The Prevalence of Invasive Bacterial Infection in Febrile Infants Presenting to Hospital Following Meningococcal B Immunisation: A Case Series
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Outcome Measures
- Urinary tract infection (UTI);
- Associated clinical features;
- Hospital admission;
- Length of stay;
- Investigations and procedures performed;
- Median CRP value;
- Mean white cell count (WCC);
- Mean absolute neutrophil count;
- Mean lymphocyte count;
- Parenteral antibiotic use.
2.4. Reference Standards and Definitions
- Pure growth >100,000 CFU/mL of a single organism from a single clean catch urine sample.
- Pure growth >10,000 CFU/mL of a single organism from either a trans-urethral bladder catheter or suprapubic aspiration urine sample.
- Pure growth, from two urine samples, >100,000 CFU/mL of the same organism from urine pad/bag samples and the presence of pyuria (>10 white blood cells per high-power field) on laboratory microscopy.
2.5. Study Procedures
2.6. Data Management
2.7. Research Ethics
2.8. Statistical Analysis
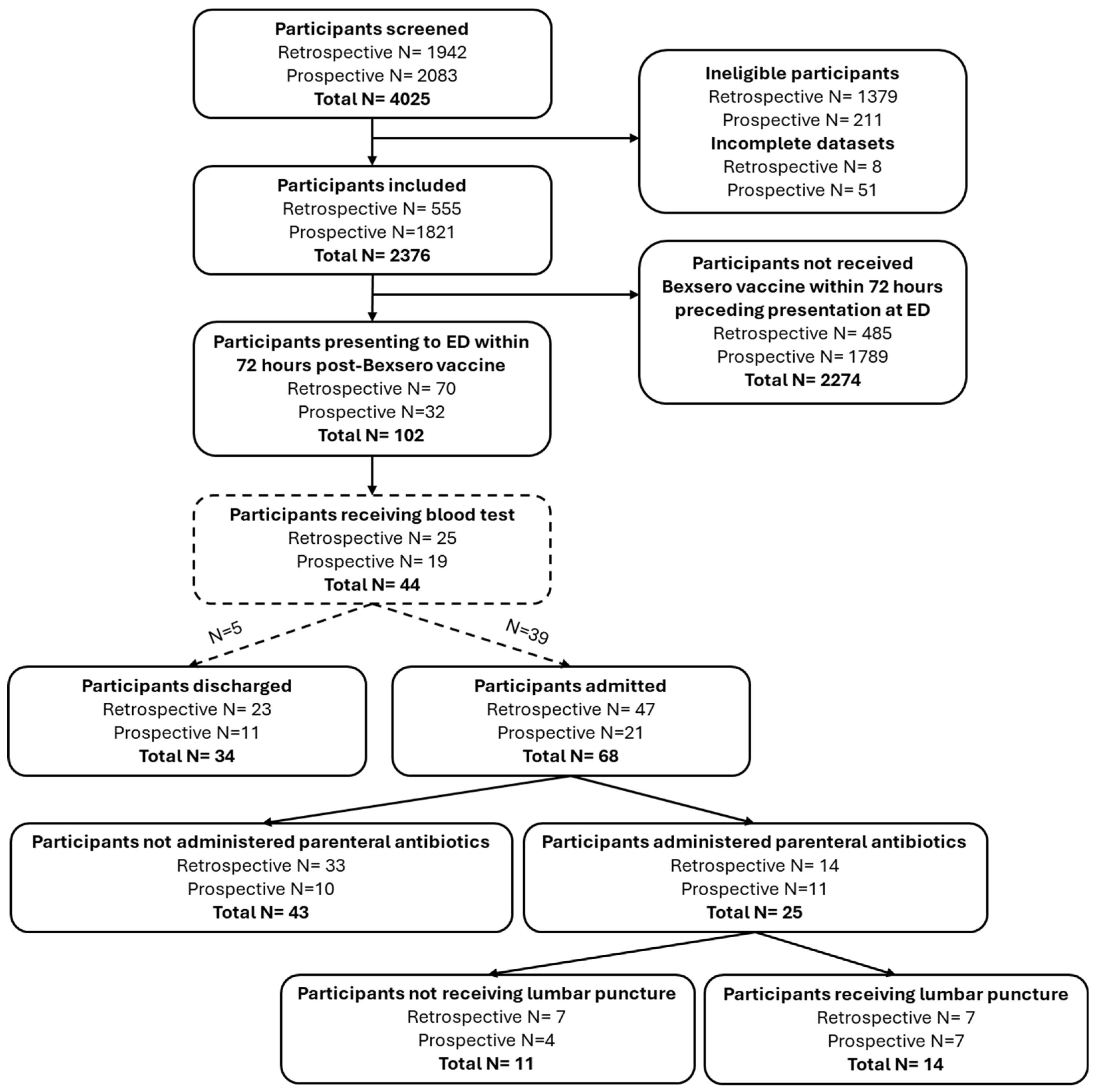
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frosi, G.; Biolchi, A.; Lo Sapio, M.; Rigat, F.; Gilchrist, S.; Lucidarme, J.; Findlow, J.; Borrow, R.; Pizza, M.; Giuliani, M.M.; et al. Bactericidal antibody against a representative epidemiological meningococcal serogroup B panel confirms that MATS underestimates 4CMenB vaccine strain coverage. Vaccine 2013, 31, 4968–4974. [Google Scholar] [CrossRef] [PubMed]
- Nainani, V.; Galal, U.; Buttery, J.; Snape, M.D. An increase in accident and emergency presentations for adverse events following immunisation after introduction of the group B meningococcal vaccine: An observational study. Arch. Dis. Child. 2017, 102, 958–962. [Google Scholar] [CrossRef] [PubMed]
- Gossger, N.; Snape, M.D.; Yu, L.M.; Finn, A.; Bona, G.; Esposito, S.; Principi, N.; Diez-Domingo, J.; Sokal, E.; Becker, B.; et al. Immunogenicity and tolerability of recombinant serogroup B meningococcal vaccine administered with or without routine infant vaccinations according to different immunization schedules: A randomized controlled trial. JAMA 2012, 307, 573–582. [Google Scholar] [CrossRef] [PubMed]
- National Health Service. Using Paracetamol to Prevent and Treat Fever After MenB Vaccination. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1110220/UKHSA-paracetamol-MenB-2022.pdf (accessed on 4 November 2024).
- Waterfield, T.; Lyttle, M.D.; Munday, C.; Foster, S.; McNulty, M.; Platt, R.; Barrett, M.; Rogers, E.; Durnin, S.; Jameel, N.; et al. Validating clinical practice guidelines for the management of febrile infants presenting to the emergency department in the UK and Ireland. Arch. Dis. Child. 2022, 107, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Gomez, B.; Mintegi, S.; Bressan, S.; Da Dalt, L.; Gervaix, A.; Lacroix, L.; European Group for Validation of the Step-by-Step Approach. Validation of the “Step-by-Step” Approach in the Management of Young Febrile Infants. Pediatrics 2016, 138, e20154381. [Google Scholar] [CrossRef]
- Pantell, R.H.; Roberts, K.B.; Adams, W.G.; Dreyer, B.P.; Kuppermann, N.; O’Leary, S.T.; Okechukwu, K.; Woods, C.R., Jr.; Subcommittee On Febrile, Infants. Evaluation and Management of Well-Appearing Febrile Infants 8 to 60 Days Old. Pediatrics 2021, 148, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Fever in Under 5s: Assessment and Initial Management. NICE Guideline [NG143]. Available online: https://www-nice-org-uk.qub.idm.oclc.org/guidance/ng143 (accessed on 8 November 2024).
- Sepsis: Recognition, Diagnosis and Early Management. NICE Guideline [NG51]. Available online: https://www-nice-org-uk.qub.idm.oclc.org/guidance/ng51 (accessed on 8 November 2024).
- Kuppermann, N.; Dayan, P.S.; Levine, D.A.; Vitale, M.; Tzimenatos, L.; Tunik, M.G.; Saunders, M.; Ruddy, R.M.; Roosevelt, G.; Rogers, A.J.; et al. A Clinical Prediction Rule to Identify Febrile Infants 60 Days and Younger at Low Risk for Serious Bacterial Infections. JAMA Pediatr. 2019, 173, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Wolff, M.; Bachur, R. Serious bacterial infection in recently immunized young febrile infants. Acad. Emerg. Med. 2009, 16, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Raba, A.A.; Krebit, I. Definite bacterial infection in recently vaccinated febrile infants. J. Paediatr. Child Health 2020, 56, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Casey, K.; Reilly, E.R.; Biggs, K.; Caskey, M.; Auten, J.D.; Sullivan, K.; Morrison, T.; Long, A.; Rudinsky, S.L. Serious bacterial infection risk in recently immunized febrile infants in the emergency department. Am. J. Emerg. Med. 2024, 80, 138–142. [Google Scholar] [CrossRef]
- Umana, E.; Mills, C.; Norman-Bruce, H.; Wilson, K.; Mitchell, H.; McFetridge, L.; Woolfall, K.; Lynn, F.A.; McKeeman, G.; Foster, S.; et al. Applying clinical decision aids for the assessment and management of febrile infants presenting to emergency care in the UK and Ireland: Febrile Infant Diagnostic Assessment and Outcome (FIDO) Study protocol. BMJ Open 2023, 13, e075823. [Google Scholar] [CrossRef] [PubMed]
- Umana, E.; Mills, C.; Norman-Bruce, H.; Mitchell, H.; McFetridge, L.; Lynn, F.A.; McKeeman, G.; Foster, S.; Barrett, M.J.; Roland, D.; et al. Performance of clinical decision aids (CDA) for the care of young febrile infants. A multicentre prospective cohort study conducted in the UK and Ireland. EClinicalMedicine 2024, 78, 102961. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- The Health Research Authority. Is My Study Research? Available online: https://www.hra-decisiontools.org.uk/research/ (accessed on 15 November 2024).
- Kapur, S.; Bourke, T.; Maney, J.A.; Moriarty, P. Emergency department attendance following 4-component meningococcal B vaccination in infants. Arch. Dis. Child. 2017, 102, 899–902. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.; Bland, R.M.; Hendry, S.J. Fever after meningococcal B immunisation: A case series. J. Paediatr. Child Health 2019, 55, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Leigh, S.; Grant, A.; Murray, N.; Faragher, B.; Desai, H.; Dolan, S.; Cabdi, N.; Murray, J.B.; Rejaei, Y.; Stewart, S.; et al. The cost of diagnostic uncertainty: A prospective economic analysis of febrile children attending an NHS emergency department. BMC Med. 2019, 17, 48. [Google Scholar] [CrossRef] [PubMed]
- Milcent, K.; Faesch, S.; Gras-Le Guen, C.; Dubos, F.; Poulalhon, C.; Badier, I.; Marc, E.; Laguille, C.; de Pontual, L.; Mosca, A.; et al. Use of Procalcitonin Assays to Predict Serious Bacterial Infection in Young Febrile Infants. JAMA Pediatr. 2016, 170, 62–69. [Google Scholar] [CrossRef]
| Guideline | Age Range | Biomarkers | Exclusions |
|---|---|---|---|
| NICE | ≤90 days | CRP, WCC, ANC | None |
| AAP | ≤60 days | PCT, CRP, ANC | Preterm |
| <2 weeks old, perinatal course complicated | |||
| Suspicion of HSV infection | |||
| Focal bacterial infection | |||
| Clinical bronchiolitis | |||
| Immuno-compromised | |||
| Neonatal course complicated | |||
| Congenital/chromosomal abnormalities | |||
| Requires technology/therapeutic intervention to sustain life | |||
| Immunisations within preceding 48 h | |||
| Step-By-Step | ≤90 days | PCT, CRP, ANC | Clear source of fever Preterm |
| Unexplained hyperbilirubinemia | |||
| Hospitalised longer than mother | |||
| Receiving current/previous antimicrobial therapy | |||
| Previous hospitalization | |||
| Chronic/underlying illness |
| Infant Characteristic | Results (n = 102), N (%) |
|---|---|
| Age, days | |
| Median (IQR; range) | 61 (58–69; 44–89) |
| Gender | |
| Male | 67 (65.7) |
| Female | 35 (34.3) |
| Temperature, °C | |
| 39–39.9 | 8 (7.8) |
| 38–38.9 | 76 (74.5) |
| 37–37.9 | 11 (10.8) |
| <37 | 7 (6.9) |
| Associated clinical features * | |
| Change in behaviour (irritability, crying, lethargy) | 18 (17.6) |
| Decreased feeding | 34 (33.3) |
| Decreased urine output | 5 (4.9) |
| Coryzal symptoms | 30 (29.4) |
| Abnormal response to social cues | 4 (3.9) |
| Abnormal cry | 53 (52.0) |
| Swollen joint/limb present | 1 (1.0) |
| Unwell-appearing | 22 (21.6) |
| Investigations | |
| C-reactive protein, mg/L (n = 44) | |
| Median (interquartile range; range) | 20.5 (5.0–38.5; 0.7–83.0) |
| White cell count (×109/L) (n = 42) | |
| Mean ± standard deviation (range) | 13.7 ± 4.5 (3.7–23.7) |
| Neutrophil count (×109/L) (n = 42) | |
| Mean ± standard deviation (range) | 7.3 ± 2.8 (1.9–14.9) |
| Lymphocyte count (×109/L) (n = 42) | |
| Mean ± standard deviation (range) | 4.7 ± 1.9 (1.0–9.2) |
| Blood culture (n = 38) | |
| Positive | 2 (contaminants) |
| Negative | 36 |
| Respiratory viral testing (n = 26) | |
| Positive | 9 |
| Negative | 17 |
| Urine culture (n = 55) | |
| Positive | 3 |
| Negative | 52 |
| Cerebrospinal fluid culture (n = 14) | |
| Positive | 0 |
| Negative | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drummond, H.; Umana, E.; Mills, C.; Waterfield, T. The Prevalence of Invasive Bacterial Infection in Febrile Infants Presenting to Hospital Following Meningococcal B Immunisation: A Case Series. Pediatr. Rep. 2025, 17, 20. https://doi.org/10.3390/pediatric17010020
Drummond H, Umana E, Mills C, Waterfield T. The Prevalence of Invasive Bacterial Infection in Febrile Infants Presenting to Hospital Following Meningococcal B Immunisation: A Case Series. Pediatric Reports. 2025; 17(1):20. https://doi.org/10.3390/pediatric17010020
Chicago/Turabian StyleDrummond, Holly, Etimbuk Umana, Clare Mills, and Thomas Waterfield. 2025. "The Prevalence of Invasive Bacterial Infection in Febrile Infants Presenting to Hospital Following Meningococcal B Immunisation: A Case Series" Pediatric Reports 17, no. 1: 20. https://doi.org/10.3390/pediatric17010020
APA StyleDrummond, H., Umana, E., Mills, C., & Waterfield, T. (2025). The Prevalence of Invasive Bacterial Infection in Febrile Infants Presenting to Hospital Following Meningococcal B Immunisation: A Case Series. Pediatric Reports, 17(1), 20. https://doi.org/10.3390/pediatric17010020






