Efficacy of Everolimus Low-Dose Treatment for Cardiac Rhabdomyomas in Neonatal Tuberous Sclerosis: Case Report and Literature Review
Abstract
1. Introduction
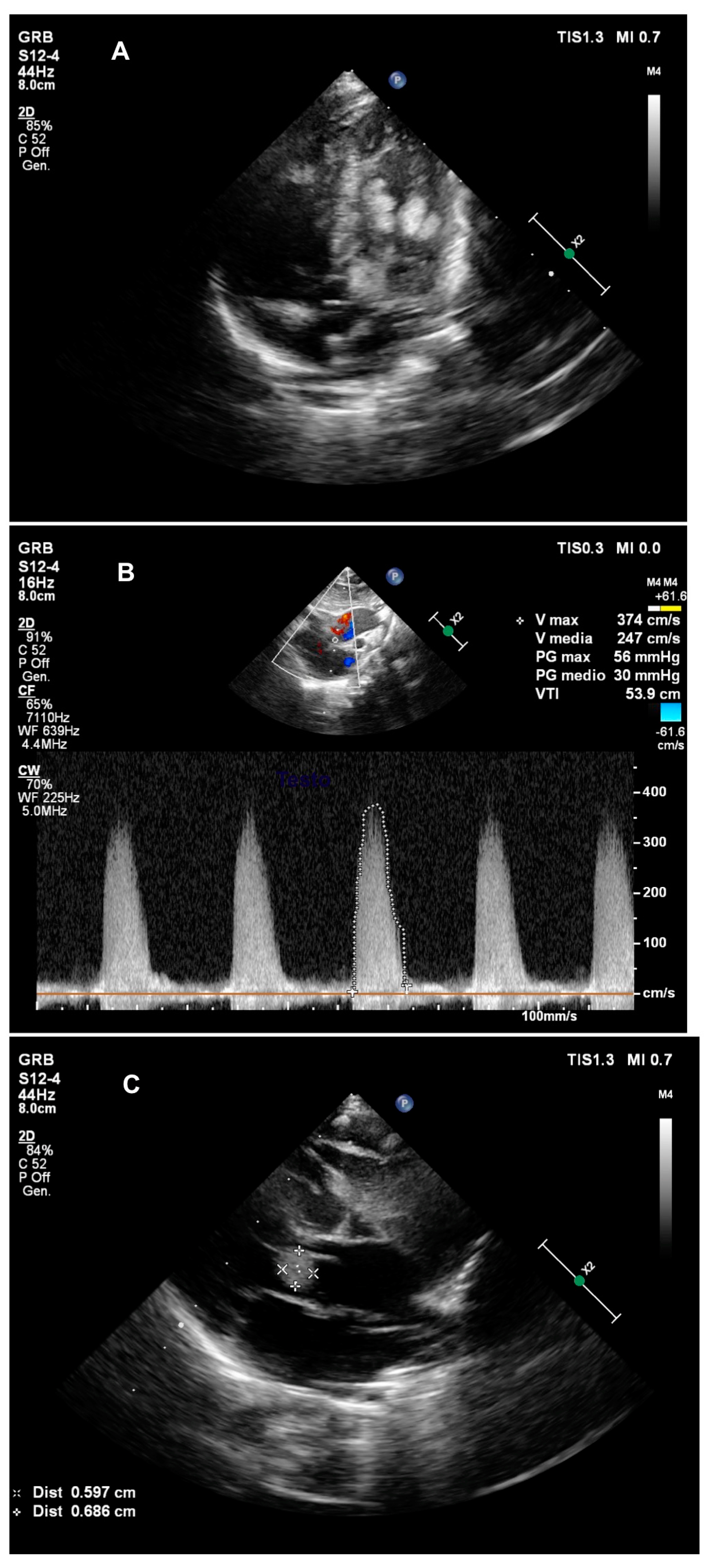
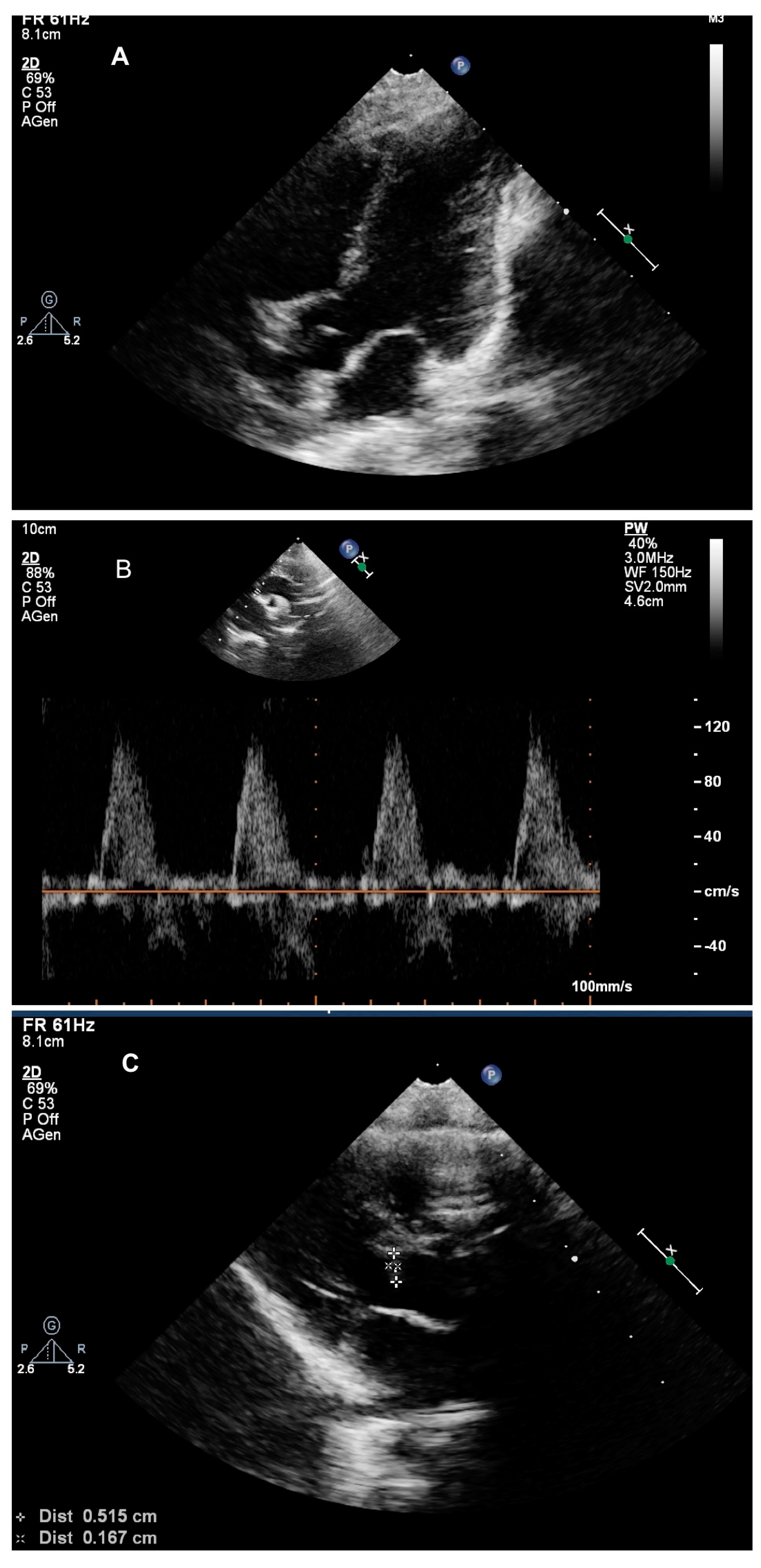
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wataya-Kaneda, M.; Uemura, M.; Fujita, K.; Hirata, H.; Osuga, K.; Kagitani-Shimono, K.; Nonomura, N. Tuberous Sclerosis Complex Board of Osaka University Hospital. Tuberous sclerosis complex: Recent advances in manifestations and therapy. Int. J. Urol. 2017, 24, 681–691. [Google Scholar] [CrossRef]
- Portocarrero, L.K.L.; Quental, K.N.; Samorano, L.P.; Oliveira, Z.N.P.; Rivitti-Machado, M.C.D.M. Tuberous sclerosis complex: Review based on new diagnostic criteria. Bras Dermatol. 2018, 93, 323–331. [Google Scholar] [CrossRef]
- Castro-Monsalve, J.; Alvarado-Socarras, J.L.; Mantilla, K.A.; Forero, L.; Moreno, A.; Prada, C.E. Cardiac Rhabdomyomas in Tuberous sclerosis complex. J. Pediatr. 2018, 192, 264-e1. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, J.; Wałdoch, A.; Meyer-Szary, J.; Potaż, P.; Grzybiak, M. Cardiac tumors in children: A 20-year review of clinical presentation, diagnostics and treatment. Adv. Clin. Exp. Med. 2017, 26, 319–326. [Google Scholar] [CrossRef]
- Aw, F.; Goyer, I.; Raboisson, M.J.; Boutin, C.; Major, P.; Dahdah, N. Accelerated Cardiac Rhabdomyoma regression with Everolimus in infants with tuberous sclerosis complex. Pediatr. Cardiol. 2017, 38, 394–400. [Google Scholar] [CrossRef]
- Franz, D.N.; Capal, J.K. mTOR inhibitors in the pharmacologic management of tuberous sclerosis complex and their potential role in other rare neurodevelopmental disorders. Orphanet J. Rare Dis. 2017, 12, 51. [Google Scholar] [CrossRef]
- Bevacqua, M.; Baldo, F.; Pastore, S.; Valencic, E.; Tommasini, A.; Maestro, A.; Rabusin, M.; Arbo, A.; Barbi, E. Off-label use of Sirolimus and Everolimus in a Pediatric Center: A case series and review of the literature. Paediatr. Drugs 2019, 21, 185–193. [Google Scholar] [CrossRef]
- Northrup, H.; Krueger, D.A. Tuberous sclerosis complex diagnostic criteria update: Recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr. Neurol. 2013, 49, 243–254. [Google Scholar] [CrossRef]
- Hinton, R.B.; Prakash, A.; Romp, R.L.; Krueger, D.A.; Knilans, T.K. Cardiovascular manifestations of tuberous sclerosis complex and summary of the revised diagnostic criteria and surveillance and management recommendations from the International Tuberous Sclerosis Consensus Group. J. Am. Heart Assoc. 2014, 3, e001493. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Gorla, S.R.; Kardon, R.E.; Swaminathan, S. Rapid Involution of Large Cardiac Rhabdomyomas With Everolimus Therapy. World J. Pediatr. Congenit Heart Surg. 2019, 2150135118822711. [Google Scholar] [CrossRef] [PubMed]
- Goyer, I.; Dahdah, N.; Major, P. Use of mTOR inhibitor everolimus in three neonates for treatment of tumors associated with tuberous sclerosis complex. Pediatr. Neurol. 2015, 52, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Dhulipudi, B.; Bhakru, S.; Rajan, S.; Doraiswamy, V.; Koneti, N.R. Symptomatic improvement using everolimus in infants with cardiac rhabdomyoma. Ann. Pediatr. Cardiol. 2019, 12, 45–48. [Google Scholar]
- Shibata, Y.; Maruyama, H.; Hayashi, T.; Ono, H.; Wada, Y.; Fujinaga, H.; Fujino, S.; Nagasawa, J.; Amari, S.; Tsukamoto, K.; et al. Effect and Complications of Everolimus Use for Giant Cardiac Rhabdomyomas with Neonatal Tuberous Sclerosis. AJP Rep. 2019, 9, e213–e217. [Google Scholar] [CrossRef]
- Martínez-García, A.; Michel-Macías, C.; Cordero-González, G.; Escamilla-Sánchez, K.I.; Aguinaga-Ríos, M.; Coronado-Zarco, A.; Cardona-Pérez, J.A. Giant left ventricular rhabdomyoma treated successfully with everolimus: Case report and review of literature. Cardiol. Young 2018, 28, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Chiou, P.Y.; Yao, S.H.; Chou, I.C.; Lin, C.Y. Regression of Neonatal Cardiac Rhabdomyoma in Two Months Through Low-Dose Everolimus Therapy: A Report of Three Cases. Pediatr. Cardiol. 2017, 38, 1478–1484. [Google Scholar] [CrossRef]
- Colaneri, M.; Quarti, A.; Pozzi, M. Everolimus-induced near-resolution of giant cardiac rhabdomyomas and large renal angiomyolipoma in a newborn with tuberous sclerosis complex. Cardiol. Young 2016, 26, 1025–1028. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, S.; Nguyen, H.H.; Anwar, S. Rapid resolution of cardiac rhabdomyomas following everolimus therapy. BMJ Case Rep. 2015, 2015, bcr2015212946. [Google Scholar]
- Wagner, R.; Riede, F.T.; Seki, H.; Hornemann, F.; Syrbe, S.; Daehnert, I.; Weidenbach, M. Oral Everolimus for Treatment of a Giant Left Ventricular Rhabdomyoma in a Neonate-Rapid Tumor Regression Documented by Real Time 3D Echocardiography. Echocardiography 2015, 32, 1876–1879. [Google Scholar] [CrossRef]
- Doğan, V.; Yeşil, Ş.; Kayalı, Ş.; Beken, S.; Özgür, S.; Ertuğrul, İ.; Bozkurt, C.; Örün, U.A.; Karademir, S. Regression of symptomatic multiple cardiac rhabdomyomas associated with tuberous sclerosis complex in a newborn receiving everolimus. J. Trop. Pediatr. 2015, 61, 74–77. [Google Scholar]
- Öztunç, F.; Atik, S.U.; Güneş, A.O. Everolimus treatment of a newborn with rhabdomyoma causing severe arrhythmia. Cardiol. Young 2015, 25, 1411–1414. [Google Scholar] [CrossRef]
- Tiberio, D.; Franz, D.N.; Phillips, J.R. Regression of a cardiac rhabdomyoma in apatient receiving everolimus. Pediatrics 2011, 127, e1335–e1337. [Google Scholar] [CrossRef] [PubMed]
- Dahdah, N. Everolimus for the Treatment of Tuberous Sclerosis Complex-Related Cardiac Rhabdomyomas in Pediatric Patients. J. Pediatr. 2017, 190, 21–26.e7. [Google Scholar] [CrossRef] [PubMed]
- Hoshal, S.G.; Samuel, B.P.; Schneider, J.R.; Mammen, L.; Vettukattil, J.J. Regression of massive cardiac rhabdomyoma on everolimus therapy. Pediatr. Int. 2016, 58, 397–399. [Google Scholar] [CrossRef] [PubMed]
| First Author, Year | Age at Treatment | Clinical Presentation | Echographic Data at Diagnosis | Everolimus Dose | Target Drug Levels | Reported Evolution | Adverse Effects | Follow Up |
|---|---|---|---|---|---|---|---|---|
| LOW-DOSE TREATMENT (≤0.1 mg/die) | ||||||||
| Garg A et al., 2019 | 4th day of life | Prenatal diagnosis of CRs At birth: Pre-excitation with ventricular tachycardia | Multiple CRs (major in RV free wall) | 0.08 mg/die | 3–7 ng/mL | Improvement of ventricular tachycardia after 4 days of treatment | None | Not reported |
| Castro-Monsalve J et al., 2018 | Not reported | Cardiac arrest | Multiple CRs on interventricular septum with LVOTO | 0.1 mg/die | 5–8 ng/mL | 60% reduction of CR at 31days—resolution at 4 weeks | None | Not reported |
| Aw F et al., 2016 | Average: 6.5 days (2–20 days) | LVOTO 3rd case: Subependymal lesion | 1st case: LVOTO e RVOTO 2nd case: Multiple CRs—major subaortic 3rd case: Multiple CRs 4th case: CRs with LVOTO | 0.1 mg/die | 5–15 ng/mL | 50% reduction of CRs in 36 days | Not reported | Treatment: Average 73 days (34–138 days) |
| Goyer I et al., 2015 | Three cases: First week of life | LVOTO In one case: Subependymal astrocytoma | 1st case: Multiple CRs (n 6) 2nd case: Multiple CRs with LVOTO 3rd case: Multiple CRs | 0.1 mg/die | 5–15 ng/mL | Regression at 1 month | Viral infection in one case | Check Everolimus plasmatic concentration every 4 days at the beginning |
| DOSE TREATMENT (>0.1 mg/die) | ||||||||
| Dhulipudi B et al., 2019 | Average: 5 days (1–90 days) | Five case reports describing cardiovascular failure due to outlet obstruction | 1st case: CR 24 mm at right outlet 2nd case: CR 13 mm in right atrium with superior vena cava obstruction 3rd case: CR 12 mm with LVOTO 4th and 5th case: Multiple CRs in right ventricle | 4.5 mg/m2/week divided in daily doses | 5–15 ng/mL | CR regression in 6.1 +/− 5.1 months | Chickenpox infection | Not reported |
| Shibata Y et al., 2019 | 4th day of life | Prenatal diagnosis of CR | Multiple CRs with LVOTO | Initial dose: 0.4 mg/die, reduced to 0.1 mg/die at day 10 | 5–15 ng/mL | Reduction of CR after 7 days of therapy | Coagulopathy at day 3 of therapy | Not reported |
| Martinez-Garcia A et al., 2018 | 36 days of life | Prenatal diagnosis of multiple CRs | Multiple CRs—major in left ventricle with LVOTO | 0.25 mg twice per day—twice per week | Not indicated | Improvement after 2 weeks of treatment | None | Not reported |
| Chang JS et al., 2017 | Not reported | Prenatal diagnosis of CRs In one case: Seizures | 1st case: CRs in both ventricles 2nd case: CR in LV with LVOTO 3rd case: Multiple CRs in left ventricle | 0.3–0.67 mg/m2/die | 3–7 ng/mL | Regression in 2 months | 1st case: Viral pneumonia 3rd case: Growth failure | Not reported |
| Colaneri M et al., 2016 | Not reported | Prenatal diagnosis of CRs At birth: Hypoplastic left heart syndrome | Multiple CRs—major on left ventricle free wall | 0.25 mg/die | 5–15 ng/mL | After 10 weeks: 80% reduction of CRs | None | Stop treatment 11 weeks post treatment |
| Bornaun H et al., 2016 | Not reported | Cardiovascular failure due to LVOTO | CR with LVOTO | 0.5 mg/die twice per week | 2.6–6.1 ng/mL | Regression of lesion in 4 weeks | TG and cholesterol levels increased. Change in lymphocyte subgroups | Stopped therapy after 7.5 months of treatment |
| Choudhry S et al., 2015 | 2 weeks of life | Cardiac and cerebral lesions | Multiple RCs | Not reported | Not reported | CR regression in 1 month | Not reported | Not noted |
| Wagner R et al., 2015 | Not reported | At birth: Heart murmur | Multiple CRs—major on LVOTO | Starting dose 1.5 mg/m2/die, reduced to 1 mg/m2/die at 4th day | 5–15 ng/mL | Progressive reduction of RCs | Slightly elevated triglycerides and transitory lymphopenia | Stop therapy at 19 days post treatment |
| Dogan V et al., 2015 | 2 days | Heart murmur and cardiovascular failure | CRs with LVOTO | 0.25 mg twice per day—twice per week | 5–15 ng/mL | Progressive reduction of RCs | Not reported | Stop therapy at 3 months |
| Oztunc F et al., 2014 | First week of life | Supraventricular tachycardia | Multiple CRs (interventricular sept—anterior wall RV) | 0.25 mg twice per day—twice per week | Not noted | Supraventricular tachycardia resolution on day 8. Regression of RCs at 15 days | None | Not reported |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nespoli, L.F.; Albani, E.; Corti, C.; Spaccini, L.; Alfei, E.; Daniele, I.; Zuccotti, G.V.; Lista, G.; Calcaterra, V.; Mannarino, S. Efficacy of Everolimus Low-Dose Treatment for Cardiac Rhabdomyomas in Neonatal Tuberous Sclerosis: Case Report and Literature Review. Pediatr. Rep. 2021, 13, 104-112. https://doi.org/10.3390/pediatric13010015
Nespoli LF, Albani E, Corti C, Spaccini L, Alfei E, Daniele I, Zuccotti GV, Lista G, Calcaterra V, Mannarino S. Efficacy of Everolimus Low-Dose Treatment for Cardiac Rhabdomyomas in Neonatal Tuberous Sclerosis: Case Report and Literature Review. Pediatric Reports. 2021; 13(1):104-112. https://doi.org/10.3390/pediatric13010015
Chicago/Turabian StyleNespoli, Luisa Federica, Elena Albani, Carla Corti, Luigina Spaccini, Enrico Alfei, Irene Daniele, Gian Vincenzo Zuccotti, Gianluca Lista, Valeria Calcaterra, and Savina Mannarino. 2021. "Efficacy of Everolimus Low-Dose Treatment for Cardiac Rhabdomyomas in Neonatal Tuberous Sclerosis: Case Report and Literature Review" Pediatric Reports 13, no. 1: 104-112. https://doi.org/10.3390/pediatric13010015
APA StyleNespoli, L. F., Albani, E., Corti, C., Spaccini, L., Alfei, E., Daniele, I., Zuccotti, G. V., Lista, G., Calcaterra, V., & Mannarino, S. (2021). Efficacy of Everolimus Low-Dose Treatment for Cardiac Rhabdomyomas in Neonatal Tuberous Sclerosis: Case Report and Literature Review. Pediatric Reports, 13(1), 104-112. https://doi.org/10.3390/pediatric13010015








