Testis-Sparing Surgery for Non-Palpable Leydig Cell Tumors in Prepubertal Children
Abstract
1. Introduction
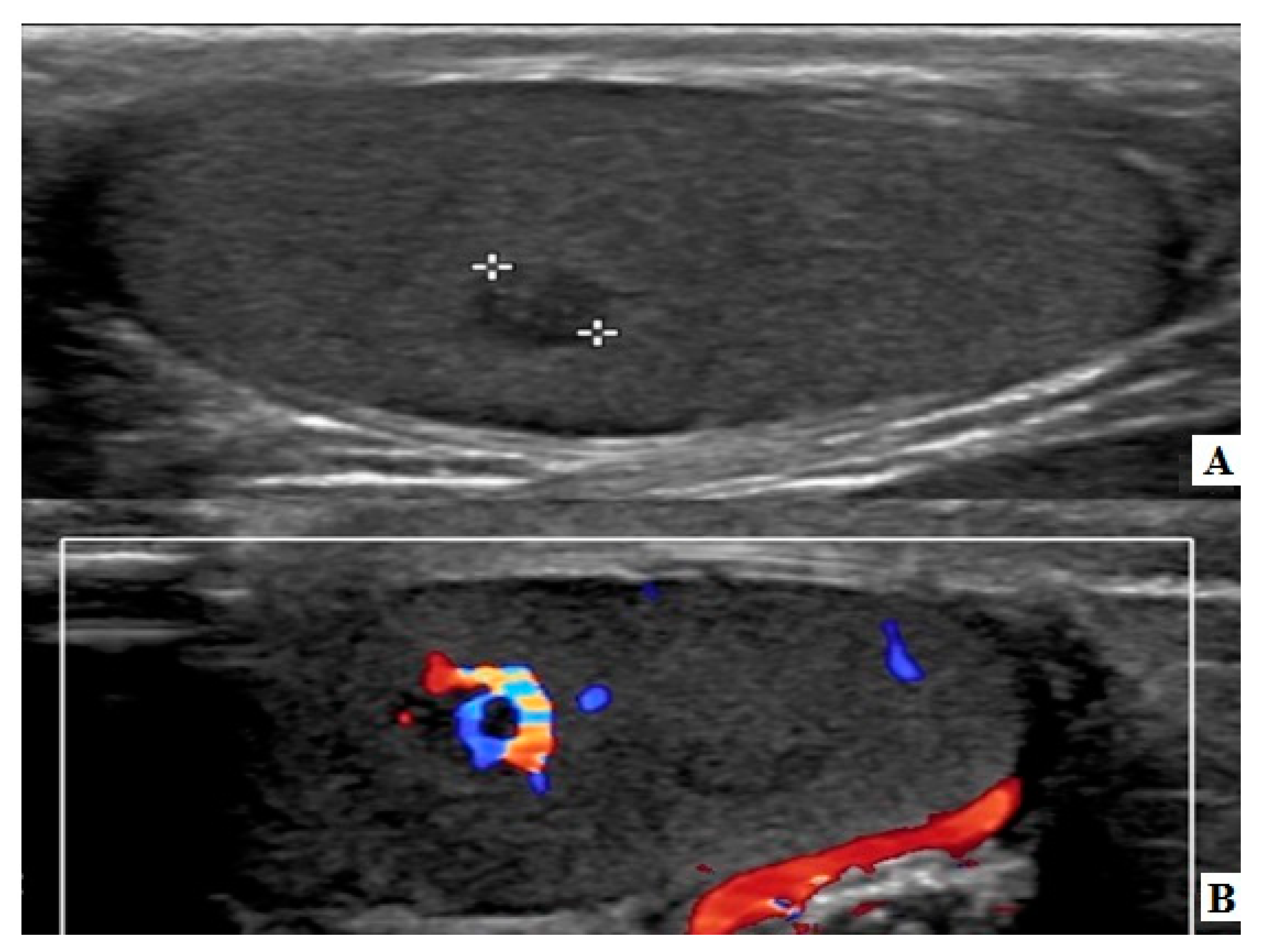
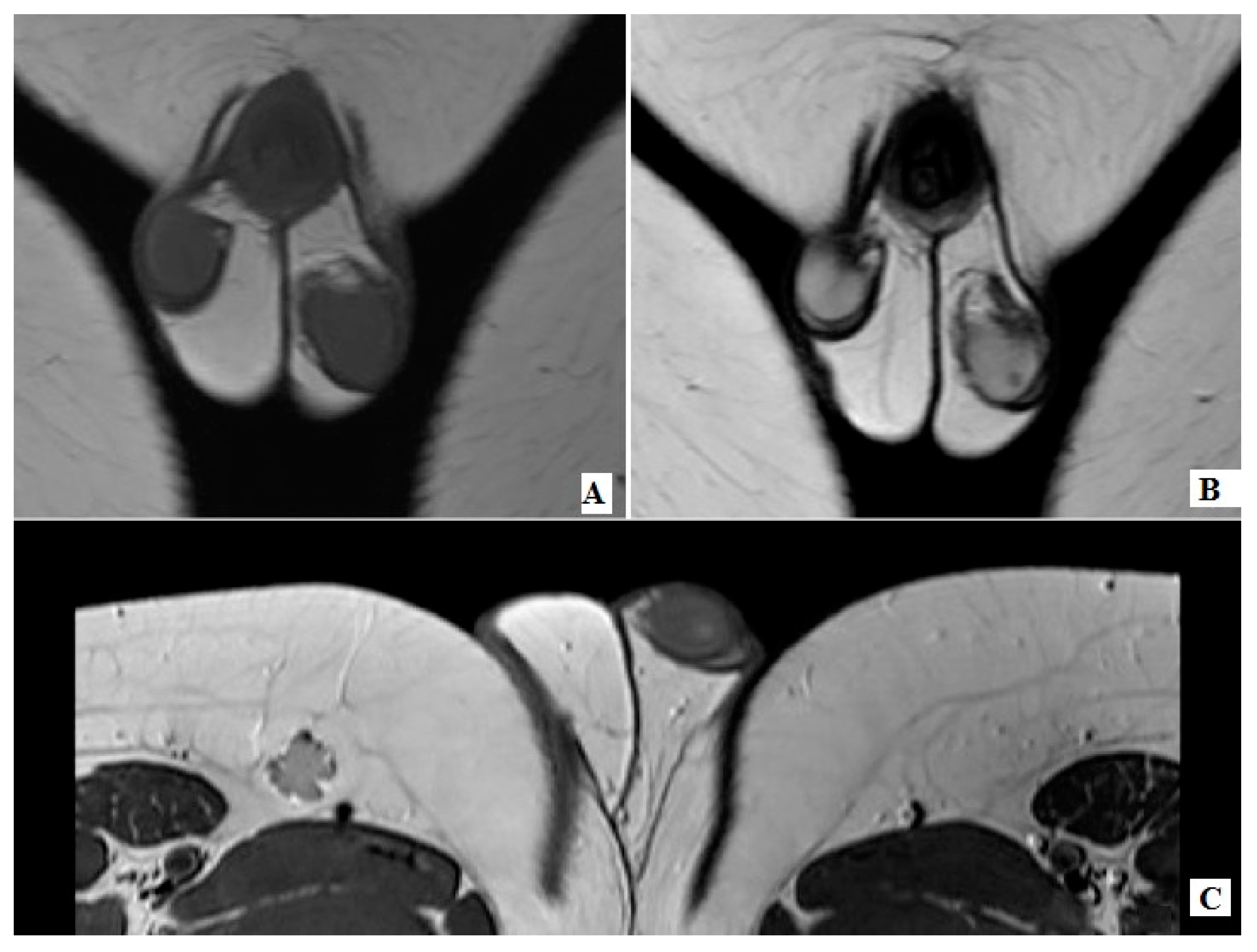
2. Case Reports
3. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Masur, Y.; Steffens, J.; Ziegler, M.; Remberger, K. Leydig-Zell-Tumoren des Hodens-Klinische und morphologische Aspekte. Urol. A 1996, 35, 468. [Google Scholar] [CrossRef] [PubMed]
- Carmignani, L.; Salvioni, R.; Gadda, F.; Colecchia, M.; Gazzano, G.; Torelli, T.; Rocco, F.; Colpi, G.M.; Pizzocaro, G. Long-term follow-up and clinical characteristics of testicular Leydig cell tumor: Experience with 24 cases. J. Urol. 2006, 176, 2040–2043. [Google Scholar] [CrossRef] [PubMed]
- Pohl, H.G.; Shukla, A.R.; Metcalf, P.D.; Cilento, B.G.; Retik, A.B.; Bagli, D.J.; Huff, D.S.; Rushton, H.G. Prepubertal testis tumors: Actual prevalence rate of histological types. J. Urol. 2004, 170, 2370–2372. [Google Scholar] [CrossRef] [PubMed]
- Cheville, J.C.; Sebo, T.J.; Lager, D.J.; Bostwick, D.G.; Farrow, G.M. Leydig cell tumor of the testis: A clinicopathologic, DNA content, and MIB-1 comparison of nonmetastasizing and metastasizing tumors. Am. J. Surg. Pathol. 1998, 22, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Young, R.H.; Scully, R.E. Leydig cell tumor of the testis: A clinicopathological analysis of 40 cases and review of the literature. Am. J. Surg. Pathol. 1985, 9, 177–192. [Google Scholar] [CrossRef]
- Ciftci, A.O.; Bingöl-Koloğlu, M.; Şenocak, M.E.; Tanyel, F.C.; Büyükpamukçu, M.; Büyükpamukçu, N. Testicular tumors in children. J. Pediatr. Surg. 2001, 36, 1796–1801. [Google Scholar] [CrossRef] [PubMed]
- Rosai, J. Ackerman’s Surgical Pathology; Mosby: Edimburgh, Scotland, 2004; pp. 1436–1437. [Google Scholar]
- Düe, W.; Dieckmann, K.P.; Loy, V.; Stein, H. Immunohistological determination of oestrogen receptor, progesterone receptor, and intermediate filaments in Leydig cell tumours, Leydig cell hyperplasia, and normal Leydig cells of the human testis. J. Pathol. 1989, 157, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, F.; Izard, V.; Ferlicot, S.; Rachas, A.; Correas, J.M.; Benoit, G.; Bellin, M.F.; Rocher, L. Colour Doppler and ultrasound characteristics of testicular Leydig cell tumours. Br. J. Radiol. 2016, 89, 20160089. [Google Scholar] [CrossRef] [PubMed]
- Manganaro, L.; Vinci, V.; Pozza, C.; Saldari, M.; Gianfrilli, D.; Pofi, R.; Bernardo, S.; Cantisani, V.; Lenzi, A.; Scialpi, M.; et al. A prospective study on contrast-enhanced magnetic resonance imaging of testicular lesions: Distinctive features of Leydig cell tumours. Eur. Radiol. 2015, 25, 3586–3595. [Google Scholar] [CrossRef] [PubMed]
- Cecchetto, G.; Alaggio, R.; Bisogno, G.; Virgone, C.; Dall’Igna, P.; Terenziani, M.; Boldrini, R.; D’Onofrio, V.; Ferrari, A.; Bernini, G. Sex cord-stromal tumors of the testis in children. A clinicopathologic report from the Italian TREP project. J. Pediatr. Surg. 2010, 45, 1868–1873. [Google Scholar] [CrossRef] [PubMed]
- Cecchetto, G.; Alaggio, R.; Bisogno, G.; Virgone, C.; Dall’Igna, P.; Terenziani, M.; Boldrini, R.; D’Onofrio, V.; Ferrari, A.; Bernini, G. Leydig cell testicular tumour presenting as isosexual precocious pseudopuberty in a 5 year-old boy with no palpable testicular mass. Clin. Pediatr. Endocrinol. 2010, 19, 19–23. [Google Scholar]
- Obembe, O.O.; Patel, M.D. Value of dynamic, contrast-enhanced MRI & intraoperative ultrasound for management of a nonpalpable, incidental, testicular Leydig-cell tumor. Radiol. Case Rep. 2015, 5, 432. [Google Scholar] [PubMed]
- Tsitouridis, I.; Maskalidis, C.; Panagiotidou, D.; Kariki, E.P. Eleven patients with testicular leydig cell tumors: Clinical, imaging, and pathologic correlation. J. Ultrasound Med. 2014, 33, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Emre, S.; Ozcan, R.; Elicevik, M.; Emir, H.; Soylet, Y.; Buyukunal, C. Testis sparing surgery for Leydig cell pathologies in children. J. Pediatr. Urol. 2017, 13, e1–e51. [Google Scholar] [CrossRef] [PubMed]
- Sönmez, N.; Ton, Ö.; Arısan, S.; Kılınç, F.; Eken, K.; Güney, S. Bilateral Leydig cell tumor of the testis: A case report. Ann. Urol. 2003, 37, 213–216. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambropoulos, V.; Theodorakopoulos, A.; Mouravas, V.; Pazarli, E.; Godosis, D.; Kepertis, C.; Anastasiadis, K.; Spyridakis, I. Testis-Sparing Surgery for Non-Palpable Leydig Cell Tumors in Prepubertal Children. Pediatr. Rep. 2020, 12, 86-92. https://doi.org/10.3390/pediatric12030020
Lambropoulos V, Theodorakopoulos A, Mouravas V, Pazarli E, Godosis D, Kepertis C, Anastasiadis K, Spyridakis I. Testis-Sparing Surgery for Non-Palpable Leydig Cell Tumors in Prepubertal Children. Pediatric Reports. 2020; 12(3):86-92. https://doi.org/10.3390/pediatric12030020
Chicago/Turabian StyleLambropoulos, Vassilis, Antonios Theodorakopoulos, Vasileios Mouravas, Elissavet Pazarli, Dimitrios Godosis, Chrysostomos Kepertis, Kleanthis Anastasiadis, and Ioannis Spyridakis. 2020. "Testis-Sparing Surgery for Non-Palpable Leydig Cell Tumors in Prepubertal Children" Pediatric Reports 12, no. 3: 86-92. https://doi.org/10.3390/pediatric12030020
APA StyleLambropoulos, V., Theodorakopoulos, A., Mouravas, V., Pazarli, E., Godosis, D., Kepertis, C., Anastasiadis, K., & Spyridakis, I. (2020). Testis-Sparing Surgery for Non-Palpable Leydig Cell Tumors in Prepubertal Children. Pediatric Reports, 12(3), 86-92. https://doi.org/10.3390/pediatric12030020




