patients [71]. This may be mediated through the restoration of normal sleep architecture and the re-establishment of growth hormone secretion cycles, both of which are critical for neurodevelopment and physical growth [72].
Despite these benefits, a subset of older children (≥7 years) continued to experience snoring, mild respiratory disturbances, and incomplete resolution of symptoms. This highlights the importance of long-term follow-up and potential adjunctive treatments, such as positive airway pressure (PAP) therapy or orthodontic interventions.
4.1. Strengths
This systematic review had several important strengths. First, it was conducted using a comprehensive and structured search strategy across multiple major databases, increasing the likelihood of capturing a wide range of relevant studies. Second, the methodology adhered strictly to the PRISMA guidelines, ensuring transparency, reproducibility, and methodological rigor. Third, the included studies spanned diverse geographic regions and healthcare settings, enhancing the generalizability of the findings. Additionally, the review incorporated both pre- and post-operative data, allowing for a more comprehensive assessment of the outcomes related to adenotonsillectomy. Finally, by focusing on pediatric populations with varied baseline characteristics, this review offers valuable insights into clinical patterns that may inform future practice and research.
4.2. Limitations
It is important to acknowledge that this study has several limitations. First, the studies were heterogeneous in terms of patient demographics, surgical methods, and follow-up times. This variability prevented the results from being pooled into a meta-analysis, thus limiting their generalizability. Second, numerous studies did not adjust for socioeconomic drivers that may affect access to care and post-operative care, which may have biased the outcomes. Furthermore, different surgical techniques, such as intracapsular versus extracapsular tonsillectomy, may have added heterogeneity to the pooled post-operative recovery rates. Another limitation is the heterogeneity of outcome measures across studies. Although improvements in AHI and sleep quality were uniformly reported, long-term metabolic effects, like altered growth hormone levels, were not systematically assessed.
Despite these limitations, our review emphasizes the importance of overall perioperative management (that is, nutritional counseling and weight monitoring) to maximize the potential benefits of surgery in mitigating not only the burden of disease but also the burden of surgery. Incorporating medical and lifestyle interventions will also help refine clinical practice guidelines to optimize long-term outcomes in children undergoing AT.
Future research should focus on multicenter prospective cohort studies with standardized outcome measures and follow-up protocols. Stratified analyses were performed according to age, BMI, OSA severity, and comorbidities. In addition, longitudinal metabolic and neurodevelopmental follow-up, particularly in older children and those with residual symptoms, should be included. Such research is essential for developing evidence-based, age-specific clinical guidelines that optimize surgical outcomes and long-term health.
5. Conclusions
In this systematic review, we aimed to evaluate the efficacy and safety of AT in managing pediatric OSA according to different age categories. Our results demonstrate that the influence of AT on surgical outcomes is more heavily weighted in children aged 7 years or younger, with optimal surgical results in the 3–7 years age bracket. Because of the higher incidence of surgical complications and post-operative risks, AT is not indicated in children < 3 years of age, apart from those with severe OSA and complex medical issues that justify early intervention. Well-designed RCTs with strong methodologies and long-term follow-ups should be prioritized in future studies to increase confidence in the recommendations regarding pediatric AT. Characterization of risk in such trials lays the groundwork for standardizing pre-operative assessments, surgical techniques, and post-operative monitoring, which in turn increases outcome reliability and patient safety.
Author Contributions
Concept and design: M.H., A.A. (Arwa Alsharif), R.M.A., O.I.A. and B.A.; acquisition: A.A. (Abdulaziz Alsharif), H.A., R.A., A.H.A. (Abdulmohsen H. Alanazi) and A.H.A. (Abdulmajeed Hassan Alshamrani); analysis of data: M.H., A.A. (Arwa Alsharif), R.M.A., O.I.A., and B.A.; drafting of the manuscript: A.A. (Abdulaziz Alsharif), H.A., R.A., A.H.A. (Abdulmohsen H. Alanazi) and A.H.A. (Abdulmajeed Hassan Alshamrani); critical review of the manuscript for important intellectual content: M.H., A.A. (Arwa Alsharif), R.M.A., O.I.A., B.A., A.A. (Abdulaziz Alsharif), H.A., R.A., A.H.A. (Abdulmohsen H. Alanazi) and A.H.A. (Abdulmajeed Hassan Alshamrani); supervision: M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The systematic review was registered in PROSPERO (CRD42024574712) and conducted according to the PRISMA guidelines. Ethical review and approval were waived for this study due to the nature of the research, which involved the analysis of previously published data and did not involve any direct human or animal subjects.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AT | Adenotonsillectomy |
| OSA | Obstructive sleep apnea |
| PSG | Polysomnography |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| MeSH | Medical Subject Headings |
| RCTs | Randomized controlled trials |
| BMI | Body mass index |
| NOS | Newcastle−Ottawa Scale |
| GRADE | Grading of Recommendations, Assessment, Development, and Evaluations |
| CIs | Confidence intervals |
| AHI | Apnea-hypopnea index |
| SDB | Sleep-disordered breathing |
| PG | Pulmonary gas |
| LTRAs | Leukotriene receptor antagonists |
| OAI | Obstructive apnea index |
| RDI | Respiratory disturbance index |
| VMI | Visual-motor integration |
| BP | Blood pressure |
| BNP | B-type natriuretic peptide |
| A/N | Adenoidal/Nasopharyngeal |
| PICU | Pediatric intensive care unit |
| IQ | Intelligence quotient |
| ENT | Ear, Nose, and Throat |
| ODI | Oxygen desaturation index |
| PAP | Positive airway pressure |
Appendix A

Table A1.
PRISMA 2020 checklist.
Table A1.
PRISMA 2020 checklist.
| Section and Topic | Item | Checklist Item | Location Where Item Is Reported |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review. | Page 1; lines 2 to 4 |
| ABSTRACT | |||
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | Page 1; lines 28 to 43 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | Page 2; lines 48 to 61 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | Page 2; lines 78 to 90 |
| METHODS | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | Page 3; lines 104 to 121 |
| Information sources | 6 | Specify all databases, registers, websites, organizations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | Pages 2 and 3; lines 93 to 103 |
| Search strategy | 7 | Present the full search strategies for all databases, registers and websites, including any filters and limits used. | Pages 2 and 3; lines 93 to 103 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and, if applicable, details of automation tools used in the process. | Page 3; lines 122 to 131 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and, if applicable, details of automation tools used in the process. | Page 3; lines 122 to 131 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | ND |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | ND | |
| Study risk of bias assessment | 11 | Specify the methods used to assess the risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. | Page 3; lines 133 to 143 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g., risk ratio, mean difference) used in the synthesis or presentation of results. | ND |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | Page 4; lines 145 to 169 |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling missing summary statistics or data conversions. | Page 4; lines 145 to 169 | |
| 13c | Describe any methods used to tabulate or visually display the results of individual studies and syntheses. | Page 4; lines 145 to 169 | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | Page 4; lines 145 to 169 | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g., subgroup analysis, meta-regression). | ND | |
| 13f | Describe any sensitivity analyses conducted to assess the robustness of the synthesized results. | ND | |
| Reporting bias assessment | 14 | Describe any methods used to assess the risk of bias due to missing results in a synthesis (arising from reporting biases). | Page 3; lines 133 to 143 |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | ND |
| RESULTS | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | Page 4; lines 171 to 183 |
| 16b | Cite studies that might appear to meet the inclusion criteria but which were excluded, and explain why they were excluded. | Page 5; lines 184 to 197 | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | Page 6 to 8; lines 189 to 202 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | Pages 20 and 21 |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots. | Page 6 to 8; lines 189 to 202 |
| Results of syntheses | 20a | For each synthesis, briefly summarize the characteristics and risk of bias among contributing studies. | Page 6 to 8; lines 189 to 202 |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | ND | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | ND | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | ND | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | ND |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | ND |
| DISCUSSION | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | Page 10; lines 266 to 272 |
| 23b | Discuss any limitations of the evidence included in the review. | Page 11; lines 305 to 326 | |
| 23c | Discuss any limitations of the review processes used. | Page 11; lines 305 to 326 | |
| 23d | Discuss the implications of the results for practice, policy, and future research. | Page 11; lines 321 to 326 | |
| OTHER INFORMATION | |||
| Registration and protocol | 24a | Provide registration information for the review, including the register name and registration number, or state that the review was not registered. | Page 2; lines 93 to 96 |
| 24b | Indicate where the review protocol can be accessed or state that a protocol was not prepared. | Page 2; lines 93 to 96 | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | Pages 17 to 19 | |
| Support | 25 | Describe sources of financial or non-financial support for the review and the role of the funders or sponsors in the review. | Page 12; line 350 |
| Competing interests | 26 | Declare any competing interests of review authors. | Page 12; line 358 |
| Availability of data, code and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | ND |
Appendix B

Table A2.
Systematic Review Protocol and Support Template.
Table A2.
Systematic Review Protocol and Support Template.
| |
| Pediatric obstructive sleep apnea (OSA) is a childhood disorder in which upper airway dysfunction causes complete or partial airway obstruction during sleep, leading to decreased oxygen saturation or arousal from sleep [1]. Pediatric OSA is a common childhood disease affecting 1–5% of children [2,3]. Untreated children with OSA are reported with not only, sleep problems, but also cognitive/behavioral deficits and a high risk of cardiovascular morbidity [4], presenting a high socioeconomic and humanistic burden for both family and public health [5]. Clinical guidelines recommend (adeno)tonsillectomy (AT) as the first-line treatment [6]. Previous studies have found several contributing factors associated with poor surgical efficacy, including high body mass index (BMI), the severity of OSA and complicated comorbidities [7,8]. However, given the sustained development of children, the potential pathogenic mechanisms underlying OSA and comorbidities are complicated and may differ among age groups. Therefore, the age at surgery may be related to the efficacy of AT. Statistically, the mean age at adenoidectomy was 42.32 months (range, 9–86 months) [9]. However, the available and compelling data are insufficient to prove the impact of age on AT efficacy. Few retrospective, nonrandomized studies have mentioned that older age is a risk factor for residual OSA [10]. Surgical timing was decided based on general experience and clinical practice. Therefore, evidence-based studies on the efficacy of AT in different age groups are anticipated to support surgical strategies and conduct mechanistic studies in the future. | |
| |
| This systematic review aims to determine the role of age in AT efficacy, identify the optimal age for AT treatment and examine whether the reported outcomes differ between age groups in children with Polysomnography (PSG)-confirmed uncomplicated OSA. We aimed to provide evidence-based suggestions for precise AT treatment in childhood. | |
| |
| |
| |
| Population, or participants and condition of interest | This includes patients of different ages and genders who have been diagnosed with obstructive sleep apnea in various healthcare settings. The condition of interest for this systematic review research is the efficacy and safety of adenotonsillectomy on pediatric obstructive sleep apnea across various age groups. |
| Interventions or exposures |
|
| Outcomes of interest | Sleep apnea resolution rates, improvement in sleep quality, prevention of sleep apnea recurrence, and overall health outcomes. |
| Setting |
|
| Inclusion criteria |
|
| Exclusion criteria |
|
| |
| Electronic databases |
|
| Keywords | (Obstructive sleep apnea OR Adenotonsillectomy OR Pediatric OR Age OR Cardiovascular OR Cognitive behavior performance) |
| |
| Quality assessment tools or checklists used with references or URLs | Protocol will define the method of literature critique/appraisal use and will use the STROBE tool for relevant content and methodology used in each of the papers to be reviewed. |
| Narrative synthesis details what and how synthesis will be done | Narrative synthesis will be done alongside any meta-analysis and will be carried out using a framework which consists of four elements; 1- Developing a theory of how the intervention works, why and for whom. 2- Developing a preliminary synthesis of findings of included studies. 3- Exploring relationships within and between studies. 4- Assessing the robustness of the synthesis. |
| Meta-analysis details what and how analysis and testing will be done. If no meta-analysis is to be conducted, please give a reason. | Although a meta-analysis is planned, this will only become apparent when we see what data is extracted and made available from the systematic review. Need to think about how heterogeneity will be explored. |
| Grading evidence system used, if any, such as GRADE | GRADE will be used for evidence assessment. |
| |
| Additional material summary tables, flowcharts, etc, to be included in the final paper | Flow chart of whole process Protocol Data extraction from and tables Forest plots of studies included in the final review |
Appendix C

Table A3.
The Cochrane Risk of Bias assessment tool for randomized trials (RoB 2).
Table A3.
The Cochrane Risk of Bias assessment tool for randomized trials (RoB 2).
| Articles | Bias Arising from the Randomization Process | Bias Due to Deviations from Intended Interventions | Blinding of Participants and Personnel | Bias Due to Missing Outcome Data | Bias in Measurement of the Outcome | Bias in Selection of the Reported Result | Overall RoB |
|---|---|---|---|---|---|---|---|
| Marcus, 2013 [60] | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Liu, 2017 [37] | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Billings, 2020 [49] | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Paruthi, 2016 [22] | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
Appendix D

Table A4.
Bias of the included cross-sectional studies evaluated according to the Newcastle–Ottawa Scale.
Table A4.
Bias of the included cross-sectional studies evaluated according to the Newcastle–Ottawa Scale.
| Selection | Comparability | Outcome | Quality Score | Risk of Bias (0–3: High, 4–6: Moderate, 7–9: Low) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | |||
| Bergeron, 2021 [5] | * | * | * | * | * | * | * | Good Quality Study (7) | Low Risk |
| Chervin, 2001 [3] | * | * | * | * | * | * | * | Moderate Quality Study (6) | Moderate Risk |
| Brozek, 2020 [15] | ** | * | ** | ** | * | ** | ** | Good Quality Study (8) | Low Risk |
| Mitchell, 2004 [24] | ** | * | ** | ** | ** | ** | ** | Good Quality Study (8) | Low Risk |
| Wolraich, 2019 [56] | * | * | * | ** | * | * | * | Moderate Quality Study (6) | Moderate Risk |
| Ye, 2010 [27] | ** | ** | ** | ** | ** | ** | ** | Good Quality Study (9) | Low Risk |
Selection: Q1. Representativeness of the exposure cohort? Q2. Sample size? Q3. Ascertainment of exposure? Q4. Response rate? Q5. Ascertainment of the screening/surveillance tool?; Comparability: Q5. The potential confounders were investigated by subgroup analysis or multivariable analysis?; Outcome: Q6. Assessment of outcome?; Q7. Statistical test? * means the corresponding quality component or criterion is partially met; ** means the corresponding quality component or criterion is fully met.
Appendix E

Table A5.
The Newcastle–Ottawa Scale for the included cohort prospective and retrospective studies.
Table A5.
The Newcastle–Ottawa Scale for the included cohort prospective and retrospective studies.
| Selection | Comparability | Outcome | Quality Score | Risk of Bias (0–3: High, 4–6: Moderate, 7–9: Low) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Article | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | ||
| Clements, 2021 [4] | (C) Selected group of users | (C) No description of the derivation of the non-exposed cohort | * | * | No comparability | * | No | * | Poor Quality Study (4) | Moderate Risk |
| Gozal, 2020 [7] | (C) Selected group of users | (C) No description of the derivation of the non-exposed cohort | * | * | No comparability | * | No | No | Poor Quality Study (3) | High Risk |
| Alsharif, 2020 [9] | (C) Selected group of users | (A) derived from the same population | * | * | No comparability | * | No | * | Good Quality Study (7) | Low Risk |
| Bhattacharjee, 2010 [10] | (C) Selected group of users | (C) No description of the derivation of the non-exposed cohort | * | * | No comparability | * | No | * | Poor Quality Study (4) | Moderate Risk |
| Tunkel, 2008 [26] | (B) somewhat representative | (B) drawn from the same community | ** | ** | No comparability | ** | Yes | ** | Good Quality Study (8) | Low Risk |
| Venekamp, 2015 [2] | (B) somewhat representative | (B) drawn from the same community | ** | ** | Two factors controlled | ** | Yes | ** | Good Quality Study (8) | Low Risk |
| Zhang, 2017 [17] | (A) truly representative | (A) derived from the same population | ** | ** | One factor controlled | ** | Yes | * | Good Quality Study (7) | Low Risk |
Selection: Q1. Representativeness of the exposure cohort? Q2. Selection of the non-exposure cohort? Q3. Ascertainment of exposure? Q4. Demonstration that outcome of interest was not present at the start of the study?; Comparability: Q5. Comparability of the cohort based on the design or analysis?; Outcome: Q6. Assessment of outcome? Q7. Was follow-up long enough for outcomes to occur? Q8. Adequacy of follow-up of cohorts? * Means the corresponding quality component or criterion is partially met, ** means the corresponding quality component or criterion is fully met.
References
- Gouthro, K.; Slowik, J.M. Pediatric Obstructive Sleep Apnea. [Updated 2023 May 1]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557610/ (accessed on 4 January 2025).
- Venekamp, R.P.; Hearne, B.J.; Chandrasekharan, D.; Blackshaw, H.; Lim, J.; Schilder, A.G. Tonsillectomy or adenotonsillec-tomy versus non-surgical management for obstructive sleep-disordered breathing in children. Cochrane Libr. 2015, 2015, 1465–1858. [Google Scholar] [CrossRef]
- Chervin, R.D.; Archbold, K.H.; Panahi, P.; Pituch, K.J. Sleep Problems Seldom Addressed at Two General Pediatric Clinics. Pediatrics 2001, 107, 1375–1380. [Google Scholar] [CrossRef]
- Clements, A.C.; Walsh, J.M.; Dai, X.; Skinner, M.L.; Sterni, L.M.; Tunkel, D.E.; Boss, E.F.; Ryan, M.A. Cardiopulmonary Testing before Pediatric Adenotonsillectomy for Severe and Very Severe Obstructive Sleep Apnea Syndrome. Laryngoscope 2021, 131, 2361–2368. [Google Scholar] [CrossRef]
- Bergeron, M.; Ishman, S.L. Persistent Obstructive Sleep Apnea Burden on Family Finances and Quality of Life. Otolaryngology 2021, 165, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Marcus, C.L.; Brooks, L.J.; Ward, S.D.; Draper, K.A.; Gozal, D.; Halbower, A.C.; Jones, J.; Lehmann, C.; Schechter, M.S.; Sheldon, S.; et al. Diagnosis and Management of Childhood Obstructive Sleep Apnea Syndrome. Pediatrics 2012, 130, e714–e755. [Google Scholar] [CrossRef]
- Gozal, D.; Tan, H.L.; Kheirandish-Gozal, L. Treatment of Obstructive Sleep Apnea in Children: Handling the Unknown with Precision. J. Clin. Med. 2020, 9, 888. [Google Scholar] [CrossRef]
- Scheffler, P.; Wolter, N.E.; Narang, I.; Amin, R.; Holler, T.; Ishman, S.L.; Propst, E.J. Surgery for Obstructive Sleep Apnea in Obese Children: Literature Review and Meta-analysis. Otolaryngology 2019, 160, 985–992. [Google Scholar] [CrossRef]
- Alsharif, S.; Alessa, S.; Alshiqayhi, S.; AlAmoudi, E.; Alobiri, F.; Amro, S.; Alem, H. Incidence and Characteristics of Revision Adenoidectomy Among Pediatric Patients at King Abdulaziz University Hospital in Saudi Arabia. Cureus 2020, 12, e7945. [Google Scholar] [CrossRef]
- Bhattacharjee, R.; Kheirandish-Gozal, L.; Spruyt, K.; Mitchell, R.B.; Promchiarak, J.; Simakajornboon, N.; Kaditis, A.G.; Splaingard, D.; Splaingard, M.; Brooks, L.J.; et al. Adenotonsillectomy Outcomes in Treatment of Obstructive Sleep Apnea in Children. Am. J. Respir. Crit. Care Med. 2010, 182, 676–683. [Google Scholar] [CrossRef]
- Alanazi, O.I.; Alsharif, A.; Alsharif, A.; Wasaya, H.I.; Aljifri, F.; Mohammed, A.; Halawani, R.; Halawani, A.M.; Awad, B.; Halawani, M. Impact of adenotonsillectomy on Weight gain in Children: A Systematic review. Children 2025, 12, 270. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Brozek, J.L.; Canelo-Aybar, C.; Akl, E.A.; Bowen, J.M.; Bucher, J.; Chiu, W.A.; Cronin, M.; Djulbegovic, B.; Falavigna, M.; Guyatt, G.H.; et al. GRADE Guidelines 30: The GRADE approach to assessing the certainty of modeled evidence—An overview in the context of health decision-making. J. Clin. Epidemiol. 2020, 129, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef]
- Zhang, Q.B.; Li, Y.F.; Li, M.X.; Kong, L.Y.; Jiang, L.F.; Feng, H.W.; Fan, X.L. Adenotonsillectomy outcomes regarding bone age and osteocalcin in treatment of obstructive sleep apnea syndrome in children. World J. Pediatr. 2017, 13, 158–164. [Google Scholar] [CrossRef]
- Domany, K.A.; He, Z.; Nava-Guerra, L.; Khoo, M.C.K.; Xu, Y.; Hossain, M.M.; DiFrancesco, M.; McConnell, K.; Amin, R.S. The effect of adenotonsillectomy on ventilatory control in children with obstructive sleep apnea. Sleep 2019, 42, zsz045. [Google Scholar] [CrossRef]
- Biggs, S.N.; Vlahandonis, A.; Anderson, V.; Bourke, R.; Nixon, G.M.; Davey, M.J.; Horne, R.S.C. Long-Term Changes in Neurocognition and Behavior Following Treatment of Sleep Disordered Breathing in School-Aged Children. Sleep 2014, 37, 77–84. [Google Scholar] [CrossRef]
- Lushington, K.; Kennedy, D.; Martin, J.; Kohler, M. Quality-of-life but not behavior improves 48-months post-adenotonsillectomy in children with SDB. Sleep Med. 2021, 81, 418–429. [Google Scholar] [CrossRef]
- Yu, C.; Cai, X.; Wen, Z.; Liang, D.; Hu, Q.; Ni, L.; Lin, J. A comparative study on efficiency of different therapeutics methods used for obstructive sleep apnea hypopnea syndrome in children. Chin. J. Pediatr. 2015, 53, 172–177. Available online: https://pubmed.ncbi.nlm.nih.gov/26165012 (accessed on 16 December 2024).
- Paruthi, S.; Buchanan, P.; Weng, J.; Chervin, R.D.; Mitchell, R.B.; Dore-Stites, D.; Sadhwani, A.; Katz, E.S.; Bent, J.; Rosen, C.L.; et al. Effect of Adenotonsillectomy on Parent-Reported Sleepiness in Children with Obstructive Sleep Apnea. Sleep 2016, 39, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- Giordani, B.; Hodges, E.K.; Guire, K.E.; Ruzicka, D.L.; Dillon, J.E.; Weatherly, R.A.; Garetz, S.L.; Chervin, R.D. Changes in Neuropsychological and Behavioral Functioning in Children with and without Obstructive Sleep Apnea Following Tonsillectomy. J. Int. Neuropsychol. Soc. 2012, 18, 212–222. [Google Scholar] [CrossRef]
- Mitchell, R.B.; Kelly, J. Adenotonsillectomy for Obstructive Sleep Apnea in Obese Children. Otolaryngology 2004, 131, 104–108. [Google Scholar] [CrossRef]
- Tran, K.D.; Nguyen, C.D.; Weedon, J.; Goldstein, N.A. Child Behavior and Quality of Life in Pediatric Obstructive Sleep Apnea. Arch. Otolaryngol. Head Neck Surg. 2005, 131, 52. [Google Scholar] [CrossRef] [PubMed]
- Tunkel, D.E.; Hotchkiss, K.S.; Carson, K.A.; Sterni, L.M. Efficacy of Powered Intracapsular Tonsillectomy and Adenoidectomy. Laryngoscope 2008, 118, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Liu, H.; Zhang, G.H.; Li, P.; Yang, Q.T.; Liu, X.; Li, Y. Outcome of Adenotonsillectomy for Obstructive Sleep Apnea Syndrome in Children. Ann. Otol. Rhinol. Laryngol. 2010, 119, 506–513. [Google Scholar] [CrossRef]
- Arima, S.; Koike, S.; Fujinaga, M.; Mihara, T.; Sato, S.; Suzuki, M.; Murakami, S.; Nakayama, M. Normalization of breathing with adenotonsillectomy in Japanese pediatric OSA. Auris Nasus Larynx 2019, 46, 758–763. [Google Scholar] [CrossRef]
- Koren, D.; Gozal, D.; Bhattacharjee, R.; Philby, M.F.; Kheirandish-Gozal, L. Impact of Adenotonsillectomy on Insulin Resistance and Lipoprotein Profile in Nonobese and Obese Children. Chest 2016, 149, 999–1010. [Google Scholar] [CrossRef]
- Tagaya, M.; Nakata, S.; Yasuma, F.; Mitchell, R.B.; Sasaki, F.; Miyazaki, S.; Morinaga, M.; Otake, H.; Teranishi, M.; Nakashima, T. Children with severe or moderate obstructive sleep apnoea syndrome show a high incidence of persistence after adenotonsillectomy. Acta Oto-Laryngol. 2012, 132, 1208–1214. [Google Scholar] [CrossRef]
- Nath, A.; Emani, J.; Suskind, D.L.; Baroody, F.M. Predictors of Persistent Sleep Apnea After Surgery in Children Younger Than 3 Years. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 1002. [Google Scholar] [CrossRef]
- Bhushan, B.; Sheldon, S.; Wang, E.; Schroeder, J.W. Clinical indicators that predict the presence of moderate to severe obstructive sleep apnea after adenotonsillectomy in children. Am. J. Otolaryngol. 2014, 35, 487–495. [Google Scholar] [CrossRef]
- Mitchell, R.B. Adenotonsillectomy for Obstructive sleep apnea in children: Outcome Evaluated by pre- and postoperative polysomnography. Laryngoscope 2007, 117, 1844–1854. [Google Scholar] [CrossRef]
- Al-Zaabi, K.; Al-Adawi, S.; Jaju, S.; Jeyaseelan, L.; Al-Sibani, N.; Al-Alawi, M.; Al-Abri, M.; Al-Abri, R. Effects of an Ade-notonsillectomy on the Cognitive and Behavioural Function of Children Who Snore: A naturalistic observational study. Sultan Qaboos Univ. Med. J. 2019, 18, 455. [Google Scholar] [CrossRef]
- Li, H.Y.; Huang, Y.S.; Chen, N.H.; Fang, T.J.; Lee, L.A. Impact of Adenotonsillectomy on Behavior in Children with Sleep-Disordered Breathing. Laryngoscope 2006, 116, 1142–1147. [Google Scholar] [CrossRef]
- Nieminen, P.; LöPpöNen, T.; Tolonen, U.; Lanning, P.; Knip, M.; LöPpöNen, H. Growth and Biochemical Markers of Growth in Children With Snoring and Obstructive Sleep Apnea. Pediatrics 2002, 109, e55. [Google Scholar] [CrossRef]
- Liu, J.F.; Tsai, C.M.; Su, M.C.; Lin, M.C.; Lin, H.C.; Lee, W.J.; Hsieh, K.S.; Niu, C.K.; Yu, H.R. Application of desaturation index in post-surgery follow-up in children with obstructive sleep apnea syndrome. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 375–382. [Google Scholar] [CrossRef]
- Lee, C.H.; Kang, K.T.; Chiu, S.N.; Chang, I.S.; Weng, W.C.; Lee, P.L.; Hsu, W.C. Association of Adenotonsillectomy With Blood Pressure Among Hypertensive and Nonhypertensive Children With Obstructive Sleep Apnea. JAMA Otolaryngol. Head Neck Surg. 2018, 144, 300. [Google Scholar] [CrossRef]
- Hsu, W.C.; Kang, K.T.; Chiu, S.N.; Weng, W.C.; Lee, P.L.; Lin, C.Y. 24-Hour Ambulatory Blood Pressure after Adenotonsillec-tomy in Childhood Sleep Apnea. J. Pediatr. 2018, 199, 112–117.e6. [Google Scholar] [CrossRef]
- De Magalhães Bertoz, A.P.; Souki, B.Q.; Lione, R.; Webber, S.A.T.; Bigliazzi, R.; Oliveira, P.M.; Moro, A.; Cozza, P. Three-dimensional airway changes after adenotonsillectomy in children with obstructive apnea: Do expectations meet reality? Am. J. Orthod. Dentofac. Orthop. 2019, 155, 791–800. [Google Scholar] [CrossRef]
- Song, I.S.; Hong, S.; Joo, J.W.; Han, M.S.; Hwang, S.J.; Seo, M.Y.; Lee, S.H. Long-term results of sleep-related quality-of-life and behavioral problems after adenotonsillectomy. Laryngoscope 2019, 130, 546–550. [Google Scholar] [CrossRef]
- Kuo, Y.; Kang, K.; Chiu, S.; Weng, W.; Lee, P.; Hsu, W. Blood Pressure after Surgery among Obese and Nonobese Children with Obstructive Sleep Apnea. Otolaryngology 2015, 152, 931–940. [Google Scholar] [CrossRef]
- Suri, J.C.; Sen, M.K.; Venkatachalam, V.P.; Bhool, S.; Sharma, R.; Elias, M.; Adhikari, T. Outcome of adenotonsillectomy for children with sleep apnea. Sleep Med. 2015, 16, 1181–1186. [Google Scholar] [CrossRef]
- Lee, S.Y.; Guilleminault, C.; Chiu, H.Y.; Sullivan, S.S. Mouth breathing, “nasal disuse,” and pediatric sleep-disordered breathing. Sleep Breath. 2015, 19, 1257–1264. [Google Scholar] [CrossRef]
- Bhattacharjee, R.; Kheirandish-Gozal, L.; Kaditis, A.G.; Verhulst, S.L.; Gozal, D. C-reactive Protein as a Potential Biomarker of Residual Obstructive Sleep Apnea Following Adenotonsillectomy in Children. Sleep 2016, 39, 283–291. [Google Scholar] [CrossRef]
- Kaditis, A.G.; Chaidas, K.; Alexopoulos, E.I.; Varlami, V.; Malakasioti, G.; Gourgoulianis, K. Effects of adenotonsillectomy on R–R interval and brain natriuretic peptide levels in children with sleep apnea: A preliminary report. Sleep Med. 2011, 12, 646–651. [Google Scholar] [CrossRef]
- Kobayashi, R.; Miyazaki, S.; Karaki, M.; Hoshikawa, H.; Nakata, S.; Hara, H.; Kodama, S.; Kikuchi, A.; Kitamura, T.; Mori, N. Evaluation of adenotonsillectomy and tonsillectomy for pediatric obstructive sleep apnea by rhinomanometry and the OSA-18 questionnaire. Acta Oto-Laryngol. 2014, 134, 818–823. [Google Scholar] [CrossRef]
- Villa, M.P.; Castaldo, R.; Miano, S.; Paolino, M.C.; Vitelli, O.; Tabarrini, A.; Mazzotta, A.R.; Cecili, M.; Barreto, M. Adenoton-sillectomy and orthodontic therapy in pediatric obstructive sleep apnea. Sleep Breath. 2013, 18, 533–539. [Google Scholar] [CrossRef]
- Billings, K.R.; Somani, S.N.; Lavin, J.; Bhushan, B. Polysomnography variables associated with postoperative respiratory issues in children <3 Years of age undergoing adenotonsillectomy for obstructive sleep apnea. Int. J. Pediatr. Otorhinolaryngol. 2020, 137, 110215. [Google Scholar] [CrossRef]
- Hamada, M.; Iida, M.; Nota, J.; Matsumoto, N.; Sawada, S.; Mukushita, N.; Washizu, Y.; Shimasaki, M.; Doi, T. Safety and efficacy of adenotonsillectomy for obstructive sleep apnea in infants, toddlers and preschool children. Auris Nasus Larynx 2015, 42, 208–212. [Google Scholar] [CrossRef]
- Chung, S.; Hodges, E.K.; Ruzicka, D.L.; Hoban, T.F.; Garetz, S.L.; Guire, K.E.; Felt, B.T.; Dillon, J.E.; Chervin, R.D.; Giordani, B. Improved behavior after adenotonsillectomy in children with higher and lower IQ. Int. J. Pediatr. Otorhinolaryngol. 2015, 80, 21–25. [Google Scholar] [CrossRef][Green Version]
- Wei, J.L.; Mayo, M.S.; Smith, H.J.; Reese, M.; Weatherly, R.A. Improved Behavior and sleep after Adenotonsillectomy in children with Sleep-Disordered Breathing. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 974. [Google Scholar] [CrossRef] [PubMed]
- Locci, C.; Cenere, C.; Sotgiu, G.; Puci, M.V.; Saderi, L.; Rizzo, D.; Bussu, F.; Antonucci, R. Adenotonsillectomy in Children with Obstructive Sleep Apnea Syndrome: Clinical and Functional Outcomes. J. Clin. Med. 2023, 12, 5826. [Google Scholar] [CrossRef]
- Arrarte, J.; Neto, J.F.L.; Fischer, G.B. The effect of adenotonsillectomy on oxygen saturation in children with sleep breathing disorders. Int. J. Pediatr. Otorhinolaryngol. 2007, 71, 973–978. [Google Scholar] [CrossRef]
- Spivey, J.F.; Uong, E.C.; Strunk, R.; Boslaugh, S.E.; DeBaun, M.R. Low daytime pulse oximetry reading is associated with nocturnal desaturation and obstructive sleep apnea in children with sickle cell anemia. Pediatr. Blood Cancer 2006, 50, 359–362. [Google Scholar] [CrossRef]
- Wolraich, M.L.; Hagan, J.F.; Allan, C.; Chan, E.; Davison, D.; Earls, M.; Evans, S.W.; Flinn, S.K.; Froehlich, T.; Frost, J.; et al. Clinical Practice Guideline for the Diagnosis, evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics 2019, 144, e20192528. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.C.; Wang, X.Y.; Xu, W.W.; Li, J.D.; Yu, Q.H. The effects of tonsillectomy by low-temperature plasma on the growth development and psychological behavior in children with obstructive sleep apnea hypopnea syndrome. Medicine 2018, 97, e13205. [Google Scholar] [CrossRef]
- Sanders, K.; Osterbauer, B.; Forman, N.; Yim, H.J.; Hochstim, C.; Bhardwaj, V.; Bansal, M.; Karnwal, A. Perioperative respiratory adverse events in children undergoing triple endoscopy. Pediatr. Anesth. 2021, 31, 1290–1297. [Google Scholar] [CrossRef]
- Mesolella, M.; Allosso, S.; Coronella, V.; Massimilla, E.A.; Mansi, N.; Motta, G.; Salerno, G.; Motta, G. Extracapsular Tonsillectomy versus Intracapsular Tonsillotomy in Paediatric Patients with OSAS. J. Pers. Med. 2023, 13, 806. [Google Scholar] [CrossRef] [PubMed]
- Marcus, C.L.; Moore, R.H.; Rosen, C.L.; Giordani, B.; Garetz, S.L.; Taylor, H.G.; Mitchell, R.B.; Amin, R.; Katz, E.S.; Arens, R.; et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N. Engl. J. Med. 2013, 368, 2366–2376. [Google Scholar] [CrossRef]
- Martins, R.O.; Castello-Branco, N.; De Barros, J.L.; Weber, S.A.T. Risk factors for respiratory complications after adenotonsillectomy in children with obstructive sleep apnea. J. Bras. Pneumol. 2015, 41, 238–245. [Google Scholar] [CrossRef]
- Paradise, J.L.; Bluestone, C.D.; Colborn, D.K.; Bernard, B.S.; Rockette, H.E.; Kurs-Lasky, M. Tonsillectomy and adenotonsillectomy for recurrent throat infection in moderately affected children. Pediatrics 2002, 110, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Wang, S.; Yang, X.; Liu, F.; Xiu, L. Effect of adenotonsillectomy on the growth, development, and comprehensive cognitive abilities of children with obstructive sleep apnea: A prospective single-arm study. BMC Pediatr. 2022, 22, 41. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, F.; Oka, Y.; Komori, K.; Tokui, Y.; Matsumoto, T.; Kawabe, K.; Ueno, S.I. Effects of adenotonsillectomy on neurocognitive function in pediatric obstructive sleep apnea syndrome. Case Rep. Psychiatry 2014, 2014, 520215. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, E.; Graf, J.; Lundeborg-Hammarstrom, I.; Hultcrantz, E. Tonsillotomy versus tonsillectomy on young children: 2 year post surgery follow-up. J. Otolaryngol.-Head Neck Surg. 2014, 43, 26. [Google Scholar] [CrossRef]
- Friedman, B.C.; Hendeles-Amitai, A.; Kozminsky, E.; Leiberman, A.; Friger, M.; Tarasiuk, A.; Tal, A. Adenotonsillectomy Improves Neurocognitive Function in Children with Obstructive Sleep Apnea Syndrome. Sleep 2003, 26, 999–1005. [Google Scholar] [CrossRef]
- Guilleminault, C.; Huang, Y.; Glamann, C.; Li, K.; Chan, A. Adenotonsillectomy and obstructive sleep apnea in children: A prospective survey. Otolaryngology 2007, 136, 169–175. [Google Scholar] [CrossRef]
- Erickson, B.K.; Larson, D.R.; St Sauver, J.L.; Meverden, R.A.; Orvidas, L.J. Changes in incidence and indications of tonsillectomy and adenotonsillectomy, 1970–2005. Otolaryngology 2009, 140, 894–901. [Google Scholar] [CrossRef]
- Nixon, G.M.; Kermack, A.S.; Davis, G.M.; Manoukian, J.J.; Brown, K.A.; Brouillette, R.T. Planning adenotonsillectomy in children with Obstructive sleep apnea: The role of Overnight oximetry. Pediatrics 2004, 113, e19–e25. [Google Scholar] [CrossRef]
- Brown, K.A.; Morin, I.; Hickey, C.; Manoukian, J.J.; Nixon, G.M.; Brouillette, R.T. Urgent adenotonsillectomy. Anesthesiology 2003, 99, 586–595. [Google Scholar] [CrossRef]
- Chervin, R.D.; Ruzicka, D.L.; Giordani, B.J.; Weatherly, R.A.; Dillon, J.E.; Hodges, E.K.; Marcus, C.L.; Guire, K.E. Sleep-Disordered Breathing, Behavior, and Cognition in Children Before and after adenotonsillectomy. Pediatrics 2006, 117, e769–e778. [Google Scholar] [CrossRef]
- Stewart, M.G.; Glaze, D.G.; Friedman, E.M.; Smith, E.O.; Bautista, M. Quality of Life and Sleep Study Findings after adenotonsillectomy in children with obstructive sleep Apnea. Arch. Otolaryngol. Head Neck Surg. 2005, 131, 308. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).