Abstract
CTX-M beta-lactamases have become the predominant extended-spectrum beta-lactamases (ESBLs) globally, contributing to increased patient morbidity, mortality, and healthcare costs. This study investigated the prevalence of biofilm formation and CTX-M genes in Klebsiella pneumoniae strains isolated from Baghdad hospitals, aiming to better understand antimicrobial resistance mechanisms and support the development of targeted interventions. A total of 300 samples were collected from various clinical and hospital sources, and antibiotic susceptibility testing was performed using the Kirby–Bauer disc diffusion method. ESBL production was also confirmed using specifically designed primers. Platanus orientalis Linn extract was evaluated for its antimicrobial and antibiofilm activity against K. pneumoniae isolates. The results showed significant resistance to the majority of antibiotics, including cefotaxime, gentamicin, levofloxacin, ceftazidime, and ceftriaxone. A high prevalence of the CTX-M gene (100%) was detected in the isolates, with the most frequent alleles being blaCTX-M-15 (65.2%) and blaCTX-M-1 (30%). Furthermore, 95.6% of the isolates were capable of forming biofilms. However, when treated with P. orientalis Linn extract, most isolates exhibited reduced biofilm production, becoming weak biofilm producers. Phytochemical analysis of P. orientalis Linn revealed significant amounts of phenolic compounds, tannins, and glycosides, as well as the presence of alkaloids and carbohydrates. Overall, this study demonstrates a correlation between CTX-M production and biofilm-forming ability in K. pneumoniae and highlights the potential role of P. orientalis Linn extract in combating antibiotic-resistant infections.
1. Introduction
Klebsiella pneumoniae, a Gram-negative opportunistic pathogen, poses a significant public health threat due to its capacity to cause a wide range of infections. It frequently infects patients utilizing medical devices, especially urinary catheters, where it can readily establish a biofilm [1]. Indeed, K. pneumoniae is a leading cause of catheter-associated urinary tract infections [2], and its involvement in infections related to burns, wounds [3], and contamination of blood and body fluids [4] is well-documented.
Previous research in Iraq indicated a 24.4% prevalence of K. pneumoniae in clinical samples [5]. The increasing antibiotic resistance among nosocomial K. pneumoniae strains, coupled with the enhanced antimicrobial resistance conferred by biofilm formation [6], presents a critical global challenge, particularly within healthcare settings. The emergence of Extended-Spectrum Beta-Lactamase (ESBLs) in bacterial pathogens, including the production of biofilms, significantly complicates treatment and infection control. ESBLs, such as TEM, SHV, OXA, and CTX-M enzymes, inactivate beta-lactam antibiotics like penicillins and cephalosporins [7]. Among ESBLs, CTX-M enzymes are most frequently associated with antibiotic resistance, with over 130 variants classified into five groups (CTX-M-1, CTX-M-2, CTX-M-8, CTX-M-9, and CTX-M-25) [8]. CTX-M15 is the predominant type encountered in both hospital and community settings [8], posing a major obstacle to effective bacterial disease control due to its ability to reduce the effectiveness of widely used antibiotics. This necessitates heightened awareness among healthcare professionals regarding ESBL presence in patient samples and the implementation of robust infection control measures to curb the spread of ESBL-producing bacteria and prevent the further development of antibiotic resistance [9]. Biofilm formation involves bacterial adhesion to surfaces, resulting in the creation of protected colonies embedded with an extracellular matrix [10]. This matrix acts as a barrier, enhancing resistance to environmental factors, including antibacterial agents [11]. Furthermore, the host inflammatory response to infection can contribute to a toxic extracellular matrix that impedes wound healing and perpetuates [12]. The significance of biofilm formation in K. pneumoniae pathogenicity is undeniable, facilitating bacterial dissemination and persistence within the host [13]. Traditional and folk medicine have long emphasized the therapeutic properties of plants. Phytotherapy, the use of plant extract for wound healing, has a rich history [14]. Modern research validates the antimicrobial, anti-inflammatory, and analgesic properties of many plant extracts, with their diverse phytochemicals influencing various stages of wound healing [15]. Platanus orientalis Linn, a member of the Plantanaceae family, is a deciduous tree native to southwest Asia [16] and found extensively in the Kurdistan region of Iraq [17,18].
Phytochemical analysis of P. orientalis L. has revealed a range of bioactive compounds, including kaempferol glycosides, platanoside, tiliroside, caffeic acid, flavonol glycosides, proanthocyanidin glycosides, fatty acids, and phytol derivatives [19]. The potent antibacterial and antioxidant properties of its polyphenolic flavonoids and tannins suggest a potential role in wound healing and the management of various health conditions [20,21].
This study aimed to investigate the prevalence of biofilm formation and ESBLs in K. pneumoniae strains isolated from Baghdad hospitals, providing insights into antimicrobial resistance mechanisms and informing the development of targeted interventions. It also explored the potential antibacterial activity of P. orientalis extract against K. pneumoniae.
2. Materials and Methods
- Ethics Statement:
The research has been conducted in vitro using bacterial isolates, without the involvement of human beings or animals.
2.1. Collection of Samples and Bacterial Identification
A total of 250 clinical samples (wound swabs, stool, burn swabs, body fluids, blood, and urine) were collected from inpatients at four hospitals in Baghdad (Al-Karkh General Hospital, Al-Shaheed Al-Sadir Hospital, Al-Yarmouk Teaching Hospital, and Al-Kindi Teaching Hospital) within 48 h of admission, using anonymized IDs to prevent duplication. In addition, 50 environmental samples (soil and water) were obtained from hospital premises. All samples were initially cultured on MacConkey agar and blood agar (Himedia, Thane, India) and incubated at 37 °C for 48 h. Presumptive K. pneumoniae colonies were further identified by standard biochemical assays, including methyl red, citrate utilization, Voges–Proskauer, urease activity, and triple sugar iron agar tests.
Genomic DNA was extracted using a commercial kit (Promega, Madison, WI, USA). Molecular identification of isolates was confirmed by PCR amplification of the 16S rRNA gene [22] using specific primers: forward 5′-AGGAAGGCGGTGAAGGTTTA-3′ and reverse 5′-CACCTGAGCGTCAGTCTTTG-3′, yielding a 334 bp product with an annealing temperature of 58 °C. Primers were designed using Primer3Plus (https://www.primer3plus.com, accessed on 16 July 2025) accessed on November 2022. E. coli ATCC 25922 and K. pneumoniae ATCC 700603 served as negative and positive controls, respectively.
PCR reactions (25 µL) consisted of 12.5 µL of Taq Green PCR Master Mix (Promega, Madison, WI, USA), 4 µL of each primer (10 pmol/µL), 4 µL of DNA template, and 4.5 µL of nuclease-free water, following the manufacturer’s protocol and Paulin-Curlee et al. [23]. The thermal cycling conditions were: initial denaturation at 94 °C for 3 min; 34 cycles of denaturation at 94 °C for 30 s, annealing at 58 °C for 30 s, and extension at 72 °C for 1 min; followed by a final extension at 72 °C for 10 min.
PCR products were analyzed by electrophoresis on 1.5% agarose gel prepared in Tris–Acetate–EDTA buffer, using a 100 bp DNA ladder as a molecular size marker. Gels were stained with ethidium bromide and visualized under UV transillumination to confirm amplicon size and specificity.
2.2. Antibiotic Susceptibility Test of Clinical Isolate
Antibiotic susceptibility testing was performed using the disk diffusion method according to CLSI (2020) guidelines. The tested antibiotics included: cefotaxime (CTX, 30 µg), amikacin (AMK, 30 µg), meropenem (MRP, 10 µg), gentamicin (CN, 10 µg), amoxicillin (AX, 25 µg), imipenem (IMP, 10 µg), ciprofloxacin (CIP, 10 µg), levofloxacin (LEV, 5 µg), augmentin (AMC, 30 µg), ceftazidime (CAZ, 30 µg), and ceftriaxone (CTR, 30 µg).
Colonies from overnight nutrient agar cultures were suspended in 3 mL of sterile saline, and turbidity was adjusted to 0.5 McFarland standard (≈1.5 × 108 CFU/mL). The suspension was evenly spread onto Mueller–Hinton agar plates, and antibiotic disks were applied [24]. Plates were incubated at 35 ± 2 °C for 16–18 h under ambient conditions, following CLSI 2020 recommendations.
All susceptibility tests were performed in triplicate. Zone diameters were measured to the nearest millimeter using digital calipers under standardized lighting. Results were interpreted as susceptible (S), intermediate (I), or resistant (R) based on CLSI zone diameter breakpoints. E. coli ATCC 25922 and K. pneumoniae ATCC 700603 (ESBL-positive) were used as quality control strains.
2.3. Molecular Analysis of CTX-M ESBL Genes in Klebsiella Pneumoniae Isolates
Genomic DNA extraction was performed using a Promega (USA) kit. The presence of blaCTX-M genes was detected by PCR using primers designed with Primer3Plus (https://www.primer3plus.com/index.html, accessed on 16 July 2025) on 22 February 2023 (Table 1). PCR reactions (25 µL) contained 12.5 µL of PCR master mix (Taq Green Polymerase, Promega, Madison, WI, USA), 4 µL of each primer (10 pmol/µL), 4.5 µL of nuclease-free water, and 4 µL of DNA template. The PCR protocol consisted of an initial denaturation at 94 °C for 3 min, followed by 34 cycles of denaturation at 94 °C for 30 s, annealing at temperatures specified in Table 1 for 30 s, extension at 72 °C for 1 min, and a final extension at 72 °C for 10 min. Non-ESBL-producing bacteria (E. coli ATCC 25922) were used as the negative control, and K. pneumoniae ATCC 700603 (ESBL-positive strain) was used as the positive control. PCR products (30 µL) were sequenced by Macrogen, Inc. (Seoul, Republic of Korea). Sequencing similarity searches were conducted using the NCBI BLAST tool version 2.13.0 (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 16 July 2025) and ClustalW version 2.1, with the NCBI reference sequence LC712935.1 used as a control for CTX-M-producing bacteria. The evolutionary history was inferred using the neighbor-joining phylogeny (Tamura–Nei model) and analyzed with the MEGA 11 software. In this study, the definition of ESBL producers was restricted to isolates carrying the blaCTX-M gene, which is the most prevalent ESBL determinant in Iraq and neighbouring regions. Other ESBL genes such as blaTEM and blaSHV were not investigated.

Table 1.
CTX-M alleles primers of K. pneumoniae.
2.4. Extraction and Antimicrobial Activity of Leaves of Platanus orientalis Linn
Leaves of P. orientalis Linn were collected from Duhok, northern Iraq (longitude 43.10–44.10° E and latitude 26.40–37.20° N), and identified by comparison with the standard botanical description provided in the Flora of Iraq [25]. After thorough washing and shade-drying, 50 g of leaves were ground using a blender and subjected to three consecutive Soxhlet extractions with 500 mL of absolute methanol for four hours at 65 °C. The extracts were then filtered, and residual methanol was removed by rotary evaporation at 40 °C [26,27]. Antibacterial activity of the methanolic extract was assessed using the agar well-diffusion method. Wells were filled with different concentrations of the extract (125, 250, and 500 mg/mL), prepared in 2% dimethyl sulfoxide (DMSO) as the solvent, and tested against bacterial suspensions in Mueller–Hinton agar medium. All assays were performed in triplicate to ensure reproducibility.
2.5. Phytochemical Screening of Platanus orientalis Linn Leaves Extract
Phytochemical screening of the P. orientalis Linn methanolic extract was carried out to detect the presence of major secondary metabolites, including tannins, carbohydrates, terpenoids, glycosides, alkaloids, phenols, and flavonoids. Tannins were detected by mixing 2 mL of the extract with 5% aqueous ferric chloride (FeCl3), resulting in a characteristic blue-black or greenish coloration. Carbohydrates were identified using Molisch’s test, in which the extract was treated with Molisch’s reagent followed by concentrated sulfuric acid (H2SO4), producing a violet ring at the interface. Glycosides were confirmed by combining the extract with glacial acetic acid and 2% ferric chloride solution, followed by careful addition of concentrated H2SO4, yielding a brown ring at the interface. Alkaloids were detected by treating the extract with 2N hydrochloric acid (HCl) and testing with Mayer’s reagent, where cream or white precipitates indicated their presence. Phenolic compounds were identified by adding 2% ferric chloride to the extract, producing a deep bluish-black coloration. Flavonoids were detected by heating powdered plant material in ethyl acetate, filtering, and then shaking the filtrate with 10% aqueous ammonia solution, which produced a yellow fluorescence under alkaline conditions [28].
2.6. MIC of Platanus orientalis Linn Leave Extract
Minimum inhibitory concentrations (MICs) were determined using the broth microdilution method [29]. A total of 200 µL of the P. orientalis Linn leaf extract stock solution (dissolved in 2% dimethyl sulfoxide, DMSO) was added to the first well, followed by serial two-fold dilutions across the row to obtain a concentration range down to 5 μg/mL. Subsequently, 100 µL of standardized bacterial suspension was added to each well, excluding sterility control wells. The following controls were included: positive control (bacteria with medium, no extract), negative control (medium only, no bacteria or extract), extract control (plant extract with medium and resazurin, no bacteria, to detect interference), and resazurin control (medium with resazurin only, no bacteria or extract). Plates were incubated at 37 °C for 24 h, after which 20 µL of resazurin dye was added to each well and incubated for an additional 2 h. A visual assessment was performed, where a color change from blue to pink indicated bacterial growth. The MIC was defined as the lowest concentration of P. orientalis Linn extract that prevented any observable color change, indicating complete inhibition of growth. All experiments were performed in triplicate.
2.7. Biofilm Formation
Biofilm formation was evaluated using the standard microtiter plate assay [30,31]. Bacterial cultures were inoculated into Brain Heart Infusion (BHI) broth supplemented with 2% sucrose and incubated at 37 °C for 24 h. Subsequently, 20 μL of bacterial suspension was added to microtiter wells containing 180 μL of fresh BHI and incubated for another 24 h. Wells were then washed three times with sterile saline to remove non-adherent cells and stained with 1% crystal violet for 15 min. Excess dye was rinsed off, and the bound stain was solubilized with 96% ethanol. Negative control wells (medium without bacterial inoculum) were included to establish the optical density cutoff (Odc). Biofilm biomass was quantified by measuring absorbance at 630 nm using a microplate reader, and results were classified as follows: non-biofilm producer (OD ≤ Odc), weak producer (Odc < OD ≤ 2× Odc), moderate producer (2× Odc < OD ≤ 4× Odc), and strong producer (OD > 4× Odc).
2.8. Antibiofilm Formation
The antibiofilm activity of P. orientalis Linn leaf extract was evaluated using the same biofilm formation method. BHI broth was supplemented with a sub-MIC concentration of the extract prior to bacterial inoculation, while the control group contained BHI without the extract. Biofilm formation was quantified by measuring the optical density (OD) at 630 nm.
2.9. Statistical Analysis
The data were analyzed using IBM SPSS Statistics 22.0, and the findings are presented as percentages. OD data were approximately normally distributed (Shapiro–Wilk test, p > 0.05) with homogeneous variances (Levene’s test, p > 0.05), supporting the use of parametric tests. Non-parametric alternatives (Mann–Whitney U test, Spearman’s rho) yielded consistent results.
The t-test was used to compare biofilm formation between control and antibiofilm agent groups, as well as for the distribution of CTX-M alleles among strains. Spearman’s correlation analysis was employed to evaluate the relationship between biofilm formation and CTX-M-producing bacteria. Statistical significance was defined at p < 0.05.
3. Results
3.1. Isolation and Identification of Bacteria
Of the 250 clinical samples, 73 (29.2%) exhibited positive growth on MacConkey and blood agar, while 177 (70.8%) showed no growth. Among the 50 soil and water samples, 16 (32%) exhibited positive growth. Microscopy, biochemical tests, and 16S rRNA PCR confirmed the presence of K. pneumoniae in 20 (27.3%) of the clinical samples and 3 (18.7%) of the hospital premises samples. Burns and blood samples were the most frequent sources of K. pneumoniae isolates (n = 5, 21.7% each), followed by stool and urine samples (n = 4, 17.4% each). Table 2 summarizes the distribution of isolated bacteria, the number of isolates from each source, and the gender of the patients.

Table 2.
Distribution of Klebsiella pneumoniae isolates by sample source and patient gender. Values represent the number of samples analyzed and percentage of isolates obtained.
3.2. Antibiotic Susceptibility
The results of the antimicrobial susceptibility tests, presented in Table 3, indicate that the majority of bacterial isolates exhibited significant resistance to a broad spectrum of antibiotics, particularly extended-spectrum cephalosporins. All isolates were fully resistant to cefotaxime, ceftazidime, ceftriaxone, amoxicillin–clavulanate (Augmentin), levofloxacin, and amoxicillin. Additionally, 2 isolates were resistant to meropenem (8.7%), 11 isolates to imipenem (47.8%), 16 isolates to gentamicin (69.6%), 14 isolates to amikacin (60.9%), and 22 isolates to ciprofloxacin (95.7%).

Table 3.
Antimicrobial susceptibility profile of Klebsiella pneumoniae isolates (n = 23) tested by disk diffusion method according to CLSI (2020) standards. Results expressed as number (%) of isolates.
3.3. ESBL Characterization
All isolates resistant to cefotaxime (100%, n = 23) and ceftriaxone (100%, n = 23) harbor blaCTX-M genes. Sequence analysis showed similarity to the CTX-M gene family using the NCBI reference sequence LC712935.1 as a control. All ESBL-positive K. pneumoniae isolates possessed at least one blaCTX-M gene. The prevalence of blaCTX-M was 100% (23/23) among ESBL-positive K. pneumoniae isolates, and the ESBL-CTX-M alleles were as follows: blaCTX-M-15 (65.2%, n = 15), blaCTX-M-1 (30%, n = 7), and blaCTX-M-3 (0%, n = 0). The distribution of CTX-M alleles among CTX-M-producing K. pneumoniae isolates is shown in Figure 1.
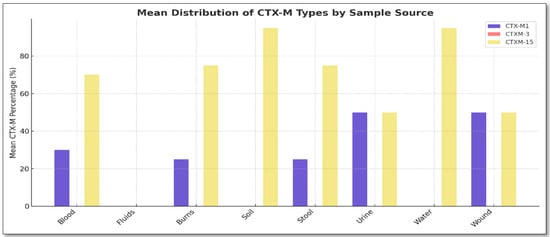
Figure 1.
Distribution of blaCTX-M alleles among the 23 ESBL-producing Klebsiella pneumoniae isolates. Prevalence: blaCTX-M-15 (65.2%), blaCTX-M-1 (30%), and blaCTX-M-3 (0%). Sources: wound (n = 2), stool (n = 4), burns (n = 5), blood (n = 5), urine (n = 4), water (n = 2), and soil (n = 1).
The evolutionary history was deduced using the Neighbor-Joining method, which showed the ideal tree with a total branch length of 1.076. Evolutionary distances were calculated using the Maximum Composite Likelihood method and are expressed as the number of base substitutions per site. The pairwise deletion method yielded a final dataset of 824 positions. Phylogenetic analysis showed that several clinical and environmental K. pneumoniae isolates clustered within the same clades, indicating close genetic relatedness. Moreover, ESBL genes (blaCTX-M-15, blaCTX-M-1, blaCTX-M-14) were distributed across multiple branches, suggesting dissemination through horizontal gene transfer rather than clonal expansion (Figure 2).
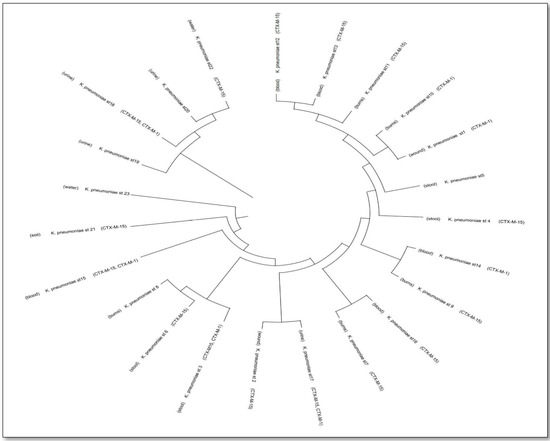
Figure 2.
Phylogenetic analysis of Klebsiella pneumoniae isolates from clinical and environmental sources highlighting clade formation and the dissemination of blaCTX-M resistance genes.
3.4. Methanolic Extract’s Antibacterial Activity
The well diffusion method demonstrated the antibacterial activity of the methanolic extract (Figure 3). A positive correlation was observed between extract concentration and the size of the inhibition zone (Figure 4). A 500 mg/mL concentration produced inhibition zones of up to 20 mm, compared to 12–15 mm for the 125 mg/mL concentration. The MIC of P. orientalis Linn extract against K. pneumoniae isolates was 64 μg/mL. This concentration inhibited visible bacterial growth and was used as a reference for subsequent antibiofilm assays.
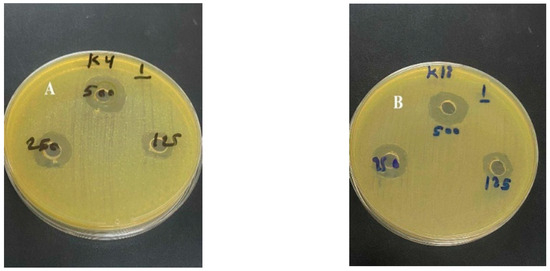
Figure 3.
Antibacterial activity of Platanus orientalis Linn methanolic leaf extract against multidrug-resistant Klebsiella pneumoniae isolates. (A) Inhibition zone observed for isolate 4; (B) inhibition zone observed for isolate 18. Wells contained different extract concentrations (125, 250, and 500 mg/mL) prepared in 2% DMSO.
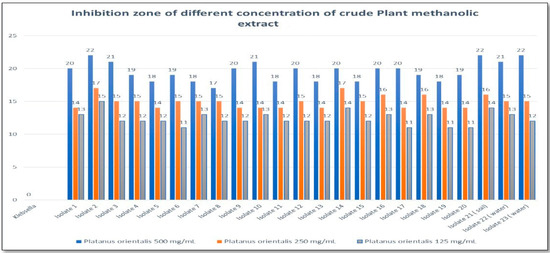
Figure 4.
Zone of inhibition (mm) for different concentrations (125, 250, and 500 mg/mL) of Platanus orientalis Linn methanolic leaf extract against multidrug-resistant Klebsiella pneumoniae isolates.
3.5. Phytochemical Screening of Platanus orientalis Linn Leaves Extract
The P. orientalis Linn extract showed the presence of phenols (indicated by a color change), alkaloids (formation of a creamy precipitate), tannins (color change and formation of a blue-black complex), glycosides (brown ring formation), carbohydrates (reddish-violet ring formation), terpenes (reddish-brown color formation), and flavonoids (yellow coloration) (Figure 5).
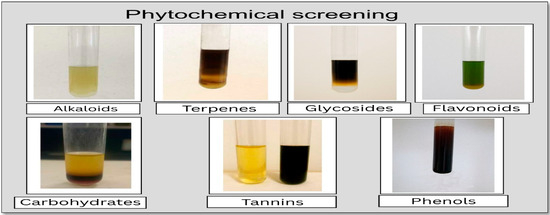
Figure 5.
Phytochemical screening of Platanus orientalis Linn methanolic leaf extract. Positive tests indicate the presence of phenols (deep blue-black color), alkaloids (cream precipitate), tannins (blue-black complex), glycosides (brown ring), carbohydrates (reddish-violet ring), terpenoids (reddish-brown color), and flavonoids (yellow coloration under alkaline conditions).
3.6. Biofilm and Anti-Biofilm Assays
The results of the biofilm assay were classified into four groups based on production capacity: strong, moderate, weak, and non-biofilm producers, according to predefined thresholds. Assumption testing confirmed approximate normality (Shapiro–Wilk test, p > 0.05) and homogeneity of variances (Levene’s test, p > 0.05), supporting the validity of subsequent statistical analyses. Optical density (OD) measurements revealed that among the K. pneumoniae isolates, 11 (47.8%) were moderate biofilm producers (Table 4). The optical density of negative controls (ODc) was 0.0523. Treatment with P. orientalis Linn methanolic extract at sub-MIC (½ MIC) concentration significantly reduced biofilm formation in K. pneumoniae isolates. Specifically, the mean OD630 of biofilm-forming isolates decreased markedly compared to untreated controls (p < 0.001). This reduction was reflected in a shift in biofilm intensity categories: moderate biofilm producers (47.8%) were completely eliminated, while non-biofilm producers increased from 4.3% to 34.8%, demonstrating the strong antibiofilm potential of the extract. Based on t-test results (t = 4.91, p = 0.000014), biofilm formation was significantly lower in isolates treated with P. orientalis extract compared to untreated isolates.

Table 4.
Biofilm formation capability of Klebsiella pneumoniae isolates (n = 23) in the absence and presence of Platanus orientalis Linn methanolic extract (at sub-MIC concentration). Results expressed as percentage (%).
Overall, in the presence of the extract, isolates predominantly exhibited non-biofilm (34.8%) and weak biofilm formation (65.2%). In contrast, untreated isolates showed higher levels of moderate (47.8%) and weak (47.8%) biofilm production. These findings suggest that P. orientalis extract effectively inhibits biofilm formation in K. pneumoniae.
3.7. Relationship Between CTX-M Production and Biofilm Formation
As shown in Figure 6, the correlation coefficient (rho) is approximately 0.493, indicating a moderate positive monotonic relationship. The corresponding p-value is very small (≈1.92 × 10−7), confirming that the correlation is statistically significant at the 0.05 level. These results suggest that as the prevalence of CTX-M-positive isolates increases, biofilm production is likely to increase as well.
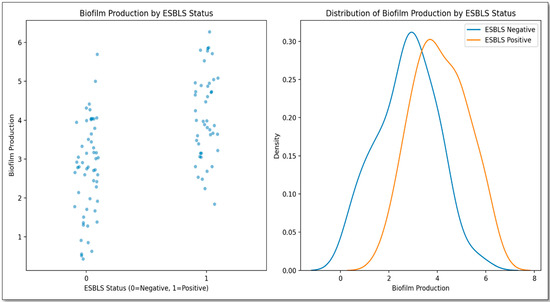
Figure 6.
Correlation between biofilm formation intensity (measured as optical density at 630 nm) and the presence of blaCTX-M genes among Klebsiella pneumoniae isolates. The scatter plot shows a positive correlation (Spearman’s rho = 0.493, p ≈ 1.92 × 10−7). Biofilm categories were classified based on OD values: non-producer (OD ≤ ODc), weak (ODc < OD ≤ 2× ODc), and moderate (2× ODc < OD ≤ 4× ODc). No strong biofilm producers were observed.
4. Discussion
This study represents the first characterization of ESBLs in Klebsiella pneumoniae from Baghdad, Iraq. The isolation rate of K. pneumoniae (8%) from clinical samples was lower than reported in some studies [32,33,34,35,36] but higher than in others [37,38]. This variation may be attributed to differences in sampling methods and the duration of patient hospitalization, as K. pneumoniae is primarily a nosocomial pathogen. Therefore, in this study, samples were collected within 48 h of admission. The prevalence of K. pneumoniae in hospital environmental samples (6%) was lower than that reported by Abbas [39].
High levels of antibiotic resistance were observed, particularly against ceftazidime (100%) and ceftriaxone (100%), consistent with previous Iraqi studies [33,40,41,42] and findings from Ethiopia [43]. In Iran, Bandari reported that 83.5% of isolates were resistant to cefotaxime [44]. The resistance patterns to amoxicillin and cephalosporins observed in our strains may be attributed to the overuse of ceftriaxone and cefotaxime in empirical hospital treatments [45,46,47]. These results highlight the widespread antibiotic resistance among K. pneumoniae isolates in Baghdad hospitals.
Meropenem showed the highest efficacy (87%), consistent with findings from a study in Sulaimani City, Iraq [45]. However, judicious use of meropenem is crucial to prevent the selection of carbapenemase-producing Enterobacteriaceae. Amikacin, although effective, is limited by its nephrotoxicity. In severe infections caused by ESBL-producing Enterobacteriaceae, carbapenems are regarded as the preferred treatment options, although partial responses to certain non-carbapenem antibiotics have occasionally been reported [48].
ESBLs are more prevalent in K. pneumoniae than in other Enterobacteriaceae [49]. The blaCTX-M gene was the predominant type (100%), consistent with findings by Tahanasab [50]. The global prevalence of blaCTX-M-producing K. pneumoniae is well established. Eskandari-Nasab investigated blaCTX-M in several countries, including Kuwait, Pakistan, the United Arab Emirates, Iran, Saudi Arabia, Turkey, and Bahrain, where prevalence rates were reported as 100%, 96.9%, 64.4%, 56.7%, 35.3%, 30%, and 10%, respectively. Different prevalence rates of blaCTX-M have also been reported in isolates from Europe, Brazil, Latin America, Russia, North America, and North Africa, with recorded percentages of 84.5%, 62.1%, 61.1%, 34.9%, 26.4%, and 7.4%, respectively [51].
In this study, blaCTX-M-15 (65.2%) and blaCTX-M-1 (30%) were the most prevalent alleles, while blaCTX-M-3 was absent. These findings differ from those reported by Salawudeen [52], Shabaa [41], and Feizabadi [53]. The clustering of clinical and environmental isolates within shared clades suggests that environmental sources may act as reservoirs for resistant K. pneumoniae, facilitating potential transmission to humans. The broad distribution of blaCTX-M variants across genetically distinct lineages further underscores the pivotal role of horizontal gene transfer in driving the dissemination of ESBL resistance.
Biofilm formation was observed in 95.6% of isolates, with 47.8% showing moderate and 47.8% showing weak biofilm production. This suggests a correlation between CTX-M production and biofilm-forming ability. Poovendran et al. [54] observed that ESBL-producing strains exhibit a significantly higher capacity for biofilm formation compared to non-ESBL producers. Moreover, the relationship between antibiotic resistance and biofilm formation has been found to be significant, increasing the risk of urinary tract infections [54], in agreement with findings from other studies [55].
Platanus orientalis Linn has recognized medicinal benefits, particularly for wound healing and the treatment of respiratory and ophthalmic conditions. These activities are attributed to its strong antibacterial and antioxidant properties, primarily due to polyphenolic flavonoids and other bioactive compounds such as kaempferol glycosides, platanoside, tiliroside, caffeic acid, flavonol glycosides, proanthocyanidin glycosides, fatty acids, and phytol derivatives, indicating its potential role in wound healing and the treatment of various health conditions [19,20,21].
In this study, the antibacterial activity of the methanolic extract of P. orientalis Linn was found to be concentration-dependent. Data presented in Figure 4 show a notable increase in inhibition zones with increasing extract concentrations. At 500 mg/mL, inhibition zones ranged between 17 and 22 mm among the tested isolates, indicating strong antibacterial activity. In contrast, lower concentrations (250 and 125 mg/mL) exhibited moderate to mild activity. The findings also demonstrated a significant reduction in biofilm formation following treatment with P. orientalis Linn extract. Our broth microdilution experiments established an MIC of 64 µg/mL for the methanolic P. orientalis leaf extract against MDR K. pneumoniae (resazurin read-out; triplicates with comprehensive controls). This MIC is congruent with the agar-well diffusion findings, where larger inhibition zones were observed at higher extract loads (17–22 mm at 500 mg/mL), reflecting dose-responsive activity.
Importantly, applying a sub-MIC (½ MIC) concentration produced a statistically significant attenuation of biofilm formation (t = 4.91, p = 0.000014), shifting isolates from moderate biofilm producers (47.8%) to predominantly weak or non-biofilm phenotypes (65.2% and 34.8%, respectively). Together, these data indicate that growth inhibition near the MIC threshold co-occurs with antibiofilm effects below the MIC, which may act via interference with quorum sensing or extracellular polymeric substance production, as suggested by our results. The observed activity is in line with the extract’s phytochemical composition (phenols, tannins, flavonoids, glycosides, alkaloids), which are known contributors to antibacterial and antibiofilm properties.
This study was limited by the relatively small sample size (n = 23), the absence of a susceptible K. pneumoniae reference strain for direct comparison, and its in vitro design without cytotoxicity or in vivo safety assessment. In addition, ESBL production was defined only by detection of blaCTX-M genes, while other determinants (e.g., TEM, SHV) were not investigated. Despite these constraints, the findings provide valuable preliminary insights with potential relevance to One Health applications and warrant further comprehensive studies.
5. Conclusions
This study provides the first comprehensive assessment in Iraq of the antimicrobial and antibiofilm activities of Platanus orientalis Linn leaf extract against multidrug-resistant Klebsiella pneumoniae. The extract exhibited significant inhibitory effects on bacterial growth and markedly reduced biofilm formation, demonstrating its potential as a promising alternative or adjunctive strategy for managing infections caused by antibiotic-resistant strains.
The detection of a 100% prevalence of blaCTX-M genes and the strong biofilm-forming capability observed in 95.6% of isolates highlight the serious clinical challenge posed by ESBL-producing K. pneumoniae in Baghdad hospitals. These findings underscore the urgent need for innovative interventions to combat the dual threat of resistance and biofilm-associated persistence. Plant-derived compounds such as those from P. orientalis, rich in phenolic compounds, flavonoids, and tannins, offer a natural and effective approach that warrants further exploration.
Overall, this research not only advances understanding of the relationship between ESBL production and biofilm formation in K. pneumoniae but also introduces P. orientalis as a potential source of bioactive compounds with significant therapeutic relevance. Further in vivo studies and toxicity assessments are essential to validate its clinical applicability and ensure safe implementation in infection management protocols.
Author Contributions
All authors contributed equally to the methodology and conceptualization of the study. A.N.A. performed the experiment and writing—original draft preparation. B.M. supervision and validation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The Data Availability Statement provided in submission is suitable. All data supporting the findings of this study are presented within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the Data Availability Statement. This change does not affect the scientific content of the article.
References
- Hu, Y.; Anes, J.; Devineau, S.; Fanning, S. Klebsiella pneumoniae: Prevalence, Reservoirs, Antimicrobial Resistance, Pathogenicity, and Infection: A Hitherto Unrecognized Zoonotic Bacterium. Foodborne Pathog. Dis. 2020, 18, 63–84. [Google Scholar] [CrossRef]
- Liu, X.; Sai, F.; Li, L.; Zhu, C.; Huang, H. Clinical characteristics and risk factors of catheter-associated urinary tract infections caused by Klebsiella pneumoniae. Ann. Palliat. Med. 2020, 9, 2668–2677. [Google Scholar] [CrossRef]
- Shariati, A.; Moradabadi, A.; Ghaznavi-Rad, E.; Dadmanesh, M.; Komijani, M.; Nojoomi, F. Investigation into antibacterial and wound healing properties of platelets lysate against Acinetobacter baumannii and Klebsiella pneumoniae burn wound infections. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 40. [Google Scholar] [CrossRef]
- Parrott, A.M.; Shi, J.; Aaron, J.; Green, D.A.; Whittier, S.; Wu, F. Detection of multiple hypervirulent Klebsiella pneumoniae strains in a New York City hospital through screening of virulence genes. Clin. Microbiol. Infect. 2020, 27, 583–589. [Google Scholar] [CrossRef]
- Mohammed, A.N.; Al–Rawi, D.F.; Buniya, H.K. Evaluation of Antibiotic Resistance of Klebsiella pneumoniae Isolated from Patients in Hospitals in Iraq. Acta Microbiol. Bulg. 2023, 39, 411–417. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Buhlin, K.; Dufrêne, Y.F.; Gomelsky, M.; Moroni, A.; Ramstedt, M.; Rumbaugh, K.P.; Schulte, T.; Sun, L.; Åkerlund, B.; et al. Biofilm formation—What we can learn from recent developments. J. Intern. Med. 2018, 284, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Sawa, T.; Kooguchi, K.; Moriyama, K. Molecular diversity of extended-spectrum β-lactamases and carbapenemases, and antimicrobial resistance. J. Intensive Care 2020, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Cantón, R.; González-Alba, J.M.; Galán, J.C. CTX-M Enzymes: Origin and diffusion. Front. Microbiol. 2012, 3, 110. [Google Scholar] [CrossRef]
- Reuter, J.; Merfort, I.; Wölfle, U.; Seelinger, G.; Schempp, C.M. Botanicals in dermatology and skin health. In Botanical Medicine: From Bench to Bedside; Mary Ann Liebert: New Rochelle, NY, USA, 2009; pp. 33–65. [Google Scholar]
- Zhao, W.-H.; Hu, Z.-Q. Epidemiology and genetics of CTX-M extended-spectrum β-lactamases in Gram-negative bacteria. Crit. Rev. Microbiol. 2012, 39, 79–101. [Google Scholar] [CrossRef]
- Maslova, E.; Eisaiankhongi, L.; Sjöberg, F.; McCarthy, R.R. Burns and biofilms: Priority pathogens and in vivo models. npj Biofilms Microbiomes 2021, 7, 73. [Google Scholar] [CrossRef]
- Dan, B.; Dai, H.; Zhou, D.; Tong, H.; Zhu, M. Relationship between drug resistance characteristics and biofilm formation in Klebsiella pneumoniae strains. Infect. Drug Resist. 2023, 16, 985–998. [Google Scholar] [CrossRef]
- Lupo, A.; Papp-Wallace, K.M.; Bonomo, R.A.; Endimiani, A. Non-Phenotypic tests to detect and characterize antibiotic resistance mechanisms in enterobacteriaceae. In Antimicrobial Resistance and Food Safety: Methods and Techniques; Academic Press: Cambridge, MA, USA, 2015; pp. 233–257. [Google Scholar] [CrossRef]
- Pazyar, N.; Yaghoobi, R.; Rafiee, E.; Mehrabian, A.; Feily, A. Skin Wound Healing and Phytomedicine: A review. Ski. Pharmacol. Physiol. 2014, 27, 303–310. [Google Scholar] [CrossRef]
- Khan, A.S. Woody Plants with Possible Anti-HIV Activity. In Medicinally Important Trees; Springer: Berlin/Heidelberg, Germany, 2017; pp. 109–131. [Google Scholar] [CrossRef]
- Carpenter, R.J.; Hill, R.S.; Jordan, G.J. Leaf cuticular morphology links Platanaceae and proteaceae. Int. J. Plant Sci. 2005, 166, 843–855. [Google Scholar] [CrossRef]
- Khalid, N. Internal Structure of Platanus (Platanus orientalis L.) Grown in Different Environments. Egypt. J. Agric. Res. 2024, 102, 103–114. [Google Scholar] [CrossRef]
- Shende, S.; Joshi, K.A.; Kulkarni, A.S.; Charolkar, C.; Shinde, V.S.; Parihar, V.S.; Kitture, R.; Banerjee, K.; Kamble, N.; Bellare, J.; et al. Platanus orientalis Leaf Mediated Rapid Synthesis of Catalytic Gold and Silver Nanoparticles. J. Nanomed. Nanotechnol. 2018, 9, 1000494. [Google Scholar] [CrossRef]
- Dogan, A.; Anuk, O.O. Investigation of the phytochemical composition and antioxidant properties of chinar (Platanus orientalis L.) leaf infusion against ethanol-induced oxidative stress in rats. Mol. Biol. Rep. 2019, 46, 3049–3061. [Google Scholar] [CrossRef] [PubMed]
- Asadbeigi, M.; Mohammadi, T.; Rafieian-Kopaei, M.; Saki, K.; Bahmani, M.; Delfan, M. Traditional effects of medicinal plants in the treatment of respiratory diseases and disorders: An ethnobotanical study in the Urmia. Asian Pac. J. Trop. Med. 2014, 7, S364–S368. [Google Scholar] [CrossRef] [PubMed]
- Niknam, S.; Rastegari, A.; Bozorgi, M.; Vahedi-Mazdabadi, Y.; Saeedi, M.; Akbarzadeh, T. In vivo evaluation of wound healing properties of Platanus orientalis L. Pharm. Sci. 2021, 28, 275–284. [Google Scholar] [CrossRef]
- Weinstein, M.P.; Lewis, J.S. The Clinical and Laboratory Standards Institute Subcommittee on Antimicrobial Susceptibility Testing: Background, organization, Functions, and Processes. J. Clin. Microbiol. 2020, 58, 10–1128. [Google Scholar] [CrossRef]
- Paulin-Curlee, G.G.; Singer, R.S.; Sreevatsan, S.; Isaacson, R.; Reneau, J.; Foster, D.; Bey, R. Genetic Diversity of Mastitis-Associated Klebsiella pneumoniae in Dairy Cows. J. Dairy. Sci. 2007, 90, 3681–3689. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2011, 18, 268–281. [Google Scholar] [CrossRef]
- Townsend, C.C. Flora of Iraq; Ministry of Agriculture, Republic of Iraq: Baghdad, Iraq, 1966; Volume 1–9. [Google Scholar]
- López-Bascón, M.A.; De Castro, M.D.L. Soxhlet Extraction. In Liquid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2019; pp. 327–354. [Google Scholar] [CrossRef]
- Loustau, E.; Rols, J.-L.; Leflaive, J.; Marcato-Romain, C.-E.; Girbal-Neuhauser, E. Comparison of extraction methods for the characterization of extracellular polymeric substances from aggregates of three biofilm-forming phototrophic microorganisms. Can. J. Microbiol. 2018, 64, 887–899. [Google Scholar] [CrossRef]
- Gokhale, M.S. Pharmacognosy; Pragati Books Pvt. Ltd.: Pune, India, 2008. [Google Scholar]
- Ohikhena, F.U.; Wintola, O.A.; Afolayan, A.J. Evaluation of the Antibacterial and Antifungal Properties of Phragmanthera capitata (Sprengel) Balle (Loranthaceae), a Mistletoe Growing on Rubber Tree, Using the Dilution Techniques. Sci. World J. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hwang, E.-H.; Park, B.-I.; Choi, N.-Y.; Kim, K.-J.; You, Y.-O. Artemisia princepsInhibits Growth, Biofilm Formation, and Virulence Factor Expression ofStreptococcus mutans. J. Med. Food 2019, 22, 623–630. [Google Scholar] [CrossRef]
- Adeosun, I.J.; Baloyi, I.T.; Cosa, S. Anti-Biofilm and Associated Anti-Virulence Activities of Selected Phytochemical Compounds against Klebsiella pneumoniae. Plants 2022, 11, 1429. [Google Scholar] [CrossRef]
- Ahmed, H.J.; Ganjo, A.R. Detection of Carbapenemase-Producing Klebsiella pneumoniae and Escherichia coli Recovered from Clinical Specimens in Erbil City Kurdistan Region of Iraq. Al-Mustansiriyah J. Sci. 2019, 30, 10–18. [Google Scholar] [CrossRef]
- Raouf, F.E.A.; Benyagoub, E.; Alkhudhairy, M.K.; Akrami, S.; Saki, M. Extended-spectrum beta-lactamases among Klebsiella pneumoniae from Iraqi patients with community-acquired pneumonia. Rev. Da Assoc. Médica Bras. 2022, 68, 833–837. [Google Scholar] [CrossRef]
- Abayneh, M.; Tesfaw, G.; Abdissa, A. Isolation of Extended-Spectrum β-lactamase- (ESBL-) Producing Escherichia coli and Klebsiella pneumoniae from Patients with Community-Onset Urinary Tract Infections in Jimma University Specialized Hospital, Southwest Ethiopia. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 4846159. [Google Scholar] [CrossRef] [PubMed]
- Nerurkar, A.; Solanky, P.; Naik, S.S. Bacterial pathogens in urinary tract infection and antibiotic susceptibility pattern. J. Pharm. Biomed. Sci. 2012, 21, 1–3. [Google Scholar]
- Jean, S.-S.; Chang, Y.-C.; Lin, W.-C.; Lee, W.-S.; Hsueh, P.-R.; Hsu, C.-W. Epidemiology, Treatment, and prevention of nosocomial bacterial pneumonia. J. Clin. Med. 2020, 9, 275. [Google Scholar] [CrossRef]
- Al-Hamed, Z.A.A.; Al-Mayahi, F.S.A. Antibiogram of Klebsiella pneumoniae that isolated from clinical and environmental samples in Al-Diwaniyah hospitals. Al-Qadisiyah J. Pure Sci. 2021, 26, 44–54. [Google Scholar] [CrossRef]
- Susethira, A.R.; Uma, A. Prevalence of klebsiella bacteriuria and antimicrobial susceptibility in a tertiary care hospital, Tiruchirapalli, India. Int. J. Pharm. Chem. Res. 2016, 8, 538–542. [Google Scholar]
- Abbas, F.M. Molecular detection of CTX-M extended spectrum beta-lactamase among carbapenem—Resistant Klebsiella pneumoniae from Al-Hillah Teaching Hospital environment, Babylon Province, Iraq. J. Phys. Conf. Ser. 2019, 1294, 062044. [Google Scholar] [CrossRef]
- Hussein, N.H.; Kareem, S.M.; Al-Kakei, S.N.H.; Taha, B.M. The predominance of Klebsiella pneumoniae carbapenemase (KPC-type) gene among high-level carbapenem-resistant Klebsiella pneumoniae isolates in Baghdad, Iraq. Mol. Biol. Rep. 2022, 49, 4653–4658. [Google Scholar] [CrossRef]
- Shabaa, R.A.H. Detection of CTX-M-1 gene Among Klebsiella pneumonia Isolates in An Najaf Province. Iraqi J. Biotechnol. 2014, 13, 128–133. [Google Scholar]
- Abed, A.N.; Ahmed, O.; Abdulmajeed, M.; Khazaal, Y.M. Evaluate Multi-Drug Resistance Pattern of Microbial Flora from Hospital Premises of Baghdad Hospitals. Teikyo Med. J. 2021, 44, 2461–2466. [Google Scholar]
- Awoke, T.; Teka, B.; Seman, A.; Sebre, S.; Yeshitela, B.; Aseffa, A.; Mihret, A.; Abebe, T. High Prevalence of Multidrug-Resistant Klebsiella pneumoniae in a Tertiary Care Hospital in Ethiopia. Antibiotics 2021, 10, 1007. [Google Scholar] [CrossRef]
- Narjes, M.B.; Keyvani, H.; Zargar, M.; Talebi, M.; Zolfaghari, M.R. Epidemiological and Genetic Overview of the Klebsiella pneumoniae Carbapenemases (KPCs) in K. pneumoniae Isolated from the Clinical Samples in Iran. Int. J. Adv. Biol. Biomed. Res. 2019, 8, 75–85. [Google Scholar] [CrossRef]
- Mohammed, A.B.; Anwar, K.A. Phenotypic and genotypic detection of extended spectrum beta lactamase enzyme in Klebsiella pneumoniae. PloS ONE 2022, 17, e0267221. [Google Scholar] [CrossRef] [PubMed]
- Van Almsick, V.; Schuler, F.; Mellmann, A.; Schwierzeck, V. The use of Long-Read Sequencing Technologies in infection control: Horizontal transfer of a BLACTX-M-27 containing LNCFII plasmid in a patient screening sample. Microorganisms 2022, 10, 491. [Google Scholar] [CrossRef] [PubMed]
- Poulou, A.; Grivakou, E.; Vrioni, G.; Koumaki, V.; Pittaras, T.; Pournaras, S.; Tsakris, A. Modified CLSI Extended-Spectrum β-Lactamase (ESBL) Confirmatory Test for Phenotypic Detection of ESBLs among Enterobacteriaceae Producing Various β-Lactamases. J. Clin. Microbiol. 2014, 52, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Schaufler, K.; Semmler, T.; Wieler, L.H.; Wöhrmann, M.; Baddam, R.; Ahmed, N.; Müller, K.; Kola, A.; Fruth, A.; Ewers, C.; et al. Clonal spread and interspecies transmission of clinically relevant ESBL-producingEscherichia coliof ST410—Another successful pandemic clone? FEMS Microbiol. Ecol. 2015, 92, fiv155. [Google Scholar] [CrossRef] [PubMed]
- Almohana, A.M.; Hadi, Z.J.; Abdul-Hadi, S. Extended spectrum ß-lactamase (ESBL) mediated resistance to third generation cephalosporins in urinary tract infection isolates of Klebseilla pneumonia. Al-Qadisiyah Med. J. 2010, 6, 142–158. [Google Scholar] [CrossRef]
- Tahanasab, Z.; Mobasherizadeh, S.; Moghadampour, M.; Rezaei, A.; Maleki, N.; Faghri, J. High Prevalence of Multiple Drug Resistance among ESBLs-Producing Klebsiella pneumoniae Isolated from Hospitalized Patients in Isfahan, Iran. J. Med. Bacteriol. 2017, 5, 29–38. [Google Scholar]
- Eskandari-Nasab, E.; Moghadampour, M.; Tahmasebi, A. Prevalence of blaCTX-M Gene among Extended- Spectrum β-Lactamases Producing Klebsiella pneumoniae Clinical Isolates in Iran: A Meta-Analysis. Iran. J. Med. Sci. 2017, 43, 347–354. [Google Scholar] [CrossRef]
- Salawudeen, A.; Desa, M.N.M.; Neoh, H.M.; Masri, S.N.; Jamaluddin, T.Z.M.T. Genomic Determinants of Resistance in Extended Spectrum Beta Lactamases (ESBLS) Klebsiella pneumoniae Isolates of a Teaching Hospital in Klang Valley. Int. J. Infect. Dis. 2023, 130, S64. [Google Scholar] [CrossRef]
- Feizabadi, M.M.; Mahamadi-Yeganeh, S.; Mirsalehian, A.; Mirafshar, S.-M.; Mahboobi, M.; Nili, F.; Yadegarinia, D. Genetic characterization of ESBL producing strains of Klebsiella pneumoniae from Tehran hospitals. J. Infect. Dev. Ctries. 2010, 4, 609–615. [Google Scholar] [CrossRef]
- Poovendran, P.; Vidhya, N.; Murugan, S. Antimicrobial susceptibility pattern of ESBL and non-ESBL producing uropathogenic Escherichia coli (UPEC) and their correlation with biofilm formation. Intl J. Microbiol. Res. 2013, 4, 56–63. [Google Scholar]
- Surgers, L.; Boyd, A.; Girard, P.-M.; Arlet, G.; Decré, D. Biofilm formation by ESBL-producing strains of Escherichia coli and Klebsiella pneumoniae. International J. Med. Microbiol. 2018, 309, 13–18. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).