A Series of 40 Congenital Lung Malformation Cases and the Informative Value of CPAM Lesion Ratios
Abstract
1. Introduction
2. Materials and Methods
3. Results
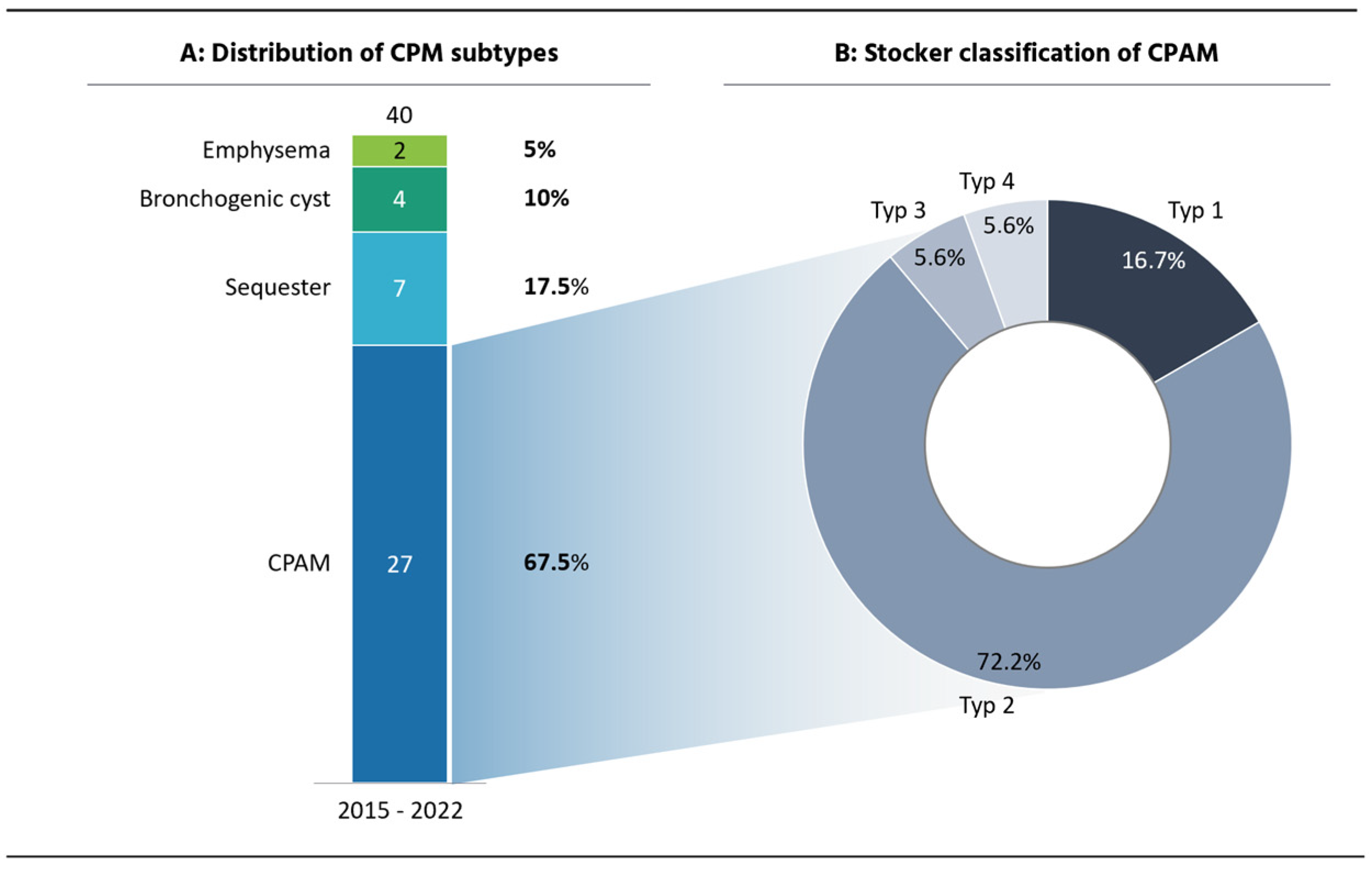
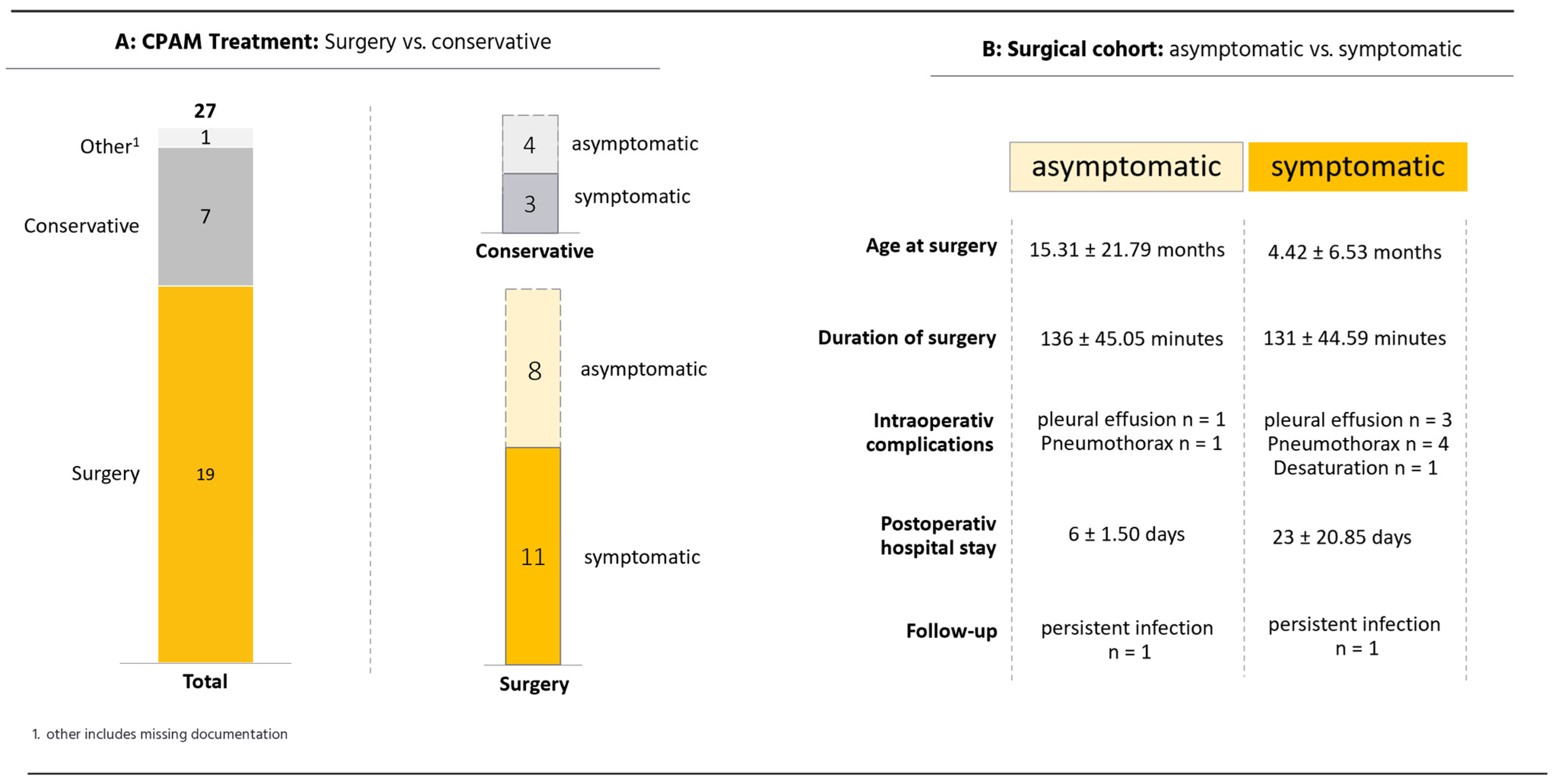
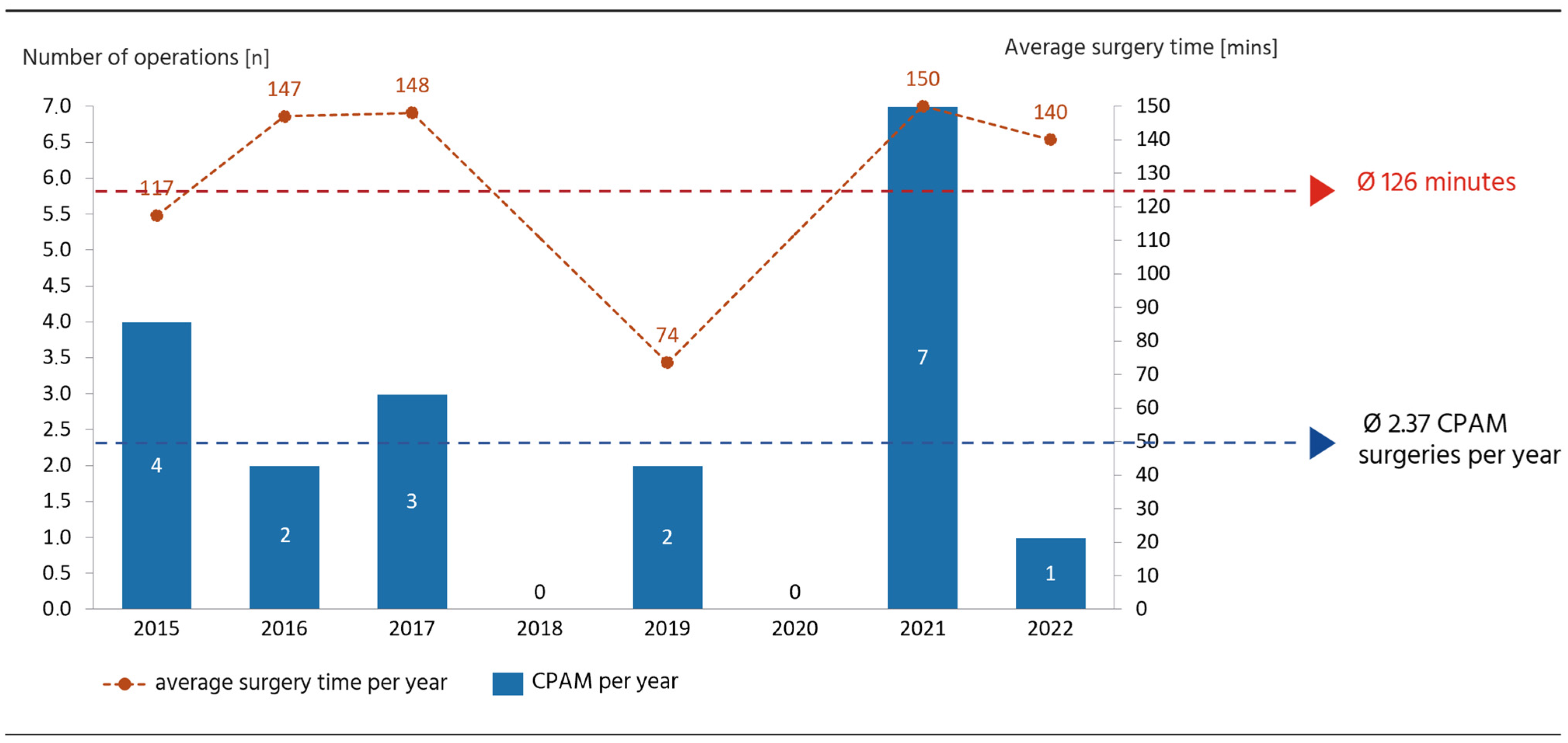
3.1. CPAM Cohort
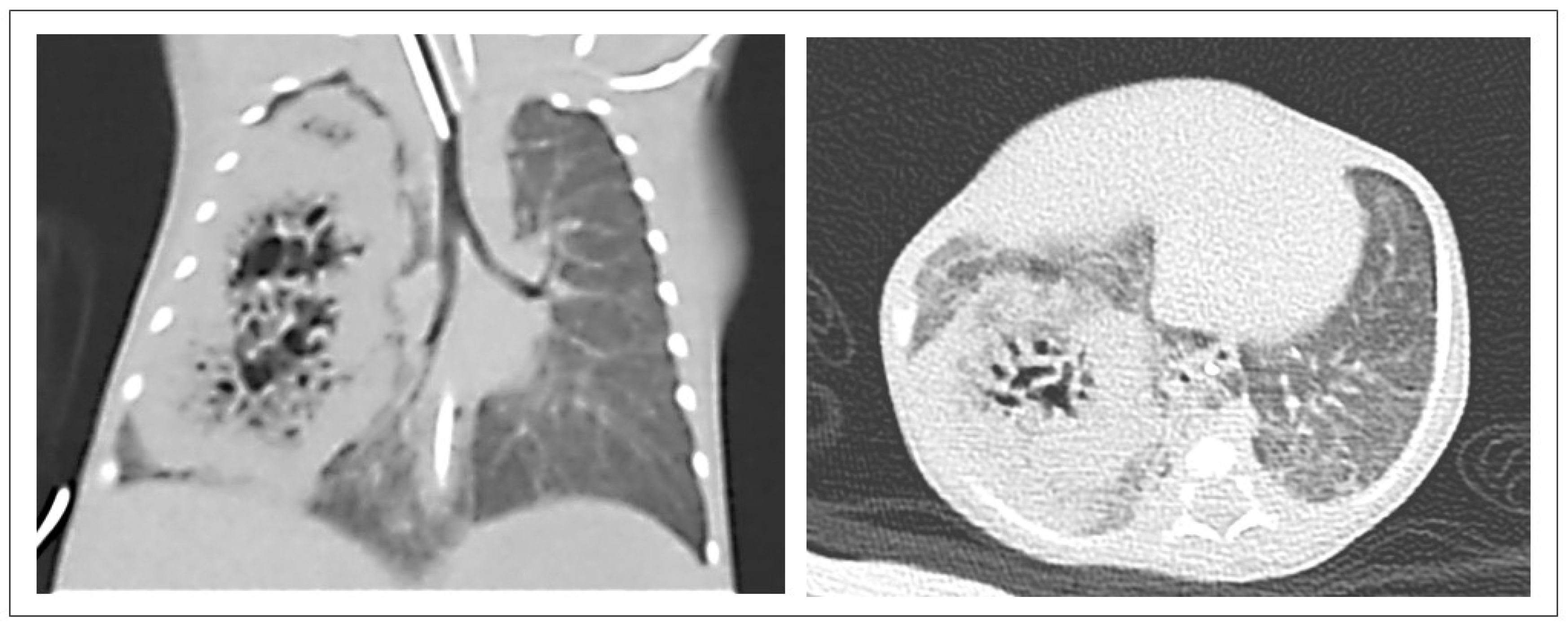
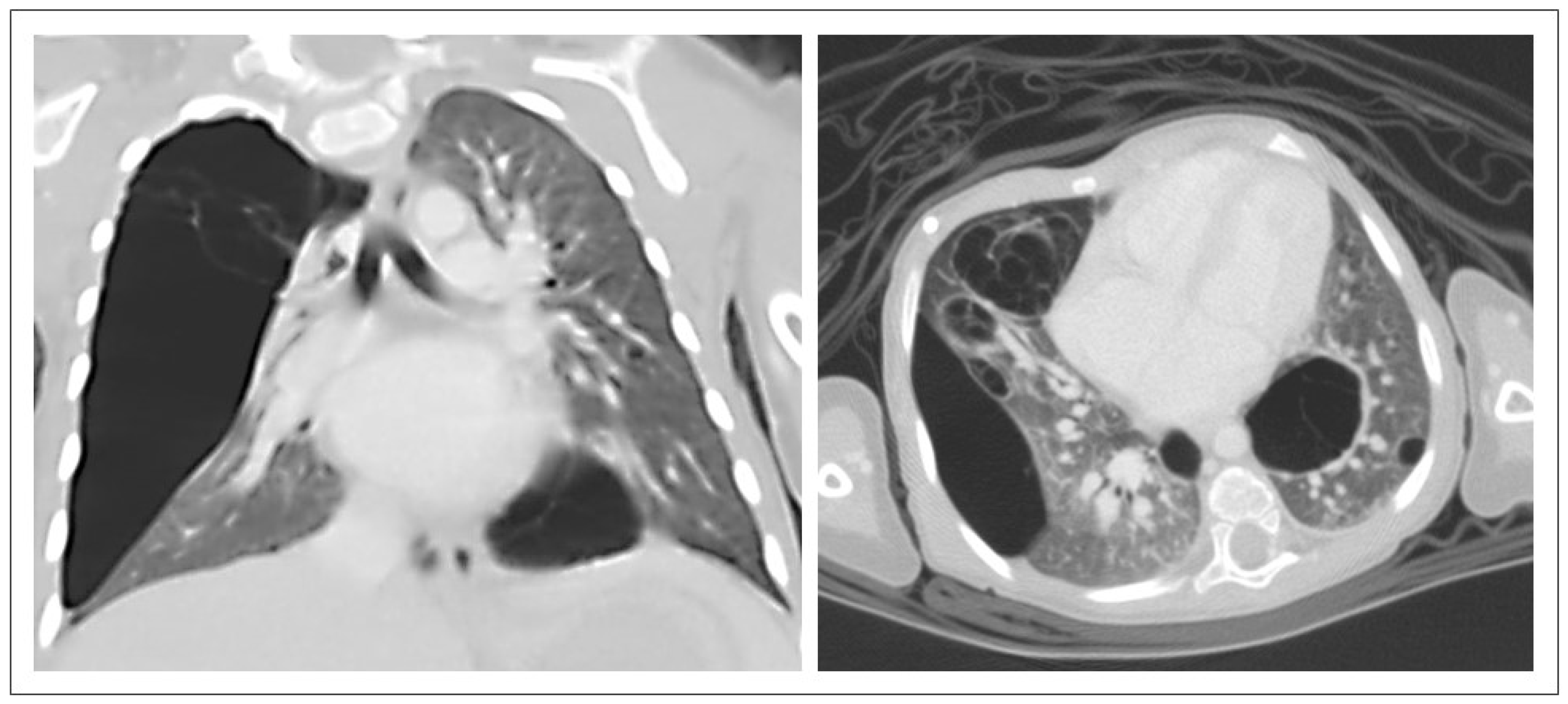
3.2. CPAM CT Findings
4. Discussion
4.1. Surgical Management
4.2. CPAM CT Findings
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kantor, N.; Wayne, C.; Nasr, A. Symptom development in originally asymptomatic CPAM diagnosed prenatally: A systematic review. Pediatr. Surg. Int. 2018, 34, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, A.; Pederiva, F. Association between Congenital Lung Malformations and Lung Tumors in Children and Adults: A Systematic Review. J. Thorac. Oncol. 2016, 11, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Liang, J.; Li, L.; Liu, W.; Tang, J.; Yin, X.; Yin, G. Surgical Treatment for Asymptomatic Congenital Pulmonary Airway Malformations in Children: Waiting or Not? Eur. J. Pediatr. Surg. 2021, 31, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.K.Y.; Flake, A.W.; Tibboel, D.; Rottier, R.J.; Tam, P.K.H. Congenital pulmonary airway malformation: Advances and controversies. Lancet Child. Adolesc. Health 2018, 2, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zeng, Q.; Chen, C.; Yu, J.; Zhang, X. Distribution, diagnosis, and treatment of pulmonary sequestration: Report of 208 cases. J. Pediatr. Surg. 2019, 54, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.R.; Abadilla, N.; Greenberg, D.R.; Sylvester, K.G.; Hintz, S.R.; Barth, R.A.; Bruzoni, M. Predicting Pathology From Imaging in Children Undergoing Resection of Congenital Lung Lesions. J. Surg. Res. 2019, 236, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Laberge, J.-M.; Bratu, I.; Flageole, H. The management of asymptomatic congenital lung malformations. Paediatr. Respir. Rev. 2004, 5 (Suppl. A), S305–S312. [Google Scholar] [CrossRef]
- Stanton, M.; Njere, I.; Ade-Ajayi, N.; Patel, S.; Davenport, M. Systematic review and meta-analysis of the postnatal management of congenital cystic lung lesions. J. Pediatr. Surg. 2009, 44, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
| Case No. | Lesion-to-Ipsilateral-Lung Ratio | Lesion-to-Both- Lungs Ratio | Lesion [mm3] | Age at CT | Symptoms | Therapy | Surgery Time [mins] | Length of Stay [days] |
|---|---|---|---|---|---|---|---|---|
| 1 | Not measurable * | Not measurable * | Lesion too large * | 1 month | Postnatal respiratory adaptation disorder, mediastinal shift | Surgery | 98 | 42 |
| 2 | Not measurable * | Not measurable * | Lesion too large * | 20 days | Respiratory insufficiency with need for intubation | Surgery | 129 | 70 |
| 3 | 1.74 | 0.64 | 68 | 5 days | Mediastinal shift, tachypnoea | Surgery | 108 | 17 |
| 4 | 0.34 | 0.34 | 145 | 10 months | Perinatal pneumothorax, repeated pulmonary infections | Observance | - | - |
| 5 | 0.29 | 0.20 | 56 | 2 months | Postnatal respiratory adaptation disorder with sufficient development in further course | Observance | - | - |
| 6 | 0.26 | 0.20 | 18.9 | 6 months | - | Surgery | 89 | 5 |
| 7 | 0.18 | 0.11 | 39 | 7 months | - | Surgery | 119 | 6 |
| 8 | 0.18 | 0.11 | 22.1 | 8 months | - | Surgery | 177 | 8 |
| 9 | 0.14 | 0.08 | 101.9 | 6 years | One singular respiratory infection with need for antibiotics | Observance | - | - |
| 10 | 0.12 | 0.06 | 15.8 | 13 months | Recurrent respiratory infections | Surgery (thoracoscopy) | 49 | 7 |
| 11 | 0.12 | 0.07 | 10 | 6 months | - | Observance | - | - |
| 12 | 0.11 | 0.05 | 9.4 | 7 months | - | Surgery | 172 | 9 |
| 13 | 0.09 | 0.05 | 20 | 13 months | - | Surgery | 210 | 6 |
| 14 | 0.07 | 0.04 | 5.5 | 8 months | - | Surgery (thoracoscopy with open conversion) | 85 | 7 |
| 15 | 0.03 | 0.01 | 2.4 | 6 months | - | Observance | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, M.; Harms, P.; Peldschus, K.; Junge, C.-M.; Tomuschat, C.; Reinshagen, K. A Series of 40 Congenital Lung Malformation Cases and the Informative Value of CPAM Lesion Ratios. Pediatr. Rep. 2025, 17, 5. https://doi.org/10.3390/pediatric17010005
Le M, Harms P, Peldschus K, Junge C-M, Tomuschat C, Reinshagen K. A Series of 40 Congenital Lung Malformation Cases and the Informative Value of CPAM Lesion Ratios. Pediatric Reports. 2025; 17(1):5. https://doi.org/10.3390/pediatric17010005
Chicago/Turabian StyleLe, Melanie, Phillip Harms, Kersten Peldschus, Carl-Martin Junge, Christian Tomuschat, and Konrad Reinshagen. 2025. "A Series of 40 Congenital Lung Malformation Cases and the Informative Value of CPAM Lesion Ratios" Pediatric Reports 17, no. 1: 5. https://doi.org/10.3390/pediatric17010005
APA StyleLe, M., Harms, P., Peldschus, K., Junge, C.-M., Tomuschat, C., & Reinshagen, K. (2025). A Series of 40 Congenital Lung Malformation Cases and the Informative Value of CPAM Lesion Ratios. Pediatric Reports, 17(1), 5. https://doi.org/10.3390/pediatric17010005






