Aggressive Neuroblastoma in a Pediatric Patient with Severe Hemophilia A
Abstract
1. Introduction
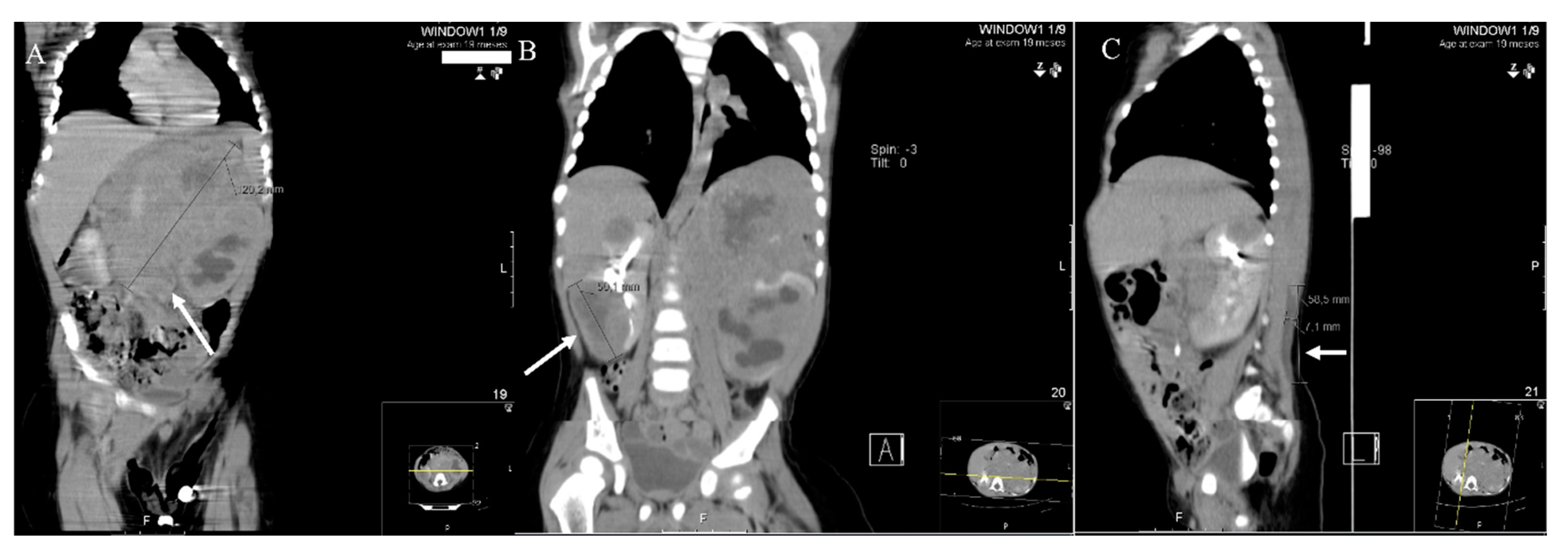
2. Results
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASCT | Autologous stem cell transplantion |
| COJEC | Cisplatin, vincristine, carboplatin, etoposide, cyclophosphamide |
| CT | Computed tomography |
| CVC | Central venous catheter |
| DMFO | Difluoromethylornithine |
| EHL | Extended half-life |
| FVIII | Factor VIII of the coagulation |
| G-CSF | Granulocyte colony stimulating factor |
| HA | Hemophilia A |
| Hb | Hemoglobin |
| ICU | Intensive care unit |
| 131I-mIBG | Metaiodobenzylguanidine |
| INSS | International Neuroblastoma Staging System |
| LDH | Lactate dehydrogenase |
| MATIN | Metaiodobenzylguanidine, topotecan |
| PBSC | Peripheral blood stem cells |
| rFVIII | Recombinant FVIII concentrate |
| RR | Reference range |
| TVD | Topotecan, vincristine, doxorubicin |
References
- Mannucci, P.M.; Tuddenham, E.G.D. The Hemophilias—From Royal Genes to Gene Therapy. N. Engl. J. Med. 2001, 344, 1773–1779. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Brewer, A.K.; Mauser-Bunschoten, E.P.; Key, N.S.; Kitchen, S.; Llinas, A.; Ludlam, C.A.; Mahlangu, J.N.; Mulder, K.; Poon, M.C.; et al. Guidelines for the management of hemophilia. Haemophilia 2013, 19, e1–e47. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D.; Fish, J.D.; Grupp, S.A. Autologous and allogeneic cellular therapies for high-risk pediatric solid tumors. Pediatr. Clin. N. Am. 2010, 57, 47–66. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tolbert, V.P.; Matthay, K.K. Neuroblastoma: Clinical and biological approach to risk stratification and treatment. Cell Tissue Res. 2018, 372, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.; Proenca, E.; Godinho, C.; Oliveira, D.; Guedes, A.; Morais, S.; Carvalho, C. Neonatal Hemophilia: A Rare Presentation. Pediatr. Rep. 2015, 7, 79–80. [Google Scholar] [CrossRef] [PubMed]
- Allen-Rhoades, W.; Whittle, S.B.; Rainusso, N. Pediatric Solid Tumors of Infancy: An Overview. Pediatr Rev. 2018, 39, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Qiao, L.; Li, D.; Liu, Z.; Zhao, F.; Yu, D. Concurrent lymphoma and hemophilia B in a pediatric patient. Medicine 2019, 98, e15474. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M. Haemophilia and cancer: A personal perspective. Blood Transfus. 2013, 11, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Park, J.R.; Kreissman, S.G.; London, W.B.; Naranjo, A.; Cohn, S.L.; Hogarty, M.D.; Tenney, S.C.; Haas-Kogan, D.; Shaw, P.J.; Kraveka, J.M.; et al. Effect of Tandem Autologous Stem Cell Transplant vs Single Transplant on Event-Free Survival in Patients With High-Risk Neuroblastoma: A Randomized Clinical Trial. JAMA 2019, 322, 746–755. [Google Scholar] [CrossRef] [PubMed]
| FVIII Replacement Scheme | Major Hemorrhagic Procedures | Immediately Before | 1st Day | 2nd Day | 8 Following Days |
|---|---|---|---|---|---|
| (by Chronologic Order) | |||||
| Chemotherapy and invasive maneuvers | Standard chemotherapy | 25 IU/kg 3×/week | |||
| (Rapid COJEC protocol + TVD cycles) | |||||
| Retroperitoneal tumor excision | 50 IU/kg | 40 IU/kg | 40 IU/lg q12 h | ||
| q8 h | |||||
| 131I-mIBG + Topotecan | 25 IU/kg 3×/week | ||||
| Insertion/replacement | 50 IU/kg | 40 IU/kg | 40 IU/kg q12 h | 25 IU/kg 3×/week | |
| of CVC | q8 h | ||||
| PBSC collection | 50 IU/kg | 50 IU/kg 1st dose + 40 UI/kg q8 h | 25 IU/kg 3×/week | ||
| (5 day mobilization with G-CSF 12 µg/kg/day + leukapheresis) * | |||||
| Double autologous hematopoietic transplant | High-dose chemotherapy (busulfan + melphalan) | 25 IU/kg 3×/week | |||
| PBSC infusion | 25 IU/kg 3×/week | 40 IU/kg q8 h | 25 IU/kg q24 h ** | ||
| (3.5 × 106 CD34 + cells/kg per transplant) | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, L.; Couto, M.E.; Moutinho, J.; Ferreira, A.M.; Costa, E.; Roncon, S.; Santos, L.L.; Cruz, E.; Morais, S. Aggressive Neuroblastoma in a Pediatric Patient with Severe Hemophilia A. Pediatr. Rep. 2021, 13, 125-130. https://doi.org/10.3390/pediatric13010018
Costa L, Couto ME, Moutinho J, Ferreira AM, Costa E, Roncon S, Santos LL, Cruz E, Morais S. Aggressive Neuroblastoma in a Pediatric Patient with Severe Hemophilia A. Pediatric Reports. 2021; 13(1):125-130. https://doi.org/10.3390/pediatric13010018
Chicago/Turabian StyleCosta, Lidia, Maria Eduarda Couto, Juliana Moutinho, Ana Maia Ferreira, Emilia Costa, Susana Roncon, Luisa Lopes Santos, Eugenia Cruz, and Sara Morais. 2021. "Aggressive Neuroblastoma in a Pediatric Patient with Severe Hemophilia A" Pediatric Reports 13, no. 1: 125-130. https://doi.org/10.3390/pediatric13010018
APA StyleCosta, L., Couto, M. E., Moutinho, J., Ferreira, A. M., Costa, E., Roncon, S., Santos, L. L., Cruz, E., & Morais, S. (2021). Aggressive Neuroblastoma in a Pediatric Patient with Severe Hemophilia A. Pediatric Reports, 13(1), 125-130. https://doi.org/10.3390/pediatric13010018





