Abstract
Linear nevus sebaceous syndrome (LNSS) is a rare neurocutaneous disorder. It is characterized by the presence of nevus sebaceous, ocular anomalies, neurological deficits, and convulsion. Renal involvement was not commonly reported. We report a 10-year-old girl with LNSS who had concomitant cystic kidney disease and diffuse aortopathy with bilateral renal artery stenosis, leading to hypertension requiring oral anti-hypertensive medications. The girl presented with chorioretinal coloboma and multiple nevus sebaceous at birth. She had aortic coarctation and received surgical repair at one week of life. She had persistent hypertension during her follow-up. Further investigations were performed to look for causes of hypertension apart from possible re-coarctation. Her magnetic resonance angiogram revealed diffuse aortopathy, which extended from the aortic arch to the abdominal aorta. Branches of the aorta, including the celiac trunk, superior mesenteric arteries, and renal arteries, were also narrowed. Multiple renal cysts were also identified in her right kidney. Interventional angioplasty over the renal arteries was not feasible due to diffuse narrowing of the aorta, especially at the origins of renal arteries. The blood pressure was controlled with oral anti-hypertensive medications. Our case illustrated that pediatricians should be aware of the possible renal involvements in LNSS, which impose a significant impact on the management and long-term prognosis of these patients.
1. Introduction
Linear nevus sebaceous syndrome (LNSS) is a rare congenital neurocutaneous disorder, with an estimated incidence of 1 per 10,000 live births [1]. In addition to the classical triad of nevus sebaceous, seizures, and mental retardation, other organ involvements of the ophthalmological, cardiovascular, and skeletal systems have also been reported [2]. However, renal involvement in this syndrome was less prevalent. We report a 10-year-old girl with LNSS with concomitant diffuse aortopathy, bilateral renal artery stenosis, and cystic kidney disease.
2. Case Presentation
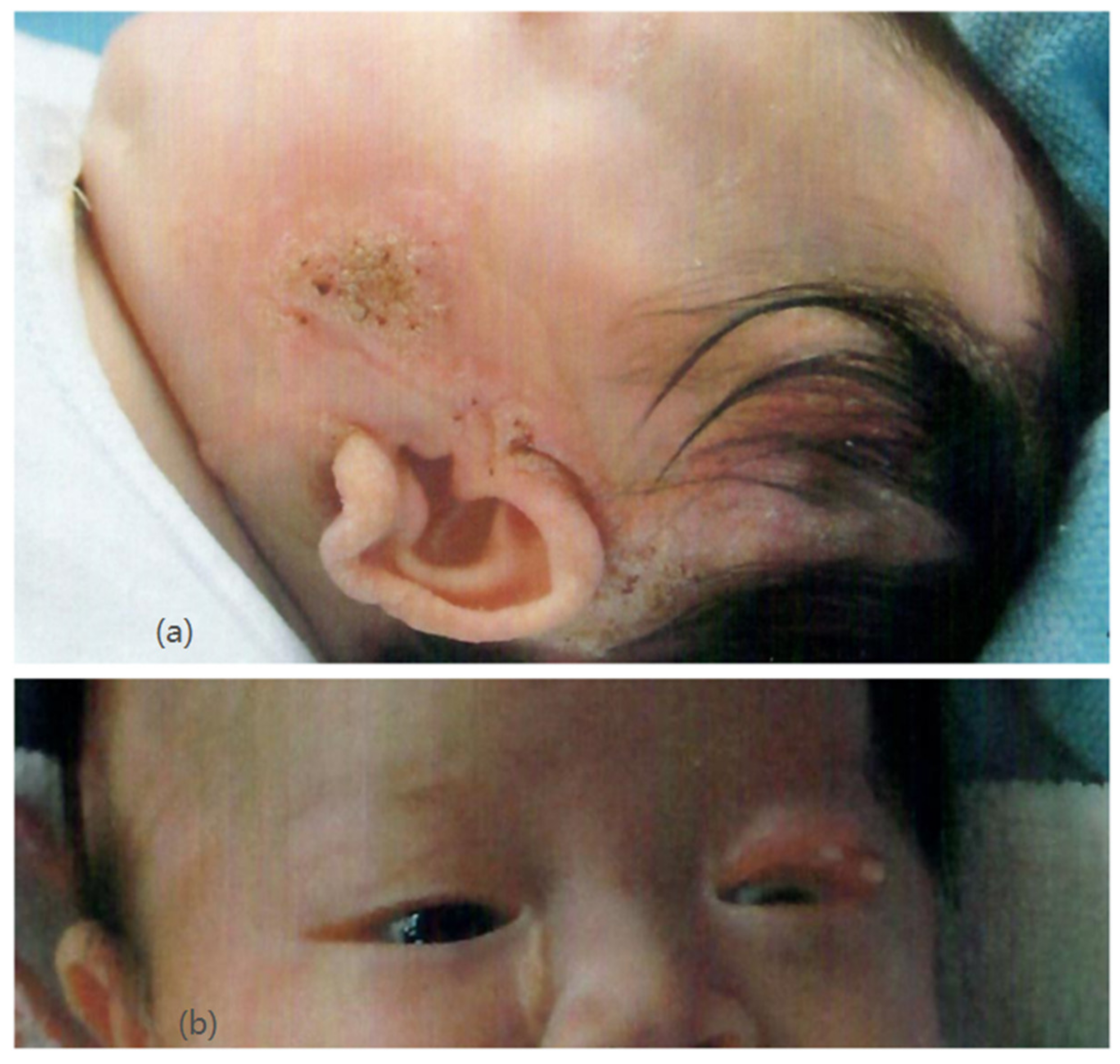
Our patient was an Asian girl who was delivered at 36 weeks of gestation with normal antenatal screening. Her family history was unremarkable. Newborn examination revealed multiple well-defined yellowish plaques over her body, involving her scalp, face, neck, right chest, and upper limb (Figure 1a). Ophthalmological examination showed chorioretinal coloboma in her right eye and a lipodermoid at her left upper eyelid (Figure 1b). She was diagnosed with linear nevus sebaceous syndrome.
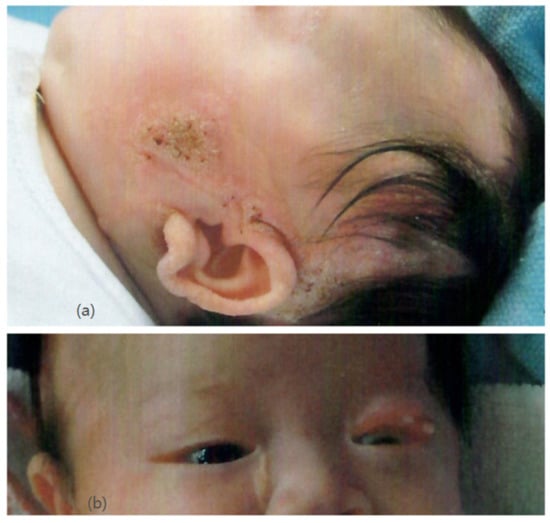
Figure 1.
(a) (upper) Nevus sebaceous over the left face and scalp. (b) (lower) Lipodermoid at the left upper eyelid.
An echocardiogram on day four of life showed aortic coarctation at the juxta-ductal region, with the narrowest point of 2 mm, and a 2.5 mm patent ductus arteriosus (PDA) with bidirectional shunt. Surgical repair of the coarctation and division of the PDA were performed on day seven of life. She developed re-coarctation at the repair site and required balloon aortoplasty six weeks after the surgery. At two months old, she also had multiple episodes of focal seizures involving her left limbs. She was put on oral carbamazepine and has remained seizure-free since six years old. The magnetic resonance imaging (MRI) of her brain and spine showed right frontotemporal lobe pachygyria and a lipomatous lesion at the thoracic spinal cord.
She was hypertensive during her follow-up, which was believed to be attributable to her narrowed aortic isthmus demonstrated in her echocardiograms. Her blood pressure was 146/83 mmHg and 137/86 mmHg over her right and left arm, respectively, at seven years old. In view of her persistent hypertension, further investigations were performed to look into other possibilities of hypertension. Her blood tests showed a normal cortisol level = 205 nmol/L (reference: 64–327 nmol/L), free thyroxine T4 level = 11.1 pmol/L (reference: 10.9–19.0 pmol/L), thyroid stimulating hormone = 2.18 mIU/L (reference: 0.35–4.94 mIU/L), and serum creatinine = 46 umol/L (reference: 34–65 umol/L). The plasma renin activity was elevated to 7.11 ng/mL/h (reference: 0.50–5.90 ng/mL/h), and aldosterone level to 1546 pmol/L (reference: < 250 pmol/L). There was no hematuria or proteinuria. The urine catecholamine levels were normal.
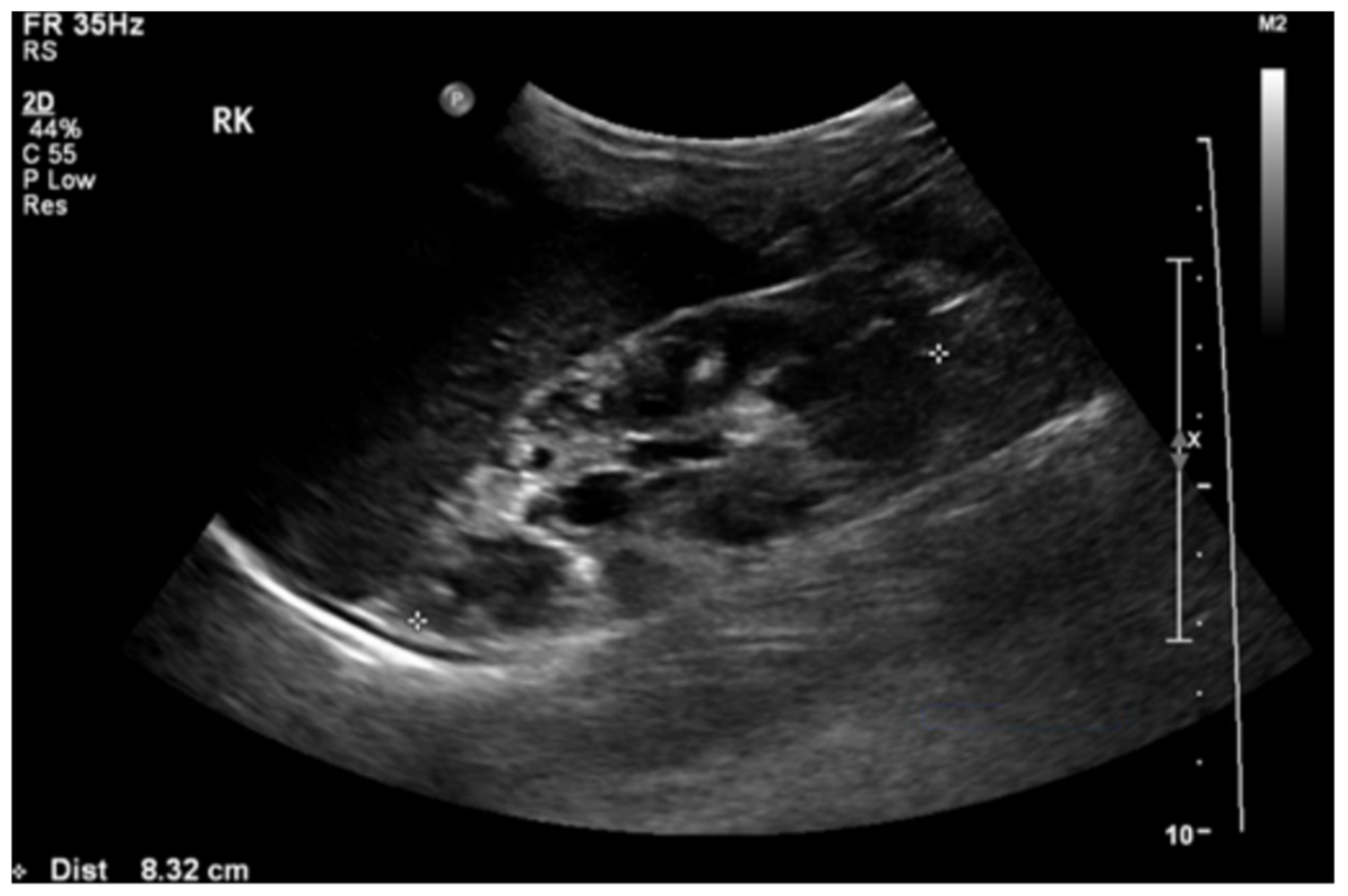
A doppler ultrasound revealed focal stenosis at the proximal and mid-portion of the left renal artery, with the raised peak systolic velocity reaching 300 cm/s. There were multiple cysts with no intra-cystic solid component in her right kidney (Figure 2). There were no cysts in the left kidney or liver. Tc99m-MAG3 (99m technetium mercaptoacetyltriglycine) scan showed impaired right renal function with a left-to-right ratio of 68%:32%.
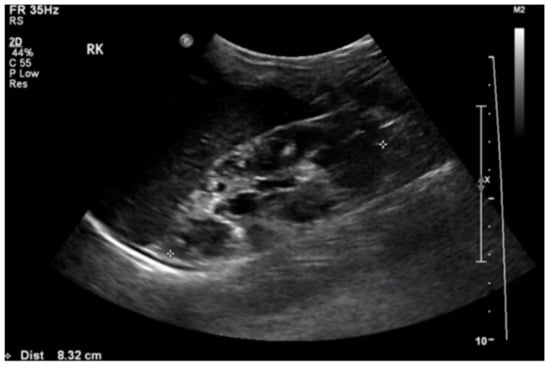
Figure 2.
Renal ultrasound showed loss of corticomedullary differentiation of the right kidney with multiple cysts of variable sizes.
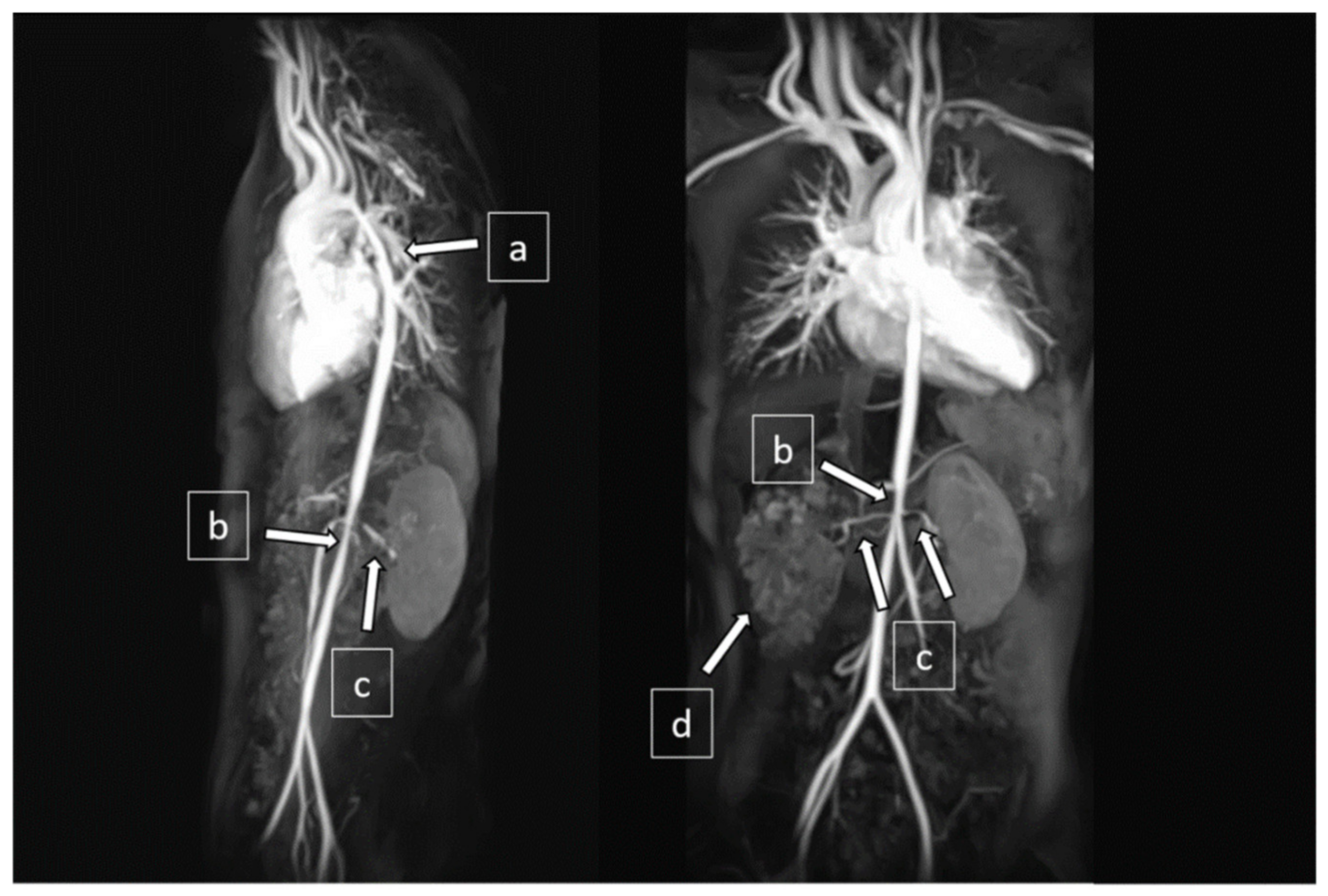
Magnetic resonance angiogram revealed diffuse aortopathy involving the aortic arch (5 mm in diameter at isthmus), thoracic aorta (7 mm), and abdominal aorta (5 mm). The main branches from the aorta were also affected, including bilateral renal artery stenoses (RAS) and narrowed celiac trunk and superior mesenteric artery (2–3 mm in diameter) (Figure 3). The Doppler ultrasound did not show focal stenosis in her common, internal, or external carotid arteries.
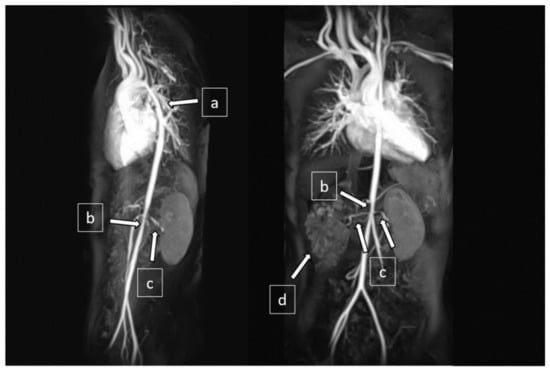
Figure 3.
Magnetic resonance angiography (MRA) showed (a) narrowing at the distal aortic arch and descending thoracic aorta, (b) narrowing at the abdominal aorta, (c) bilateral renal artery stenoses, and (d) right cystic kidney.
Our patient received oral amlodipine and atenolol to control her blood pressure. Interventional angioplasty over the renal arteries was not feasible due to the diffuse narrowing of the aorta, especially near the origins of the renal arteries. She remained asymptomatic, and her blood pressure was controlled at around the 95th percentile for her height. The latest blood tests showed normal serum urea = 4.7 mmol/L (reference: 1.8–6.4 mmol/L) and creatinine level = 47 umol/L (reference: 34–65 umol/L).
3. Discussion
Linear nevus sebaceous syndrome (LNSS), also known as Schimmelpenning syndrome, is a sporadic congenital disorder with no gender predilection. It is characterized by craniofacial nevus sebaceous in association with extracutaneous features, including neurological, ocular, and cardiovascular anomalies [2]. The term epidermal nevus syndrome is sometimes used interchangeably to describe the same condition [3]. It was suggested that there are other subtypes of epidermal nevus syndrome besides LNSS, which can be distinguished by different phenotypic and genetic features [2].
Nevus sebaceous is the hallmark of LNSS. It is a type of epidermal nevus that involves hyperplasia of epidermis, hair follicles, and sebaceous and apocrine glands. It typically presents as verrucous, granulated, yellow-orange plaques at birth [4]. The lesions most frequently occur in the scalp in two-thirds of patients, followed by the facial region in one-third [5].
Neurological and ophthalmological manifestations affect 72% and 59% of LNSS patients, respectively [6]. The syndrome is associated with structural brain malformation, including hemimegalencephaly, ventriculomegaly, and pachygyria. Coloboma and choristoma are the common ophthalmological presentations. Other eye involvements include strabismus, retinal anomalies, and vitreous and corneal opacities [7]. Other associations of LNSS, including vitamin D resistant rickets [8], lymphatic malformation [9], and intraspinal lipoma [10], have also been reported.
Our patient presented at birth with distinctive external features of nevus sebaceous over the facial region and body, as well as coloboma and lipodermoid in her eyes. She had neurological abnormalities, including pachygyria, epilepsy, and intraspinal lipoma. In addition to the typical features, our patient also had diffuse narrowing of the aorta with bilateral renal artery stenoses and unilateral cystic kidney disease. The association between nevus sebaceous and renal abnormalities was less described in the literature, possibly due to under-recognition and under-reporting. The previously reported renal associations are summarized in Table 1.

Table 1.
Summary of the associations between epidermal nevus/nevus sebaceous and renal manifestations.
Renal vasculopathy constituted the majority of the abnormalities. Renal artery stenoses were reported in four cases [10,11,12,13]. The stenoses were in conjunction with the narrowing of aorta, which varied in extent and severity. These patients had renovascular hypertension related to the stenoses. Percutaneous renal angioplasty was attempted in one patient, but balloon inflation was unsuccessful [12]. Another patient had a focal renal artery aneurysm with narrowing at the descending thoracic aorta [14]. Oral anti-hypertensive medication was the mainstay of treatment in these patients.
Renal parenchymal involvements were less reported. Nickavar et al. reported a two-year-old boy who had Wilm’s tumor in a non-functioning multi-cystic dysplastic kidney, in addition to skin and neurological manifestations [15]. Bourdeaut et al. reported another patient who had an incidental finding of bilateral polycystic kidneys during a computed tomography (CT) scan [16]. The differential functions of the kidneys and subsequent treatment were not mentioned in this report. In both cases, the renal arteries were not affected. Renal dysplasia and horseshoe kidney were described in other patients [17,18]. The concomitant occurrence of renal vasculopathy and renal cysts in our patient was not previously described.
Most patients with LNSS are recognized at birth due to the distinctive phenotypes. Besides cardiac and neurological complications, physicians should also be aware of possible renal manifestations in these patients, which carry a significant impact on management and long-term prognosis. Blood pressure should be monitored regularly in LNSS patients. Renal doppler ultrasonography should be considered as a non-invasive screening modality for detecting renal structural and vascular abnormalities. Further evaluation by computed tomography angiography or magnetic resonance angiography is warranted in individual cases to delineate the anatomy and extent of the aortopathy for formulating a management plan.
Author Contributions
All authors are involved in the patient’s care and drafting the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Not applicable.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent was obtained from the patient and her parents for publication.
Data Availability Statement
All data are reported in the case.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jiang, L.; Gao, F.; Mao, S.; Xu, J.; Jiang, K. Somatic KRAS mutation in an infant with linear nevus sebaceous syndrome associated with lymphatic malformations: A case report and literature review. Medicine 2017, 96, e8016. [Google Scholar]
- Happle, R. The group of epidermal nevus syndromes Part I. Well defined phenotypes. J. Am. Acad Dermatol. 2010, 63, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Laura, F.-S. Epidermal nevus syndrome. Handb. Clin. Neurol. 2013, 111, 349–368. [Google Scholar] [CrossRef] [PubMed]
- Eisen, D.B.; Michael, D.J. Sebaceous lesions and their associated syndromes: Part I. J. Am. Acad Dermatol. 2009, 61, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Sugarman, J.L. Epidermal nevus syndromes. Semin. Cutan. Med. Surg. 2007, 26, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Van de Warrenburg, B.P.; van Gulik, S.; Renier, W.O.; Lammens, M.; Doelman, J.C. The linear naevus sebaceus syndrome. Clin. Neurol. Neurosurg. 1998, 100, 126–132. [Google Scholar] [CrossRef]
- Kausar, A.; Zafar, S.N.; Altaf, S.; Khan, A. Ophthalmic manifestations of linear nevus sebaceous/organoid nevus syndrome. J. Coll. Physicians Surg. Pak. 2015, 25, 220–222. [Google Scholar] [PubMed]
- Hosalkar, H.S.; Jones, D.H.; Offiah, A.; Hall, C. Linear sebaceous naevus syndrome and resistant rickets. J. Bone Jt. Surg. Br. Vol. 2003, 85, 578–583. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Greene, A.K.; Rogers, G.F.; Mulliken, J.B. Schimmelpenning Syndrome: An Association with Vascular Anomalies. Cleft Palate-Craniofacial J. 2007, 44, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Mall, V.; Heinen, F.; Uhl, M.; Wellens, E.; Korinthenberg, R. CNS Lipoma in Patients with Epidermal Nevus Syndrome. Neuropediatrics 2000, 31, 175–179. [Google Scholar] [CrossRef]
- Nagatsuma, M.; Takasawa, K.; Yamauchi, T.; Nakagawa, R.; Mizuno, T.; Tanaka, E.; Yamamoto, K.; Uemura, N.; Kashimada, K.; Morio, T. A postzygotic KRAS mutation in a patient with Schimmelpenning syndrome presenting with lipomatosis, renovascular hypertension, and diabetes mellitus. J. Hum. Genet. 2019, 64, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, K.; Nakamura, T.; Ohyama, Y.; Saito, Y.; Hoshino, J.; Kanda, T.; Sumino, H.; Nagai, R. Renal artery stenosis associated with epidermal nevus syndrome. Nephron 2000, 84, 67–70. [Google Scholar] [CrossRef]
- Alsohim, F.; Abou-Jaoude, P.; Ninet, J.; Pracros, J.-P.; Phan, A.; Cochat, P. Bilateral renal artery stenosis and epidermal nevus syndrome in a child. Pediatr. Nephrol. 2011, 26, 2081–2084. [Google Scholar] [CrossRef] [PubMed]
- Juan, Y.-H.; Nagpal, P.; Saboo, S.S.; Lin, Y.-C.; Khandelwal, A. Vascular manifestations and post-treatment changes of epidermal nevus syndrome. QJM Int. J. Med. 2014, 108, 135–137. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nickavar, A.; Atefi, N.; Hesari, K.K. Epidermal nevus syndrome and dysplatic kidney disease. Acta Med. Iran. 2014, 52, 647–649. [Google Scholar] [PubMed]
- Bourdeaut, F.; Hérault, A.; Gentien, D.; Pierron, G.; Ballet, S.; Reynaud, S.; Paris, R.; Schleiermacher, G.; Baumann, C.; Philippe-Chomette, P.; et al. Mosaicism for oncogenic G12D KRAS mutation associated with epidermal nevus, polycystic kidneys and rhabdomyosarcoma. J. Med. Genet. 2010, 47, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Almefty, K.K.; Ducruet, A.F.; Crowley, R.W.; Bristol, R.; LaVine, S.D.; Albuquerque, F.C. Spinal arteriovenous malformation associated with Schimmelpenning syndrome. J. Neurosurg. Pediatr. 2013, 11, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Mollica, F.; Pavone, L.; Nuciforo, G. Linear Sebaceous Nevus Syndrome in a Newborn. Arch. Pediatr. Adolesc. Med. 1974, 128, 868. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).