Biofilm and Antimicrobial Resistance: Mechanisms, Implications, and Emerging Solutions
Abstract
1. Introduction
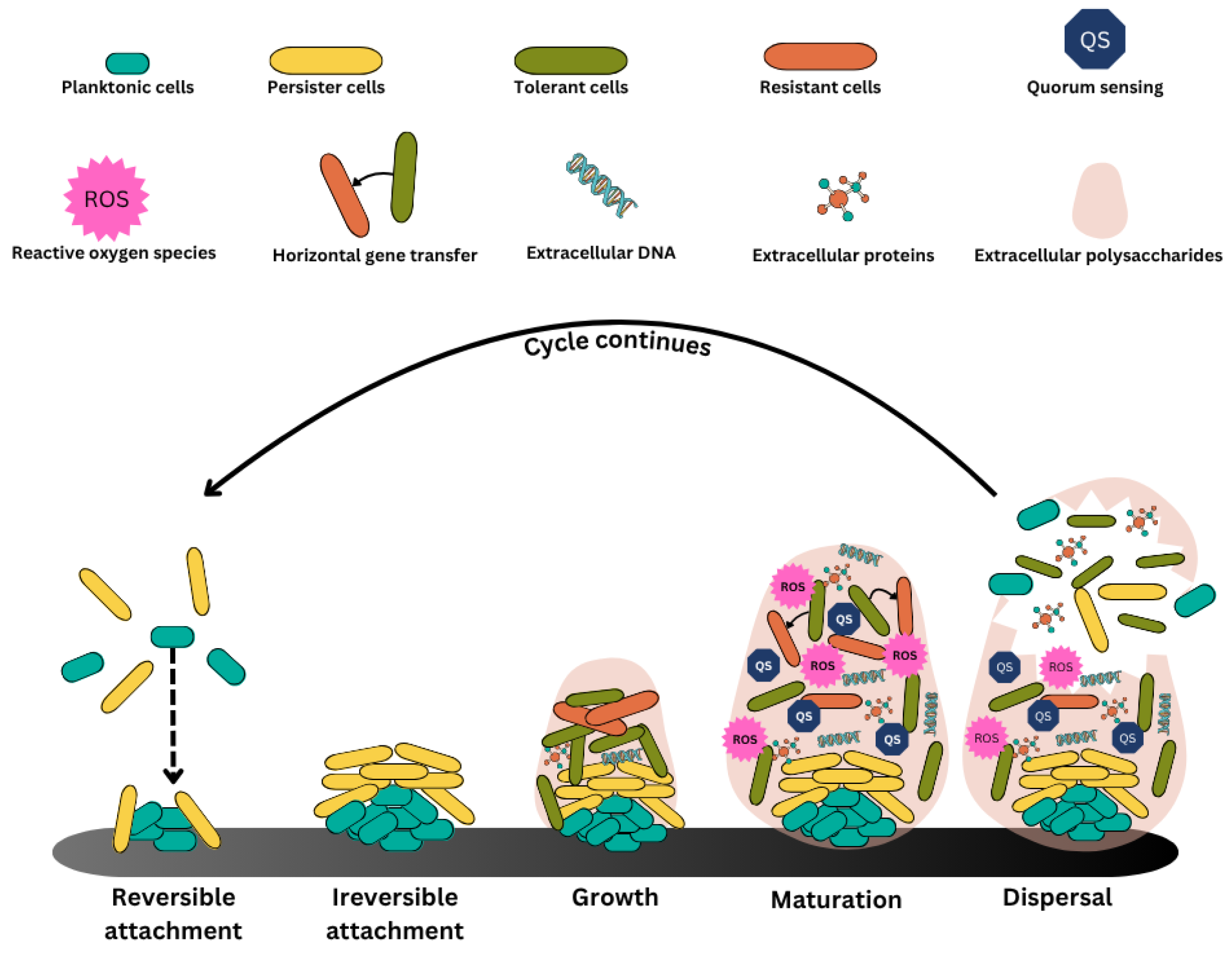
2. Formation of Biofilms
3. Quorum Sensing (QS)
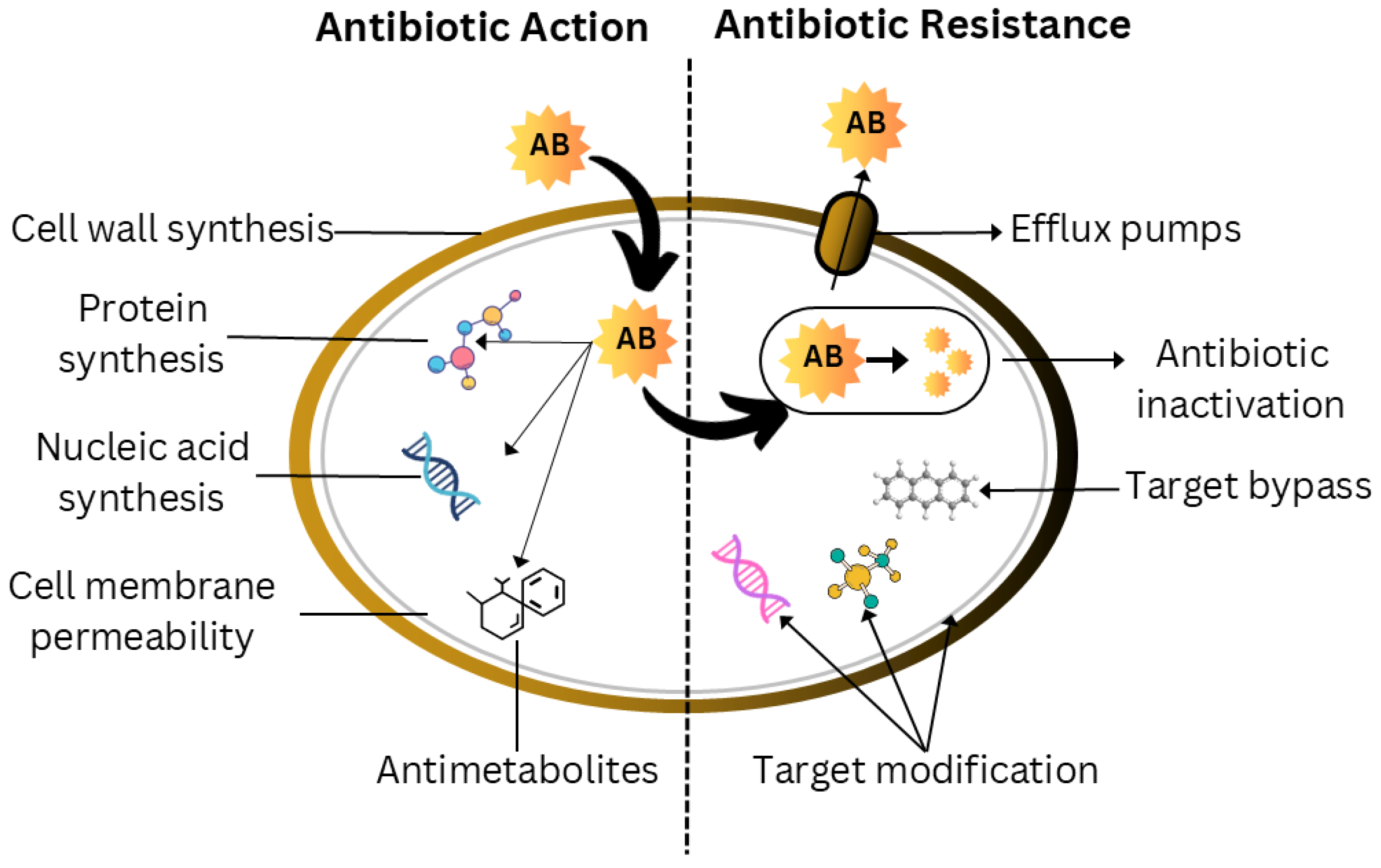
4. Biofilm Antibiotic Tolerance (BAT)
5. Antibiofilm Agents
5.1. Physical Agents
5.1.1. Nanomaterials
5.1.2. Photoinactivation
5.1.3. Organic Nanoparticles
5.1.4. Metal–Polymer Nanocomposites
Dendrimers
Cyclodextrins
5.1.5. Lipid-Based NPs and Microemulsions
5.1.6. Nanofibers
5.1.7. Responsive Smart Nanoparticles
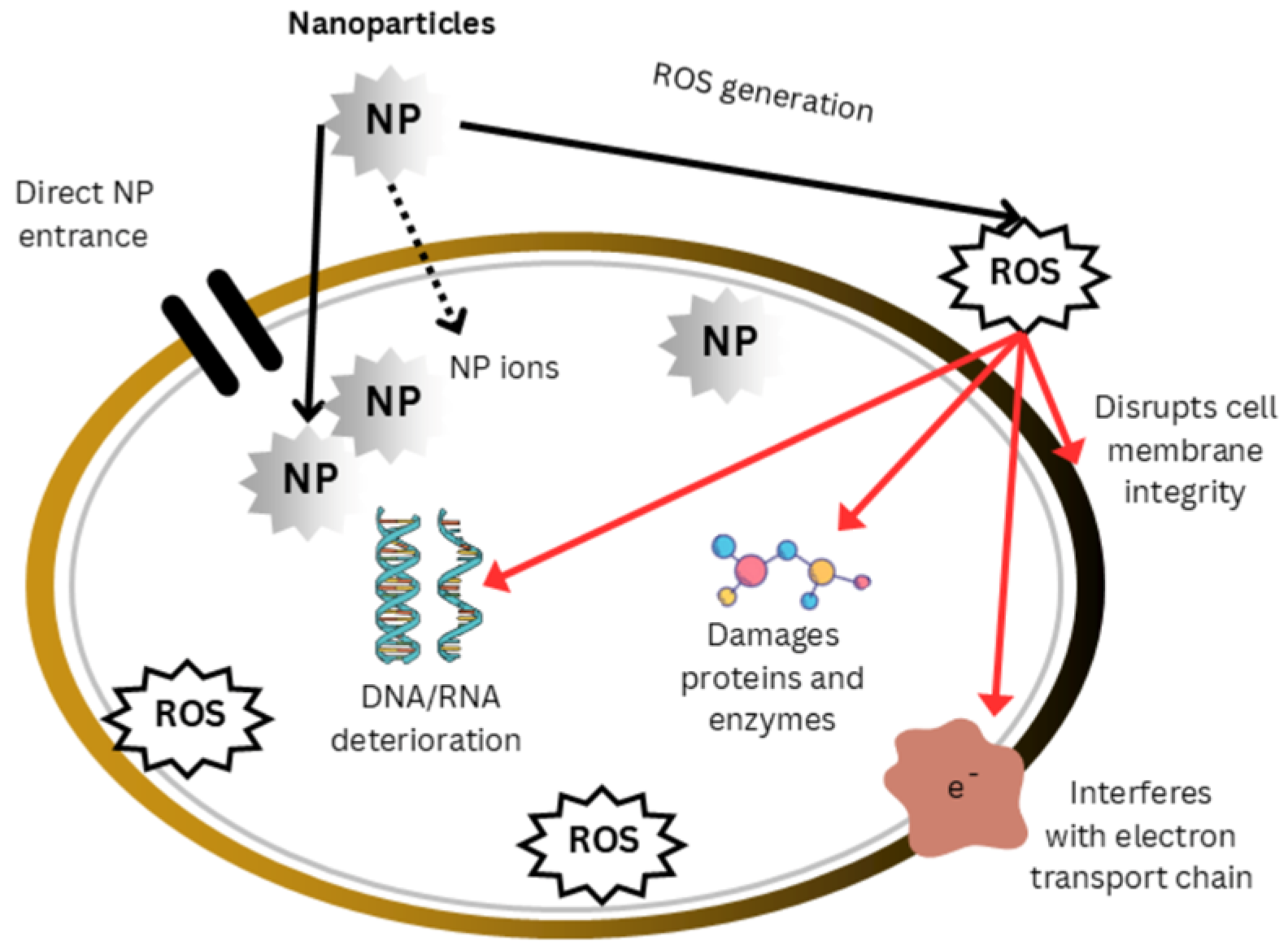
5.1.8. Mechanisms of Antimicrobial and Antibiofilm Nanoparticles
5.2. Chemical Agents
5.2.1. Phytochemicals
5.2.2. Biosurfactants
5.2.3. Quaternary Ammonium Compounds
5.2.4. Phenazines and Quinolines
5.2.5. Antibiotics
5.2.6. Nanoparticles of Natural Products for the Eradication of Biofilms
5.3. Biological Agents
5.3.1. Antimicrobial Peptides
5.3.2. Antimicrobial Lipids
5.3.3. Bacteriophages
5.3.4. Collaborative Therapy Strategy
5.3.5. CRISPR
6. Challenges and Prospects
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marshall, K.C.; Stout, R.; Mitchell, R. Mechanism of the Initial Events in the Sorption of Marine Bacteria to Surfaces. J. Gen. Microbiol. 1971, 68, 337–348. [Google Scholar] [CrossRef]
- Geesey, G.G.; Mutch, R.; Costerton, J.W.; Green, R.B. Sessile bacteria: An important component of the microbial population in small mountain streams 1. Limnol. Oceanogr. 1978, 23, 1214–1223. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wuertz, S. Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- de Vos, W.M. Microbial biofilms and the human intestinal microbiome. NPJ Biofilms Microbiomes 2015, 1, 15005. [Google Scholar] [CrossRef] [PubMed]
- Mahto, K.U.; Das, S. Bacterial biofilm and extracellular polymeric substances in the moving bed biofilm reactor for wastewater treatment: A review. Bioresour. Technol. 2022, 345, 126476. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Datta, S.; Narayanan, K.B.; Rajnish, K.N. Bacterial exo-polysaccharides in biofilms: Role in antimicrobial resistance and treatments. J. Genet. Eng. Biotechnol. 2021, 19, 140. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, J.M.; Rai, A.K.; Sharma, M.; Tripathi, M.; Prasad, R. Microbial biofilms: Recent advances and progress in environmental bioremediation. Sci. Total. Environ. 2022, 824, 153843. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. Extracellular DNA (eDNA). A Major Ubiquitous Element of the Bacterial Biofilm Architecture. Int. J. Mol. Sci. 2021, 22, 9100. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef]
- Vandana; Das, S. Genetic regulation, biosynthesis and applications of extracellular polysaccharides of the biofilm matrix of bacteria. Carbohydr. Polym. 2022, 291, 119536. [Google Scholar] [CrossRef]
- Santore, M.M. Interplay of physico-chemical and mechanical bacteria-surface interactions with transport processes controls early biofilm growth: A review. Adv. Colloid Interface Sci. 2022, 304, 102665. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.; Jangra, B.; Ashok, G.; Ravichandiran, V.; Mohan, U. Current Strategies for Combating Biofilm-Forming Pathogens in Clinical Healthcare-Associated Infections. Indian J. Microbiol. 2024, 64, 781–796. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Tang, X.; Dong, W.; Sun, N.; Yuan, W. A Review of Biofilm Formation of Staphylococcus aureus and Its Regulation Mechanism. Antibiotics 2022, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Asma, S.T.; Imre, K.; Morar, A.; Herman, V.; Acaroz, U.; Mukhtar, H.; Arslan-Acaroz, D.; Shah, S.R.A.; Gerlach, R. An Overview of Biofilm Formation–Combating Strategies and Mechanisms of Action of Antibiofilm Agents. Life 2022, 12, 1110. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-H. Nanoparticle-Based Therapies for Wound Biofilm Infection: Opportunities and Challenges. IEEE Trans. NanoBiosci. 2016, 15, 294–304. [Google Scholar] [CrossRef]
- Singh, S.; Bhatia, S. Quorum Sensing Inhibitors: Curbing Pathogenic Infections through Inhibition of Bacterial Communication. Iran J. Pharm Res. 2021, 20, 486–514. [Google Scholar] [CrossRef]
- Wu, X.; Wang, H.; Xiong, J.; Yang, G.-X.; Hu, J.-F.; Zhu, Q.; Chen, Z. Staphylococcus aureus biofilm: Formulation, regulatory, and emerging natural products-derived therapeutics. Biofilm 2024, 7, 100175. [Google Scholar] [CrossRef] [PubMed]
- Erkihun, M.; Asmare, Z.; Endalamew, K.; Getie, B.; Kiros, T.; Berhan, A. Medical Scope of Biofilm and Quorum Sensing during Biofilm Formation: Systematic Review. Bacteria 2024, 3, 118–135. [Google Scholar] [CrossRef]
- Mahto, K.U.; Kumari, S.; Das, S. Unraveling the complex regulatory networks in biofilm formation in bacteria and relevance of biofilms in environmental remediation. Crit. Rev. Biochem. Mol. Biol. 2022, 57, 305–332. [Google Scholar] [CrossRef]
- D’aquila, P.; De Rose, E.; Sena, G.; Scorza, A.; Cretella, B.; Passarino, G.; Bellizzi, D. Quorum Quenching Approaches against Bacterial-Biofilm-Induced Antibiotic Resistance. Antibiotics 2024, 13, 619. [Google Scholar] [CrossRef] [PubMed]
- Shree, P.; Singh, C.K.; Sodhi, K.K.; Surya, J.N.; Singh, D.K. Biofilms: Understanding the structure and contribution towards bacterial resistance in antibiotics. Med. Microecol. 2023, 16, 10084. [Google Scholar] [CrossRef]
- Dutt, Y.; Dhiman, R.; Singh, T.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Raj, V.S.; Chang, C.-M.; Priyadarshini, A. The Association between Biofilm Formation and Antimicrobial Resistance with Possible Ingenious Bio-Remedial Approaches. Antibiotics 2022, 11, 930. [Google Scholar] [CrossRef]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 495483. [Google Scholar] [CrossRef]
- Davenport, E.K.; Call, D.R.; Beyenal, H. Differential Protection from Tobramycin by Extracellular Polymeric Substances from Acinetobacter baumannii and Staphylococcus aureus Biofilms. Antimicrob. Agents Chemother. 2014, 58, 4755–4761. [Google Scholar] [CrossRef] [PubMed]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef]
- Wood, T.K.; Knabel, S.J.; Kwan, B.W. Bacterial Persister Cell Formation and Dormancy. Appl. Environ. Microbiol. 2013, 79, 7116–7121. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Sheikh, H.I.; Sarkar, T.; Edinur, H.A.; Pati, S.; Ray, R.R. Microbiologically-Synthesized Nanoparticles and Their Role in Silencing the Biofilm Signaling Cascade. Front. Microbiol. 2021, 12, 636588. [Google Scholar] [CrossRef]
- Raha, S. Ahmaruzzaman ZnO nanostructured materials and their potential applications: Progress, challenges and perspectives. Nanoscale Adv. 2022, 4, 1868–1925. [Google Scholar] [CrossRef]
- Moradi, F.; Ghaedi, A.; Fooladfar, Z.; Bazrgar, A. Recent advance on nanoparticles or nanomaterials with anti-multidrug resistant bacteria and anti-bacterial biofilm properties: A systematic review. Heliyon 2023, 9, e22105. [Google Scholar] [CrossRef]
- Naik, K.; Kowshik, M. Anti-biofilm efficacy of low temperature processed AgCl–TiO2 nanocomposite coating. Mater. Sci. Eng. C 2014, 34, 62–68. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, Y.-G.; Cho, M.H.; Lee, J. ZnO nanoparticles inhibit Pseudomonas aeruginosa biofilm formation and virulence factor production. Microbiol. Res. 2014, 169, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Sathiyaseelan, A.; Naveen, K.V.; Zhang, X.; Han, K.; Wang, M.-H. Research progress on chitosan-zinc oxide nanocomposites fabrication, characterization, biomedical and environmental applications. Coord. Chem. Rev. 2023, 496, 215398. [Google Scholar] [CrossRef]
- Webster, T.J.; Geilich, B.M. Reduced adhesion of Staphylococcus aureus to ZnO/PVC nanocomposites. Int. J. Nanomed. 2013, 8, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Ahire, J.J.; Hattingh, M.; Neveling, D.P.; Dicks, L.M.T.; Omri, A. Copper-Containing Anti-Biofilm Nanofiber Scaffolds as a Wound Dressing Material. PLoS ONE 2016, 11, e0152755. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Gul, A.; Zia, M.; Mukhtiar, A. Toxicity of copper oxide nanoparticles: A review study. IET Nanobiotechnol. 2020, 14, 1–13. [Google Scholar] [CrossRef]
- Ahmed, M.E.; Faiq, N.H.; Almutairi, H.H.; Alam, M.W. Biosynthesized ZnO-CuO Nanocomposite for Biofilm Formation of Proteus mirabilis upon LuxS Gene Expression. Inorganics 2025, 13, 65. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Chakrabartty, I.; Mishra, A.K.; Chopra, H.; Mahanta, S.; Avula, S.K.; Patowary, K.; Ahmed, R.; Mishra, B.; Mohanta, T.K.; et al. Nanotechnology in combating biofilm: A smart and promising therapeutic strategy. Front. Microbiol. 2023, 13, 1028086. [Google Scholar] [CrossRef]
- Chifiriuc, M.C.; Grumezescu, A.M.; Andronescu, E.; Ficai, A.; Cotar, A.I.; Grumezescu, V.; Bezirtzoglou, E.; Lazar, V.; Radulescu, R. Water dispersible magnetite nanoparticles influence the efficacy of antibiotics against planktonic and biofilm embedded Enterococcus faecalis cells. Anaerobe 2013, 22, 14–19. [Google Scholar] [CrossRef]
- Yu, Q.; Li, J.; Zhang, Y.; Wang, Y.; Liu, L.; Li, M. Inhibition of gold nanoparticles (AuNPs) on pathogenic biofilm formation and invasion to host cells. Sci. Rep. 2016, 6, 26667. [Google Scholar] [CrossRef]
- Mu, H.; Tang, J.; Liu, Q.; Sun, C.; Wang, T.; Duan, J. Potent Antibacterial Nanoparticles against Biofilm and Intracellular Bacteria. Sci. Rep. 2016, 6, srep18877. [Google Scholar] [CrossRef] [PubMed]
- Enwemeka, C.S.; Baker, T.L.; Bumah, V.V. The role of UV and blue light in photo-eradication of microorganisms. J. Photochem. Photobiol. 2021, 8, 100064. [Google Scholar] [CrossRef]
- Amos-Tautua, B.M.; Songca, S.P.; Oluwafemi, O.S. Application of Porphyrins in Antibacterial Photodynamic Therapy. Molecules 2019, 24, 2456. [Google Scholar] [CrossRef]
- Ferrer-Espada, R.; Liu, X.; Goh, X.S.; Dai, T. Antimicrobial Blue Light Inactivation of Polymicrobial Biofilms. Front. Microbiol. 2019, 10, 721. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Naskar, A.; Acharya, K.; Mandal, S. Antibiofilm and antivirulence efficacy of Pleurotus platypus methanolic extract against Staphylococcus aureus through ROS generation and cell membrane disruption. Microb. Pathog. 2024, 196, 106992. [Google Scholar] [CrossRef]
- Fulaz, S.; Vitale, S.; Quinn, L.; Casey, E. Nanoparticle–Biofilm Interactions: The Role of the EPS Matrix. Trends Microbiol. 2019, 27, 915–926. [Google Scholar] [CrossRef]
- Ramasamy, M.; Lee, J. Recent Nanotechnology Approaches for Prevention and Treatment of Biofilm-Associated Infections on Medical Devices. BioMed Res. Int. 2016, 2016, 1–17. [Google Scholar] [CrossRef]
- Haktaniyan, M.; Bradley, M. Polymers showing intrinsic antimicrobial activity. Chem. Soc. Rev. 2022, 51, 8584–8611. [Google Scholar] [CrossRef]
- Hora, J.; Hall, C.; Evans, D.; Charrault, E. Inorganic Thin Film Deposition and Application on Organic Polymer Substrates. Adv. Eng. Mater. 2017, 20, 1700868. [Google Scholar] [CrossRef]
- Depan, D.; Misra, R. On the determining role of network structure titania in silicone against bacterial colonization: Mechanism and disruption of biofilm. Mater. Sci. Eng. C 2014, 34, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.P.S.; Yazdanpanah, A.; Mozafari, M. Polymer–metal nanocomposites with antimicrobial activity. In Biocidal Polymers; De Gruyter: Berlin, Germany, 2019; pp. 83–106. [Google Scholar]
- Paul, S.; Verma, S.; Chen, Y.-C. Peptide Dendrimer-Based Antibacterial Agents: Synthesis and Applications. ACS Infect. Dis. 2024, 10, 1034–1055. [Google Scholar] [CrossRef]
- Alfei, S.; Caviglia, D. Prevention and Eradication of Biofilm by Dendrimers: A Possibility Still Little Explored. Pharmaceutics 2022, 14, 2016. [Google Scholar] [CrossRef]
- Mirza, M.A.; Aggarwal, G.; Bharti, S.; Zakir, F. Antifungal Biofilm Strategies: A Less Explored Area in Wound Management. Curr. Pharm. Biotechnol. 2022, 23, 1497–1513. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Fernandez, B.; Lopez-Viota, M.; Concheiro, A.; Alvarez-Lorenzo, C. Synergistic performance of cyclodextrin–agar hydrogels for ciprofloxacin delivery and antimicrobial effect. Carbohydr. Polym. 2011, 85, 765–774. [Google Scholar] [CrossRef]
- Alhariri, M.; Omri, A. Efficacy of Liposomal Bismuth-Ethanedithiol-Loaded Tobramycin after Intratracheal Administration in Rats with Pulmonary Pseudomonas aeruginosa Infection. Antimicrob. Agents Chemother. 2013, 57, 569–578. [Google Scholar] [CrossRef]
- Al-Adham, I.S.; Ashour, H.; Al-Kaissi, E.; Khalil, E.; Kierans, M.; Collier, P.J. Studies on the kinetics of killing and the proposed mechanism of action of microemulsions against fungi. Int. J. Pharm. 2013, 454, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Ahire, J.J.; Dicks, L.M.T. Nisin Incorporated With 2,3-Dihydroxybenzoic Acid in Nanofibers Inhibits Biofilm Formation by a Methicillin-Resistant Strain of Staphylococcus aureus. Probiotics Antimicrob. Proteins 2015, 7, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Ahire, J.J.; Dicks, L.M.T. 2,3-Dihydroxybenzoic Acid-Containing Nanofiber Wound Dressings Inhibit Biofilm Formation by Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 2098–2104. [Google Scholar] [CrossRef]
- Ahire, J.J.; Neveling, D.P.; Hattingh, M.; Dicks, L.M.T.; Coenye, T. Ciprofloxacin-Eluting Nanofibers Inhibits Biofilm Formation by Pseudomonas aeruginosa and a Methicillin-Resistant Staphylococcus aureus. PLoS ONE 2015, 10, e0123648. [Google Scholar] [CrossRef]
- An, X.; Erramilli, S.; Reinhard, B.M. Plasmonic nano-antimicrobials: Properties, mechanisms and applications in microbe inactivation and sensing. Nanoscale 2021, 13, 3374–3411. [Google Scholar] [CrossRef]
- Escudero, A.; Carrillo-Carrión, C.; Castillejos, M.C.; Romero-Ben, E.; Rosales-Barrios, C.; Khiar, N. Photodynamic therapy: Photosensitizers and nanostructures. Mater. Chem. Front. 2021, 5, 3788–3812. [Google Scholar] [CrossRef]
- Ribeiro, M.; Gomes, I.; Saavedra, M.; Simões, M. Photodynamic therapy and combinatory treatments for the control of biofilm-associated infections. Lett. Appl. Microbiol. 2022, 75, 548–564. [Google Scholar] [CrossRef]
- Khan, A.; Azam, A.; Alam, F. Gold nanoparticles enhance methylene blue– induced photodynamic therapy: A novel therapeutic approach to inhibit Candida albicans biofilm. Int. J. Nanomed. 2012, 7, 3245–3257. [Google Scholar] [CrossRef]
- Shrestha, A.; Kishen, A. Antibiofilm Efficacy of Photosensitizer-functionalized Bioactive Nanoparticles on Multispecies Biofilm. J. Endod. 2014, 40, 1604–1610. [Google Scholar] [CrossRef]
- Ahmad, F.; Salem-Bekhit, M.M.; Khan, F.; Alshehri, S.; Khan, A.; Ghoneim, M.M.; Wu, H.-F.; Taha, E.I.; Elbagory, I. Unique Properties of Surface-Functionalized Nanoparticles for Bio-Application: Functionalization Mechanisms and Importance in Application. Nanomaterials 2022, 12, 1333. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, X.; Zhen, L.; Ares, J.N.; Vackier, T.; Lange, H.; Crestini, C.; Steenackers, H.P. Effect of chemical modifications of tannins on their antimicrobial and antibiofilm effect against Gram-negative and Gram-positive bacteria. Front. Microbiol. 2023, 13, 987164. [Google Scholar] [CrossRef] [PubMed]
- Elkhalifa, M.E.; Ashraf, M.; Ahmed, A.; Usman, A.; Hamdoon, A.A.; A Elawad, M.; Almalki, M.G.; Mosa, O.F.; Niyazov, L.N.; Ayaz, M. Polyphenols and Their Nanoformulations as Potential Antibiofilm Agents Against Multidrug-Resistant Pathogens. Futur. Microbiol. 2024, 19, 255–279. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, T.H.; van Gennip, M.; Phipps, R.K.; Shanmugham, M.S.; Christensen, L.D.; Alhede, M.; Skindersoe, M.E.; Rasmussen, T.B.; Friedrich, K.; Uthe, F.; et al. Ajoene, a Sulfur-Rich Molecule from Garlic, Inhibits Genes Controlled by Quorum Sensing. Antimicrob. Agents Chemother. 2012, 56, 2314–2325. [Google Scholar] [CrossRef]
- Reiter, J.; Levina, N.; Van der Linden, M.; Gruhlke, M.; Martin, C.; Slusarenko, A.J. Diallylthiosulfinate (Allicin), a Volatile Antimicrobial from Garlic (Allium sativum), Kills Human Lung Pathogenic Bacteria, Including MDR Strains, as a Vapor. Molecules 2017, 22, 1711. [Google Scholar] [CrossRef]
- Yan, X.; Gu, S.; Shi, Y.; Cui, X.; Wen, S.; Ge, J. The effect of emodin on Staphylococcus aureus strains in planktonic form and biofilm formation in vitro. Arch. Microbiol. 2017, 199, 1267–1275. [Google Scholar] [CrossRef]
- Adeosun, I.J.; Baloyi, I.T.; Cosa, S. Anti-Biofilm and Associated Anti-Virulence Activities of Selected Phytochemical Compounds against Klebsiella pneumoniae. Plants 2022, 11, 1429. [Google Scholar] [CrossRef]
- Vikram, A.; Jesudhasan, P.R.; Pillai, S.D.; Patil, B.S. Isolimonic acid interferes with Escherichia coli O157:H7 biofilm and TTSS in QseBC and QseA dependent fashion. BMC Microbiol. 2012, 12, 261. [Google Scholar] [CrossRef]
- Vikram, A.; Jesudhasan, P.R.; Jayaprakasha, G.K.; Pillai, S.D.; Patil, B.S. Citrus limonoids interfere with Vibrio harveyi cell–cell signalling and biofilm formation by modulating the response regulator LuxO. Microbiology 2011, 157, 99–110. [Google Scholar] [CrossRef]
- Raorane, C.J.; Lee, J.-H.; Kim, Y.-G.; Rajasekharan, S.K.; García-Contreras, R.; Lee, J. Antibiofilm and Antivirulence Efficacies of Flavonoids and Curcumin Against Acinetobacter baumannii. Front. Microbiol. 2019, 10, 990. [Google Scholar] [CrossRef]
- Shehabeldine, A.M.; Ashour, R.M.; Okba, M.M.; Saber, F.R. Callistemon citrinus bioactive metabolites as new inhibitors of methicillin-resistant Staphylococcus aureus biofilm formation. J. Ethnopharmacol. 2020, 254, 112669. [Google Scholar] [CrossRef]
- Samoilova, Z.; Tyulenev, A.; Muzyka, N.; Smirnova, G.; Oktyabrsky, O. Tannic and gallic acids alter redox-parameters of the medium and modulate biofilm formation. AIMS Microbiol. 2019, 5, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Gartika, M.; Pramesti, H.T.; Kurnia, D.; Satari, M.H. A terpenoid isolated from sarang semut (Myrmecodia pendans) bulb and its potential for the inhibition and eradication of Streptococcus mutans biofilm. BMC Complement. Altern. Med. 2018, 18, 151. [Google Scholar] [CrossRef] [PubMed]
- Vashistt, J. Study on Natural Antimicrobial Compounds Against Bacterial Isolates. Ph.D. Thesis, Jaypee University, Solan, India, 2022. [Google Scholar]
- Ćirić, A.; Petrović, J.; Glamočlija, J.; Smiljković, M.; Nikolić, M.; Stojković, D.; Soković, M.D. Natural products as biofilm formation antagonists and regulators of quorum sensing functions: A comprehensive review update and future trends. S. Afr. J. Bot. 2019, 120, 65–80. [Google Scholar] [CrossRef]
- Lu, L.; Hu, W.; Tian, Z.; Yuan, D.; Yi, G.; Zhou, Y.; Cheng, Q.; Zhu, J.; Li, M. Developing natural products as potential anti-biofilm agents. Chin. Med. 2019, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-B.; Wang, S.; Wang, C.; Huang, Q.-Y.; Bai, J.-W.; Chen, J.-Q.; Chen, X.-Y.; Li, Y.-H. Emodin affects biofilm formation and expression of virulence factors in Streptococcus suis ATCC700794. Arch. Microbiol. 2015, 197, 1173–1180. [Google Scholar] [CrossRef]
- Mishra, R.; Panda, A.K.; De Mandal, S.; Shakeel, M.; Bisht, S.S.; Khan, J. Natural Anti-biofilm Agents: Strategies to Control Biofilm-Forming Pathogens. Front. Microbiol. 2020, 11, 566325. [Google Scholar] [CrossRef]
- Adnan, M.; Patel, M.; Deshpande, S.; Alreshidi, M.; Siddiqui, A.J.; Reddy, M.N.; Emira, N.; De Feo, V. Effect of Adiantum philippense Extract on Biofilm Formation, Adhesion With Its Antibacterial Activities Against Foodborne Pathogens, and Characterization of Bioactive Metabolites: An in vitro-in silico Approach. Front. Microbiol. 2020, 11, 823. [Google Scholar] [CrossRef]
- Yahya, M.F.Z.R. Anti-biofilm Potential and Mode of Action of Malaysian Plant Species: A Review. Sci. Lett. 2020, 14, 34. [Google Scholar] [CrossRef]
- Satputea, S.K.; Banpurkar, A.G.; Banat, I.M.; Sangshetti, J.N.; Patil, R.H.; Gade, W.N. Multiple Roles of Biosurfactants in Biofilms. Curr. Pharm. Des. 2016, 22, 1429–1448. [Google Scholar] [CrossRef]
- Yan, X.; Gu, S.; Cui, X.; Shi, Y.; Wen, S.; Chen, H.; Ge, J. Antimicrobial, anti-adhesive and anti-biofilm potential of biosurfactants isolated from Pediococcus acidilactici and Lactobacillus plantarum against Staphylococcus aureus CMCC26003. Microb. Pathog. 2019, 127, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Ohadi, M.; Forootanfar, H.; Dehghannoudeh, G.; Eslaminejad, T.; Ameri, A.; Shakibaie, M.; Adeli-Sardou, M. Antimicrobial, anti-biofilm, and anti-proliferative activities of lipopeptide biosurfactant produced by Acinetobacter junii B6. Microb. Pathog. 2020, 138, 103806. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, M.M.; Al-Omar, M.S.; Mohammed, H.A.; Emam, T.M. In Vitro and Ex Vivo Antibiofilm Activity of a Lipopeptide Biosurfactant Produced by the Entomopathogenic Beauveria bassiana Strain against Microsporum canis. Microorganisms 2020, 8, 232. [Google Scholar] [CrossRef]
- Janek, T.; Drzymała, K.; Dobrowolski, A. In vitro efficacy of the lipopeptide biosurfactant surfactin-C15 and its complexes with divalent counterions to inhibit Candida albicans biofilm and hyphal formation. Biofouling 2020, 36, 210–221. [Google Scholar] [CrossRef]
- Karlapudi, A.P.; Venkateswarulu, T.C.; Srirama, K.; Kota, R.K.; Mikkili, I.; Kodali, V.P. Evaluation of anti-cancer, anti-microbial and anti-biofilm potential of biosurfactant extracted from an Acinetobacter M6 strain. J. King Saud Univ.—Sci. 2020, 32, 223–227. [Google Scholar] [CrossRef]
- Abdollahi, S.; Tofighi, Z.; Babaee, T.; Shamsi, M.; Rahimzadeh, G.; Rezvanifar, H.; Saeidi, E.; Amiri, M.M.; Ashtiani, Y.S.; Samadi, N. Evaluation of Anti-oxidant and Anti-biofilm Activities of Biogenic Surfactants Derived from Bacillus amyloliquefaciens and Pseudomonas aeruginosa. IJ Pharm. Res. 2018, 19, 115–126. [Google Scholar]
- Elshikh, M.; Funston, S.; Chebbi, A.; Ahmed, S.; Marchant, R.; Banat, I.M. Rhamnolipids from non-pathogenic Burkholderia thailandensis E264: Physicochemical characterization, antimicrobial and antibiofilm efficacy against oral hygiene related pathogens. New Biotechnol. 2017, 36, 26–36. [Google Scholar] [CrossRef]
- Sacco, L.P.; Castellane, T.C.L.; Polachini, T.C.; Lemos, E.G.d.M.; Alves, L.M.C. Exopolysaccharides produced by Pandoraea shows emulsifying and anti-biofilm activities. J. Polym. Res. 2019, 26, 91. [Google Scholar] [CrossRef]
- Sharahi, J.Y.; Azimi, T.; Shariati, A.; Safari, H.; Tehrani, M.K.; Hashemi, A. Advanced strategies for combating bacterial biofilms. J. Cell. Physiol. 2019, 234, 14689–14708. [Google Scholar] [CrossRef]
- Satpute, S.K.; Mone, N.S.; Das, P.; Banat, I.M.; Banpurkar, A.G. Inhibition of pathogenic bacterial biofilms on PDMS based implants by L. acidophilus derived biosurfactant. BMC Microbiol. 2019, 19, 39. [Google Scholar] [CrossRef]
- Saverina, E.A.; Frolov, N.A.; Kamanina, O.A.; Arlyapov, V.A.; Vereshchagin, A.N.; Ananikov, V.P. From Antibacterial to Antibiofilm Targeting: An Emerging Paradigm Shift in the Development of Quaternary Ammonium Compounds (QACs). ACS Infect. Dis. 2023, 9, 394–422. [Google Scholar] [CrossRef]
- Jennings, M.C.; Ator, L.E.; Paniak, T.J.; Minbiole, K.P.C.; Wuest, W.M. Biofilm-Eradicating Properties of Quaternary Ammonium Amphiphiles: Simple Mimics of Antimicrobial Peptides. ChemBioChem 2014, 15, 2211–2215. [Google Scholar] [CrossRef] [PubMed]
- Forman, M.E.; Jennings, M.C.; Wuest, W.M.; Minbiole, K.P.C. Building a Better Quaternary Ammonium Compound (QAC): Branched Tetracationic Antiseptic Amphiphiles. ChemMedChem 2016, 11, 1401–1405. [Google Scholar] [CrossRef]
- Nadar, S.; Khan, T.; Patching, S.G.; Omri, A. Development of Antibiofilm Therapeutics Strategies to Overcome Antimicrobial Drug Resistance. Microorganisms 2022, 10, 303. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, L.A.; Newman, D.K. Both toxic and beneficial effects of pyocyanin contribute to the lifecycle of Pseudomonas aeruginosa. Mol. Microbiol. 2018, 110, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, L.E.P.; Okegbe, C.; Price-Whelan, A.; Sakhtah, H.; Hunter, R.C.; Newman, D.K. Bacterial Community Morphogenesis Is Intimately Linked to the Intracellular Redox State. J. Bacteriol. 2013, 195, 1371–1380. [Google Scholar] [CrossRef]
- Garrison, A.T.; Bai, F.; Abouelhassan, Y.; Paciaroni, N.G.; Jin, S.; Iii, R.W.H. Bromophenazine derivatives with potent inhibition, dispersion and eradication activities against Staphylococcus aureus biofilms. RSC Adv. 2015, 5, 1120–1124. [Google Scholar] [CrossRef]
- Garrison, A.T.; Abouelhassan, Y.; Kallifidas, D.; Bai, F.; Ukhanova, M.; Mai, V.; Jin, S.; Luesch, H.; Huigens, R.W. Halogenated Phenazines that Potently Eradicate Biofilms, MRSA Persister Cells in Non-Biofilm Cultures, and Mycobacterium tuberculosis. Angew. Chem. Int. Ed. Engl. 2015, 54, 14819–14823. [Google Scholar] [CrossRef]
- Basak, A.; Abouelhassan, Y.; Norwood, V.M.; Bai, F.; Nguyen, M.T.; Jin, S.; Huigens, R.W. Synthetically Tuning the 2-Position of Halogenated Quinolines: Optimizing Antibacterial and Biofilm Eradication Activities via Alkylation and Reductive Amination Pathways. Chem.—Eur. J. 2016, 22, 9181–9189. [Google Scholar] [CrossRef]
- Zhang, F.; Cheng, W. The Mechanism of Bacterial Resistance and Potential Bacteriostatic Strategies. Antibiotics 2022, 11, 1215. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, I.; Favini-Stabile, S.; Dessen, A. Resistance to antibi—Otics targeted to the bacterial cell wall. Protein Sci. 2014, 23, 243–259. [Google Scholar] [CrossRef]
- Husna, A.; Rahman, M.; Badruzzaman, A.T.M.; Sikder, M.H.; Islam, M.R.; Rahman, T.; Alam, J.; Ashour, H.M. Extended-Spectrum β-Lactamases (ESBL): Challenges and Opportunities. Biomedicines 2023, 11, 2937. [Google Scholar] [CrossRef]
- Lee, V.C. The Antibiotic Resistance Crisis Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Tsai, K.; Stojković, V.; Lee, D.J.; Young, I.D.; Szal, T.; Klepacki, D.; Vázquez-Laslop, N.; Mankin, A.S.; Fraser, J.S.; Fujimori, D.G. Structural basis for context-specific inhibition of translation by oxazolidinone antibiotics. Nat. Struct. Mol. Biol. 2022, 29, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Zou, W.; Zhong, Y. Sulfonamides repress cell division in the root apical meristem by inhibiting folates synthesis. J. Hazard. Mater. Adv. 2022, 5, 100045. [Google Scholar] [CrossRef]
- Peng, L.; Zeng, L.; Jin, H.; Yang, L.; Xiao, Y.; Lan, Z.; Yu, Z.; Ouyang, S.; Zhang, L.; Sun, N. Discovery and antibacterial study of potential PPK1 inhibitors against uropathogenic E. coli. J. Enzym. Inhib. Med. Chem. 2020, 35, 1224–1232. [Google Scholar] [CrossRef]
- Díaz-Puertas, R.; Álvarez-Martínez, F.J.; Falco, A.; Barrajón-Catalán, E.; Mallavia, R. Phytochemical-Based Nanomaterials against Antibiotic-Resistant Bacteria: An Updated Review. Polymers 2023, 15, 1392. [Google Scholar] [CrossRef]
- Nwafor, I.R.; Alhassan, Y.; Udoh, J.I.; Odanibeh, D.; Oyaniyi, J.; Efoli-Bam, V.K.; Azubuike, E.O.; Ojobor, J.-F.C.; Nwokafor, C.V. Plant-derived Bioactive Compounds and Their Mechanistic Roles in Combating Microbial Biofilms. Microbiol. Res. J. Int. 2024, 34, 74–85. [Google Scholar] [CrossRef]
- Asfour, H.Z. Anti-quorum sensing natural compounds. J. Microsc. Ultrastruct. 2018, 6, 1–10. [Google Scholar] [CrossRef]
- Karaca, A.C.; Rezaei, A.; Qamar, M.; Assadpour, E.; Esatbeyoglu, T.; Jafari, S.M. Lipid-based nanodelivery systems of curcumin: Recent advances, approaches, and applications. Food Chem. 2024, 463, 141193. [Google Scholar] [CrossRef]
- Hirt, H.; Hall, J.W.; Larson, E.; Gorr, S.-U.; Kreth, J. A D-enantiomer of the antimicrobial peptide GL13K evades antimicrobial resistance in the Gram positive bacteria Enterococcus faecalis and Streptococcus gordonii. PLoS ONE 2018, 13, e0194900. [Google Scholar] [CrossRef] [PubMed]
- Shahrour, H.; Ferrer-Espada, R.; Dandache, I.; Bárcena-Varela, S.; Sánchez-Gómez, S.; Chokr, A.; Martinez-de-Tejada, G. AMPs as Anti-biofilm Agents for Human Therapy and Prophylaxis. In Antimicrobial Peptides: Basics for Clinical Application; Springer: Berlin/Heidelberg, Germany, 2019; pp. 257–279. [Google Scholar]
- Yuan, Y.; Zai, Y.; Xi, X.; Ma, C.; Wang, L.; Zhou, M.; Shaw, C.; Chen, T. A novel membrane-disruptive antimicrobial peptide from frog skin secretion against cystic fibrosis isolates and evaluation of anti-MRSA effect using Galleria mellonella model. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2019, 1863, 849–856. [Google Scholar] [CrossRef]
- Mohammadi, M.; Taheri, B.; Momenzadeh, N.; Salarinia, R.; Nabipour, I.; Farshadzadeh, Z.; Bargahi, A. Identification and Characterization of Novel Antimicrobial Peptide from Hippocampus comes by In Silico and Experimental Studies. Mar. Biotechnol. 2018, 20, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xi, X.; Ma, C.; Chen, X.; Zhou, M.; Burrows, J.F.; Chen, T.; Wang, L. A Novel Dermaseptin Isolated from the Skin Secretion of Phyllomedusa tarsius and Its Cationicity-Enhanced Analogue Exhibiting Effective Antimicrobial and Anti-Proliferative Activities. Biomolecules 2019, 9, 628. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Wu, Y.; Wang, L.; Ma, C.; Xi, X.; Bininda-Emonds, O.R.; Shaw, C.; Chen, T.; Zhou, M. Evaluation of the bioactivity of a mastoparan peptide from wasp venom and of its analogues designed through targeted engineering. Int. J. Biol. Sci. 2018, 14, 599–607. [Google Scholar] [CrossRef]
- Khozani, R.S.; Shahbazzadeh, D.; Harzandi, N.; Feizabadi, M.M.; Bagheri, K.P. Kinetics Study of Antimicrobial Peptide, Melittin, in Simultaneous Biofilm Degradation and Eradication of Potent Biofilm Producing MDR Pseudomonas aeruginosa Isolates. Int. J. Pept. Res. Ther. 2019, 25, 329–338. [Google Scholar] [CrossRef]
- de Barros, E.; Gonçalves, R.M.; Cardoso, M.H.; Santos, N.C.; Franco, O.L.; Cândido, E.S. Snake Venom Cathelicidins as Natural Antimicrobial Peptides. Front. Pharmacol. 2019, 10, 1415. [Google Scholar] [CrossRef]
- Basso, V.; Tran, D.Q.; Schaal, J.B.; Tran, P.; Eriguchi, Y.; Ngole, D.; Cabebe, A.E.; Park, A.Y.; Beringer, P.M.; Ouellette, A.J.; et al. Rhesus Theta Defensin 1 Promotes Long Term Survival in Systemic Candidiasis by Host Directed Mechanisms. Sci. Rep. 2019, 9, 16905. [Google Scholar] [CrossRef]
- von Borowski, R.G.; Chat, S.; Schneider, R.; Nonin-Lecomte, S.; Bouaziz, S.; Giudice, E.; Zimmer, A.R.; Gnoatto, S.C.; Macedo, A.J.; Gillet, R. First-in-class matrix anti-assembly peptide prevents staphylococcal biofilm in vitro and in vivo. bioRxiv. 2020. [Google Scholar] [CrossRef]
- Casciaro, B.; Cappiello, F.; Loffredo, M.R.; Ghirga, F.; Mangoni, M.L. The Potential of Frog Skin Peptides for Anti-Infective Therapies: The Case of Esculentin-1a(1-21)NH2. Curr. Med. Chem. 2020, 27, 1405–1419. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Santhakumari, S.; Poonguzhali, P.; Geetha, M.; Dyavaiah, M.; Xiangmin, L. Bacterial Biofilm Inhibition: A Focused Review on Recent Therapeutic Strategies for Combating the Biofilm Mediated Infections. Front. Microbiol. 2021, 12, 676458. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.-J. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef] [PubMed]
- Schlievert, P.M.; Peterson, M.L.; Kaufmann, G.F. Glycerol Monolaurate Antibacterial Activity in Broth and Biofilm Cultures. PLoS ONE 2012, 7, e40350. [Google Scholar] [CrossRef]
- Lopes, L.Q.S.; Vaucher, R.d.A.; Giongo, J.L.; Gündel, A.; Santos, R.C.V. Characterisation and anti-biofilm activity of glycerol monolaurate nanocapsules against Pseudomonas aeruginosa. Microb. Pathog. 2019, 130, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhou, Z.; Dong, J.; Zhang, J.; Xia, Y.; Shu, R. Antibacterial and antibiofilm activities of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) against periodontopathic bacteria. Microb. Pathog. 2016, 99, 196–203. [Google Scholar] [CrossRef]
- Sun, M.; Dong, J.; Xia, Y.; Shu, R. Antibacterial activities of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) against planktonic and biofilm growing Streptococcus mutans. Microb. Pathog. 2017, 107, 212–218. [Google Scholar] [CrossRef]
- Jameel, F.; Agarwal, P.; Ahmad, R.; Siddiqui, S.; Serajuddin, M. Synergistic anticancer effects of omega-3 fatty acids (EPA/DHA) and anticancer drug Doxorubicin against human lung adenocarcinoma. Food Biosci. 2024, 61, 104710. [Google Scholar] [CrossRef]
- Bai, J.; Kim, Y.-T.; Ryu, S.; Lee, J.-H. Biocontrol and Rapid Detection of Food-Borne Pathogens Using Bacteriophages and Endolysins. Front. Microbiol. 2016, 7, 474. [Google Scholar] [CrossRef]
- Palafox-Rivera, P.; Tapia-Rodriguez, M.R.; Lopez-Romero, J.C.; Lugo-Flores, M.A.; Quintero-Cabello, K.P.; Silva-Espinoza, B.A.; Cruz-Valenzuela, M.R.; Nazzaro, F.; Ayala-Zavala, J.F. Exploring the potential of hydrolytic enzymes combined with antibacterial agents to disrupt pathogenic biofilms and disinfect released cells. Biofouling 2025, 41, 131–143. [Google Scholar] [CrossRef]
- Alipour-Khezri, E.; Skurnik, M.; Zarrini, G. Pseudomonas aeruginosa Bacteriophages and Their Clinical Applications. Viruses 2024, 16, 1051. [Google Scholar] [CrossRef]
- Ferriol-González, C.; Domingo-Calap, P. Phages for Biofilm Removal. Antibiotics 2020, 9, 268. [Google Scholar] [CrossRef]
- Vrancianu, C.O.; Gheorghe, I.; Dobre, E.-G.; Barbu, I.C.; Cristian, R.E.; Popa, M.; Lee, S.H.; Limban, C.; Vlad, I.M.; Chifiriuc, M.C. Emerging Strategies to Combat β-Lactamase Producing ESKAPE Pathogens. Int. J. Mol. Sci. 2020, 21, 8527. [Google Scholar] [CrossRef]
- Davies, D.T.; Everett, M. Designing Inhibitors of β-Lactamase Enzymes to Overcome Carbapenem Resistance in Gram-Negative Bacteria. Accounts Chem. Res. 2021, 54, 2055–2064. [Google Scholar] [CrossRef] [PubMed]
- Pirlar, R.F.; Halili, N.; Travnik, T.; Trampuz, A.; Karbysheva, S. In vitro activity of cefiderocol against Gram-negative aerobic bacilli in planktonic and biofilm form–alone and in combination with bacteriophages. Sci. Rep. 2025, 15, 17105. [Google Scholar] [CrossRef]
- El-Din, E.M.R.S.; Zaki, B.M.; El-Aziz, A.M.A.; Ali, Y.M. Bacteriophage-Antibiotic Synergy Enhances Therapeutic Efficacy Against Multidrug-Resistant Klebsiella pneumoniae Infections. J. Appl. Microbiol. 2025, 136, lxaf131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.; Huang, X.-H.; Wong, W.-L.; Luo, J.-R.; Guo, X.-C.; Liu, W.; Hou, J.; She, M.-T.; Jiang, W.-H.; Sun, N.; et al. A red fluorescent small-molecule for visualization of higher-order cyclic dimeric guanosine monophosphate (c-di-GMP) structure in live bacterial cells and real-time monitoring of biofilm formation on biotic and abiotic surfaces. Sens. Actuators B Chem. 2022, 376, 132992. [Google Scholar] [CrossRef]
- Gou, Y.; Liu, W.; Wang, J.J.; Tan, L.; Hong, B.; Guo, L.; Liu, H.; Pan, Y.; Zhao, Y. CRISPR-Cas9 knockout of qseB induced asynchrony between motility and biofilm formation in Escherichia coli. Can. J. Microbiol. 2019, 65, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Zuberi, A.; Ahmad, N.; Khan, A.U. CRISPRi Induced Suppression of Fimbriae Gene (fimH) of a Uropathogenic Escherichia coli: An Approach to Inhibit Microbial Biofilms. Front. Immunol. 2017, 8, 1552. [Google Scholar] [CrossRef] [PubMed]
- Zuberi, A.; Misba, L.; Khan, A.U. CRISPR Interference (CRISPRi) Inhibition of luxS Gene Expression in E. coli: An Approach to Inhibit Biofilm. Front. Cell. Infect. Microbiol. 2017, 7, 214. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.; Nilyanimit, P.; Kozlova, E.; Anderson, E.R.; Narra, H.P.; Sahni, S.K.; Heinz, E.; Hughes, G.L.; Bonizzoni, M. CRISPR/Cas9-mediated gene deletion of the ompA gene in symbiotic Cedecea neteri impairs biofilm formation and reduces gut colonization of Aedes aegypti mosquitoes. PLOS Neglected Trop. Dis. 2019, 13, e0007883. [Google Scholar] [CrossRef]
| Compound | Source | Pathogen | Minimum Inhibitory Concentration (MIC) | References |
|---|---|---|---|---|
| Ajoene | Allium sativum L. | P. aeruginosa S. aureus | 80 μg/mL | [69] |
| Allicin | Allium sativum L. | P. aeruginosa | 64 μg/mL | [70] |
| Emodin | Polygonum cuspidatum | S. aureus | 4–8 μg/mL | [71] |
| Phytol | Piper betle L. | K. pneumoniae | 0.125 mg/mL | [72] |
| Isolimonic acid and ichangin | Citrus Species | EHEC | 19.7 μM & 28.3 μM respectively | [73,74] |
| Curcumin | Curcuma longa L. | A. baumannii, C. albicans | Less than 500 μg/mL | [75] |
| Pulverulentone A | Callistemon citrinus | MRSA | 125 μg/mL | [76] |
| Tannic acid | - | E. coli | 1 mg/mL | [77] |
| Diterpene derivative | Myrmecodia pendens | Streptococcus mutans | 40 ppm | [78] |
| Biosurfactant | Source | Pathogen | Inhibitory Concentration | References |
|---|---|---|---|---|
| Lipopeptide | Acinetobacter junii | S. aureus, P. mirabilis, and P. aeruginosa | 1250 μg/mL | [88] |
| Lipopeptide | Beauveria bassiana | Microsporum canis | 1.95 μg/mL | [89] |
| Lipopeptide surfactin—C15 | B. subtilis | C. albicans | 960 μg/mL | [90] |
| Glycolipoprotein | Acinetobacter indicus M6 | MRSA | 500 μg/mL | [91] |
| Rhamnolipids | P. aeruginosa MN1 | S. mutans | 12.5 mg/mL | [92] |
| Rhamnolipids | B. thailandensis E264 | S. oralis, A. naeslundii, N. mucosa, and S. sanguinis | 0.39 mg/mL | [93] |
| Exopolysaccharides | P. pnomenusa MS5 | B. cepacia | 0.25 mg/mL | [94] |
| AMP | Amino Acids Sequence | Source | Effect | References |
|---|---|---|---|---|
| Japonicin-2LF | FIVPSIFLLK KAFCIALKKC | Frog skin | Eradicates MRSA biofilm matrix | [119] |
| Moronecidin-like | FFRNLWKGAK AAFRAGHAAWRA | Sea horse | Inhibit S. aureus biofilms | [120] |
| Dermaseptin-PT9 | GLWSKIKDAAKT AGKAALGFVNEMV | Frog skin | Inhibits S. aureus, MRSA, and E. coli biofilm formation | [121] |
| Mastoporan | LNLKALL AVAKKIL | European hornet venom | Inhibit S. aureus and P. aeruginosa biofilm | [122] |
| Melittin | GIGAVLKVLTTG LPALISWIKRKRQQ | Honeybee venom | Inhibit MDR aeruginosa | [123] |
| NA-CATH | KRFKKFFKKLKNSV KKRAKKFFKKPKVIGVTFPF | Chinese cobra | Inhibit Burkholderia thailandensis biofilm | [124] |
| Rhesus theta defensin-1 | GFCRCLCRRGVCRCICTR | Monkey leukocytes | Inhibit C. albicans | [125] |
| Capsicumicine | RSCQQQIQQ AQQLSSCQQYLKQ | Red pepper | Inhibit S. epidermis biofilm | [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, B.; Dahiya, M.; Kumar, V.; Ayyagari, A.; Chaudhari, D.N.; Ahire, J.J. Biofilm and Antimicrobial Resistance: Mechanisms, Implications, and Emerging Solutions. Microbiol. Res. 2025, 16, 183. https://doi.org/10.3390/microbiolres16080183
Singh B, Dahiya M, Kumar V, Ayyagari A, Chaudhari DN, Ahire JJ. Biofilm and Antimicrobial Resistance: Mechanisms, Implications, and Emerging Solutions. Microbiology Research. 2025; 16(8):183. https://doi.org/10.3390/microbiolres16080183
Chicago/Turabian StyleSingh, Bharmjeet, Manju Dahiya, Vikram Kumar, Archana Ayyagari, Deepti N. Chaudhari, and Jayesh J. Ahire. 2025. "Biofilm and Antimicrobial Resistance: Mechanisms, Implications, and Emerging Solutions" Microbiology Research 16, no. 8: 183. https://doi.org/10.3390/microbiolres16080183
APA StyleSingh, B., Dahiya, M., Kumar, V., Ayyagari, A., Chaudhari, D. N., & Ahire, J. J. (2025). Biofilm and Antimicrobial Resistance: Mechanisms, Implications, and Emerging Solutions. Microbiology Research, 16(8), 183. https://doi.org/10.3390/microbiolres16080183







