Assessing the Efficacy of Chemical and Green-Synthesized CuO Nanoparticles in Combatting Clinical Candida Species: A Comparative Study
Abstract
1. Introduction
2. Material and Methods
2.1. Material
2.2. Methods
2.2.1. Biosynthesis of CuONPs
2.2.2. X-Ray Diffraction Analysis
2.2.3. CuO Characterization Methods
2.2.4. Biofilm Forming Assay
2.2.5. Effect of Nanoparticles on Planktonic Candida Viability
Well Diffusion Method
Microdilution Method
2.2.6. Antibiofilm Activity
Antiadhesive Activity
Biofilm Eradication Assay
3. Results
3.1. Biosynthesis of CuONPs
3.2. CuONPs Characterization
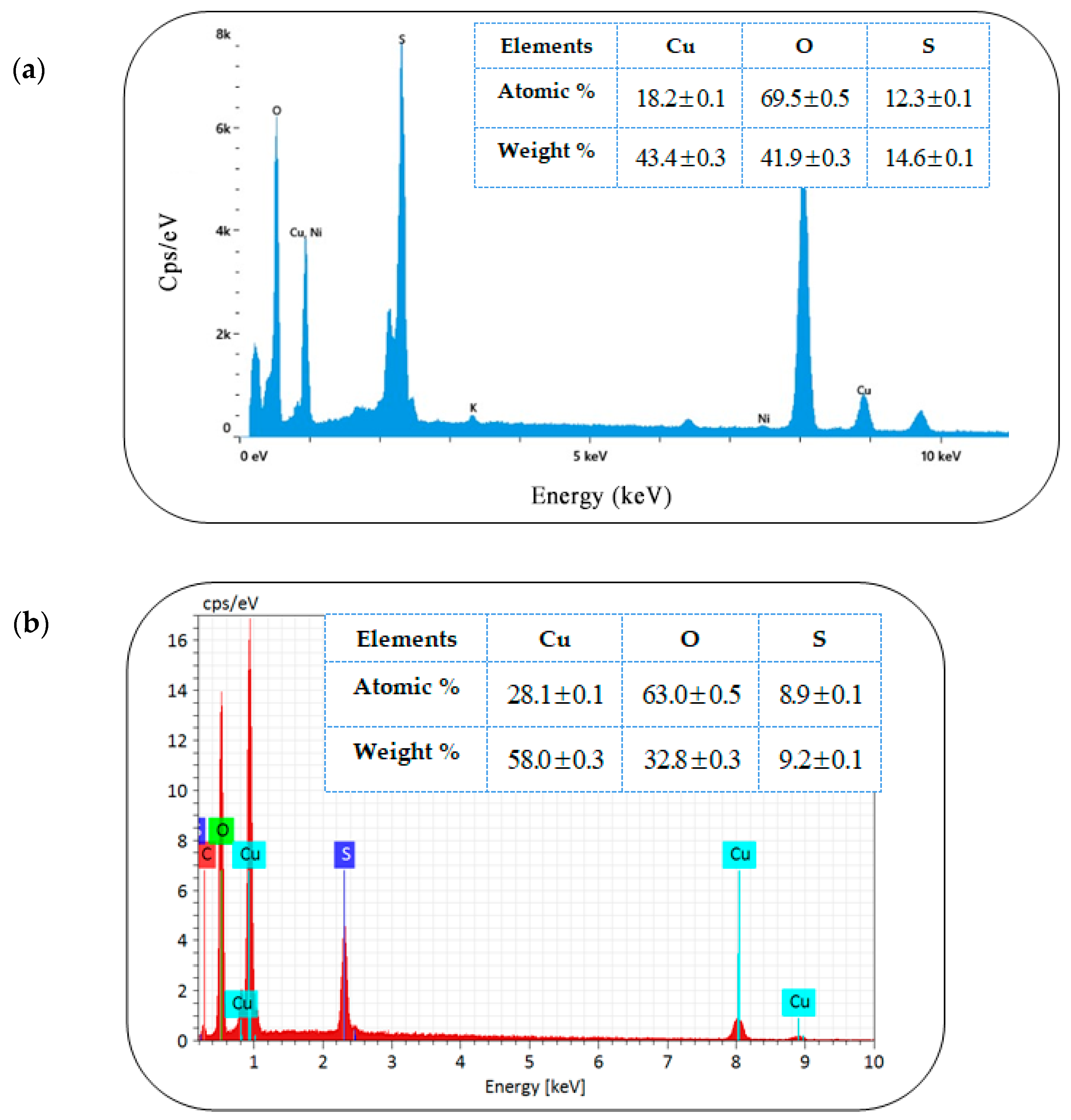
3.2.1. Energy-Dispersive Spectroscopy (EDS) Analysis
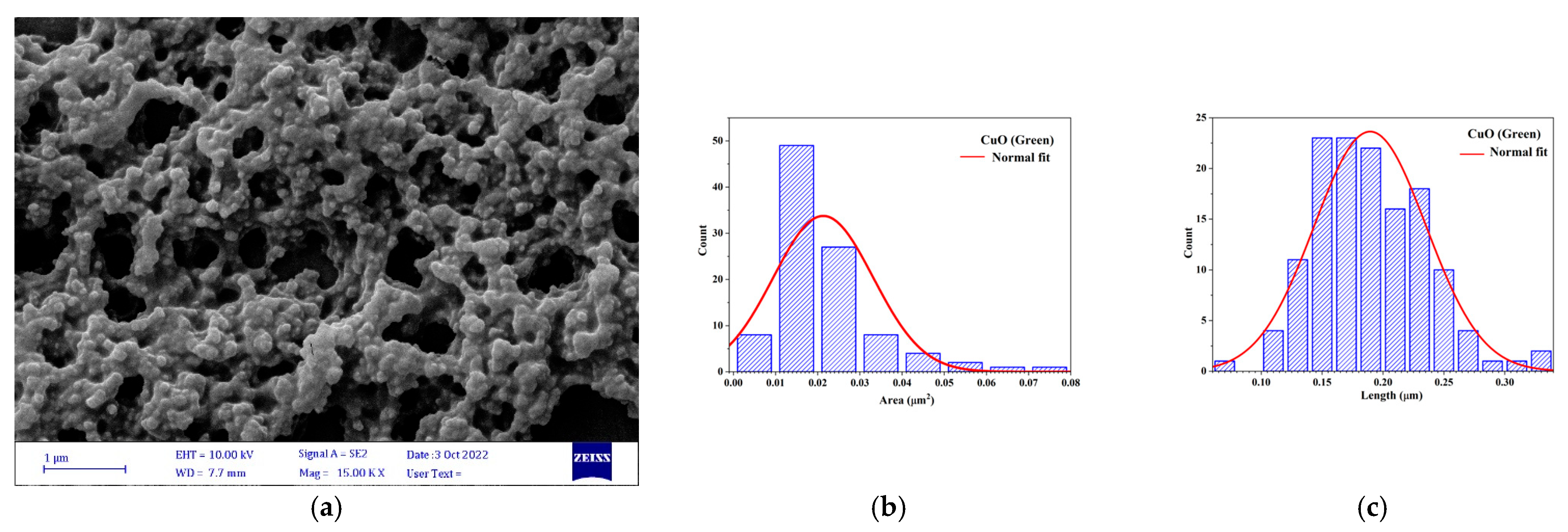
3.2.2. Scanning Electron Microscopy
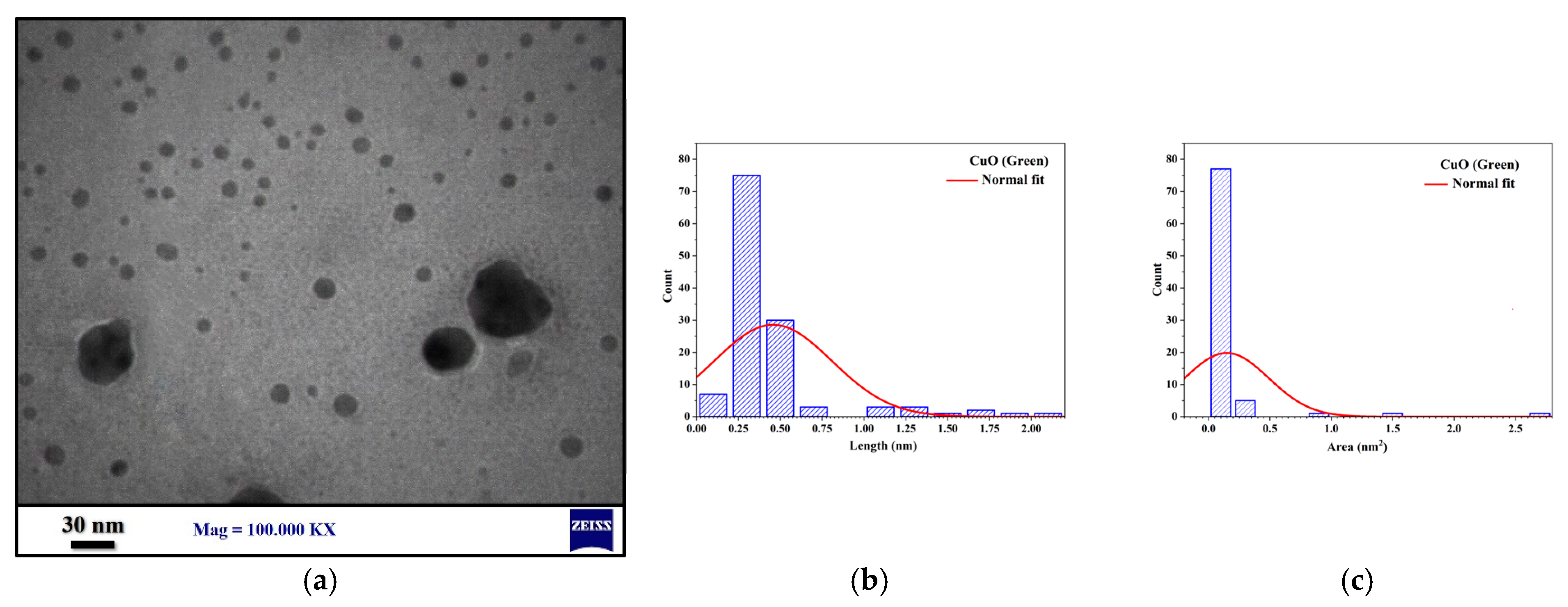
3.2.3. TEM
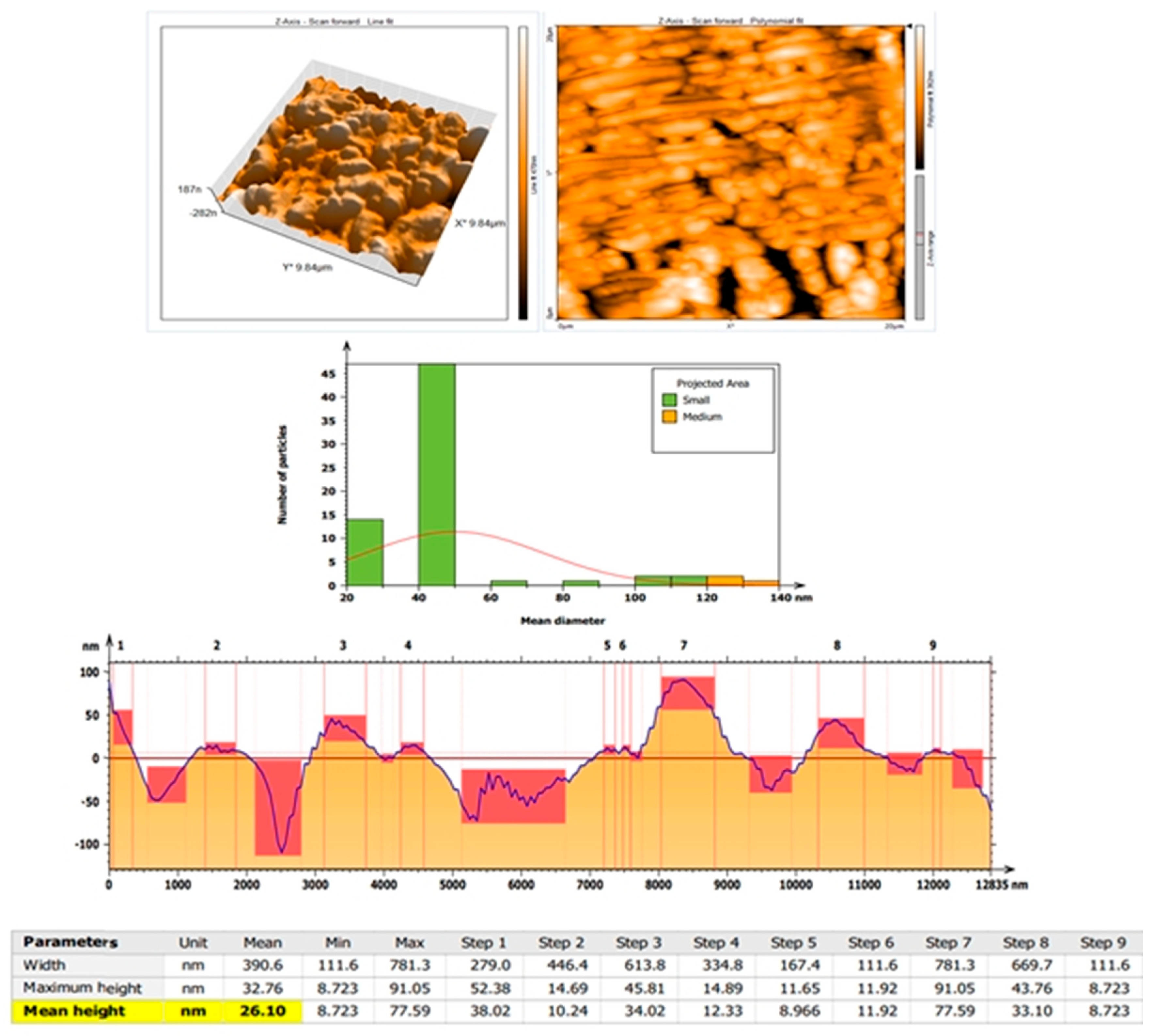
3.2.4. AFM Analysis
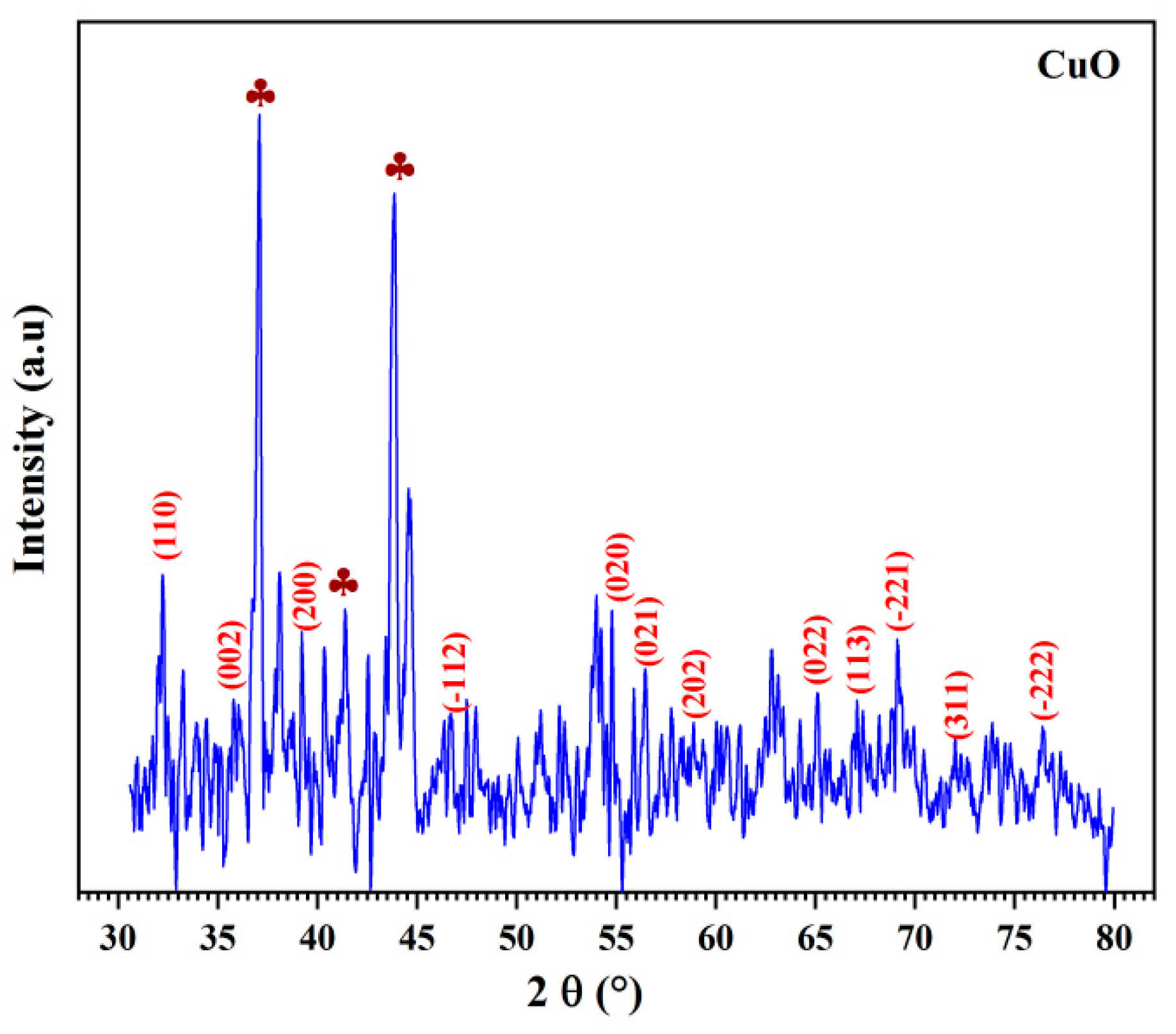
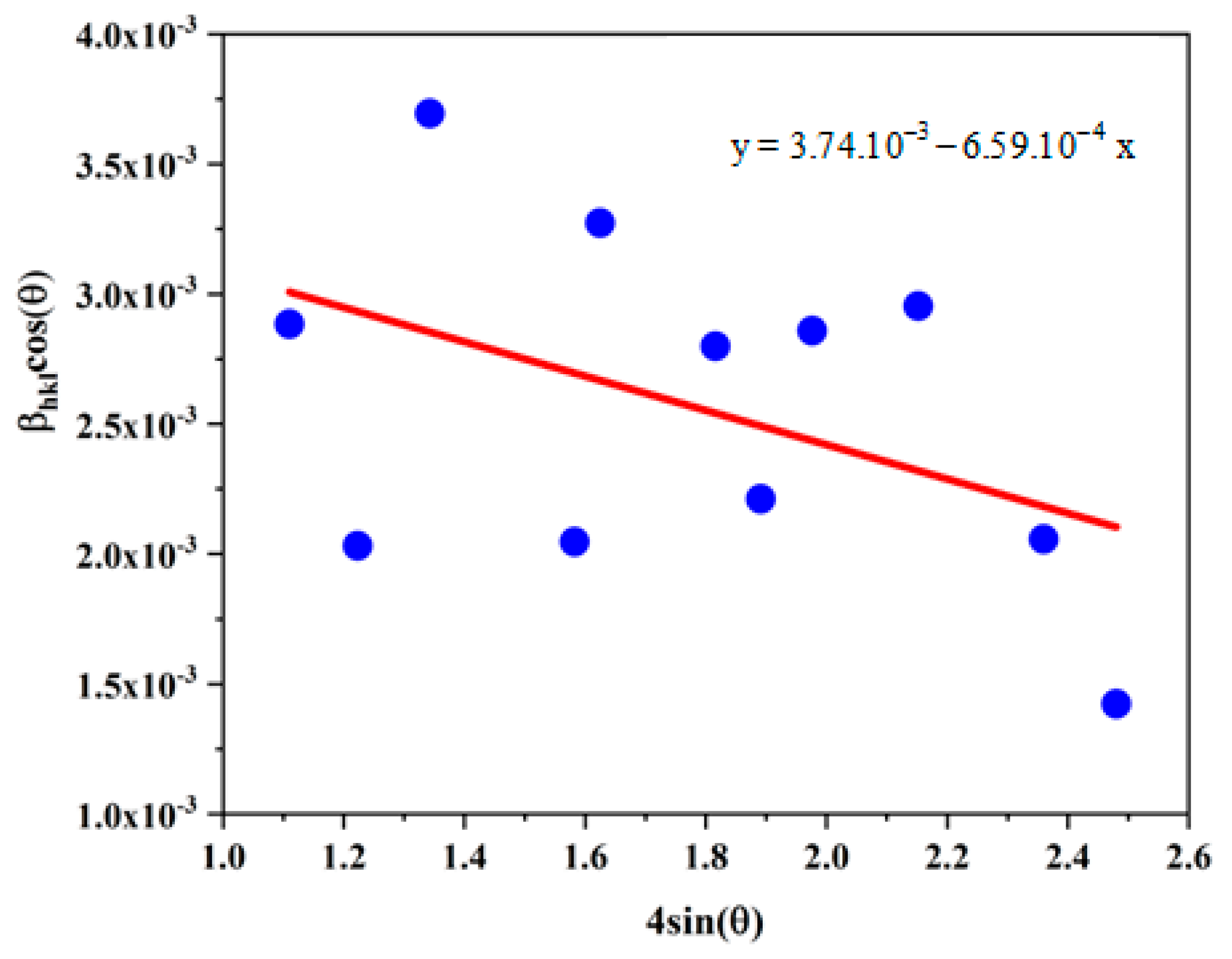
3.2.5. X Ray Diffraction Study
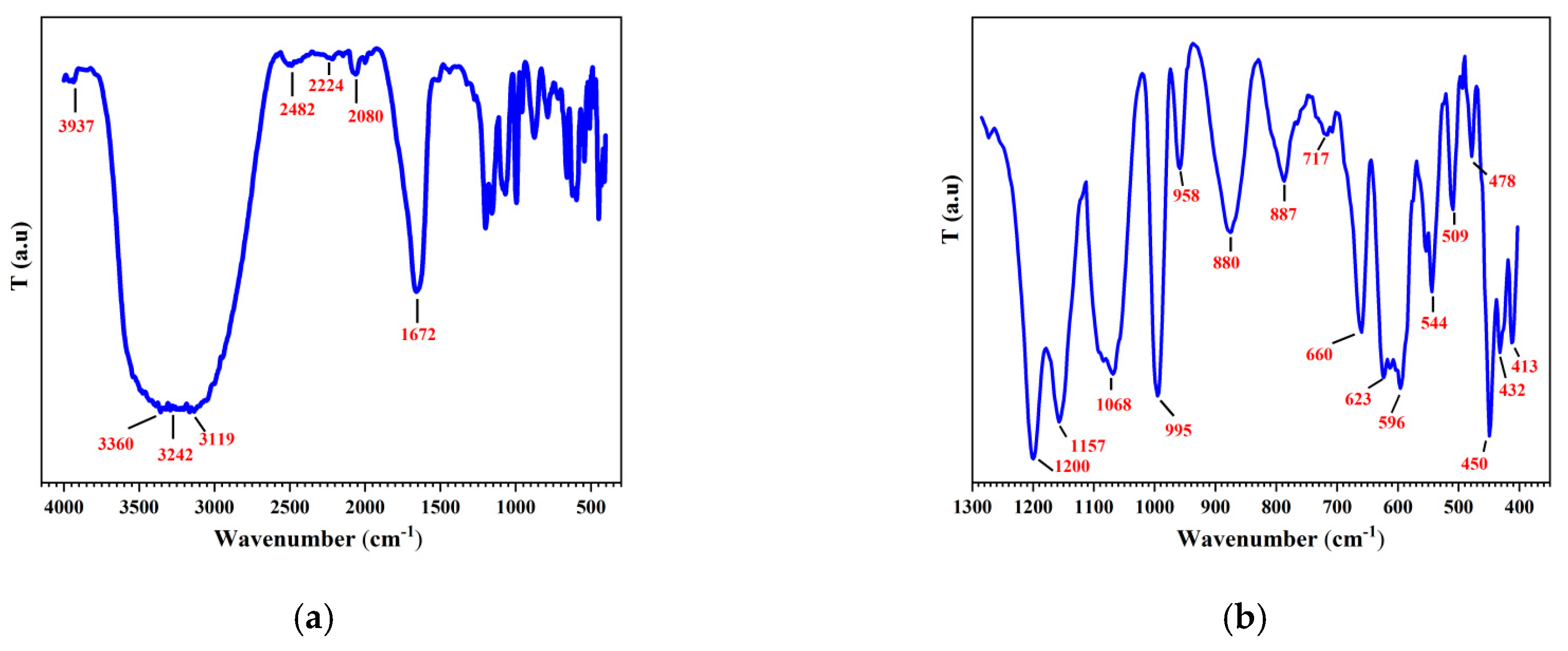
3.2.6. FT-IR Spectrum of CuONPs
3.3. Effect of NPs on Planktonic Candida Viability
3.3.1. Well Diffusion Method
3.3.2. Microdilution Method
3.4. Antibiofilm Assay
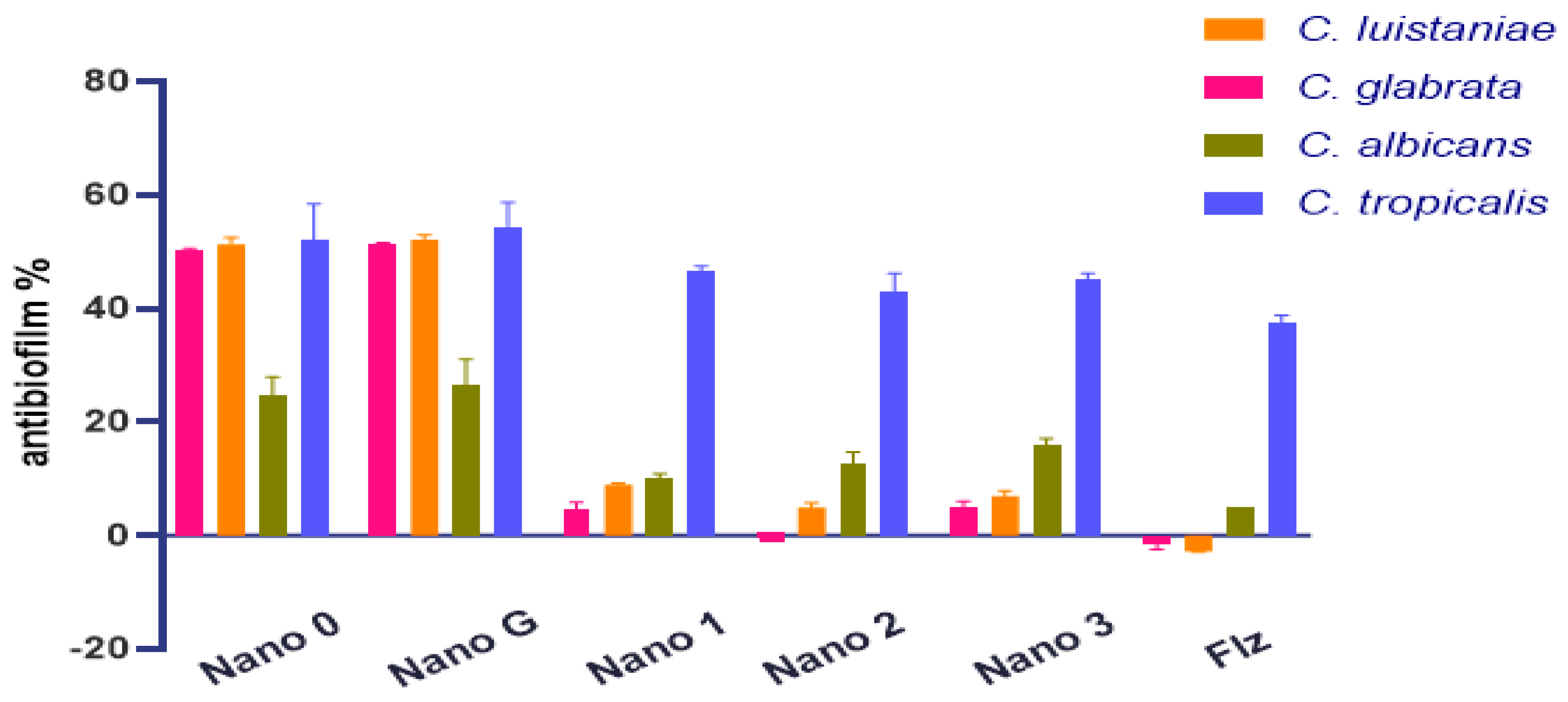
3.4.1. Antibiofilm Activity of Nanoparticles
3.4.2. Biofilm Eradication Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cornely, O.A.; Sprute, R.; Bassetti, M.; Chen, S.C.-A.; Groll, A.H.; Kurzai, O.; Lass-Flörl, C.; Ostrosky-Zeichner, L.; Rautemaa-Richardson, R.; Revathi, G.; et al. Global guideline for the diagnosis and management of candidiasis: An initiative of the ECMM in cooperation with ISHAM and ASM. Lancet Infect. Dis. 2025, 25, e280–e293, Erratum in: Lancet Infect. Dis. 2025, 25, e203. Erratum in: Lancet Infect. Dis. 2025, 25, e261. [Google Scholar] [CrossRef] [PubMed]
- Kreulen, I.A.M.; de Jonge, W.J.; van den Wijngaard, R.M.; van Thiel, I.A.M. Candida spp. in Human Intestinal Health and Disease: More than a Gut Feeling. Mycopathologia 2023, 188, 845–862. [Google Scholar] [CrossRef]
- Bays, D.J.; Jenkins, E.N.; Lyman, M.; Chiller, T.; Strong, N.; Ostrosky-Zeichner, L.; Hoenigl, M.; Pappas, P.G.; Thompson, G. Epidemiology of Invasive Candidiasis. Clin. Epidemiol. 2024, 16, 549–566. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Re, V.L.; Carbonari, D.M.; Lewis, J.D.; Forde, K.A.; Goldberg, D.S.; Reddy, K.R.; Haynes, K.; Roy, J.A.; Sha, D.; Marks, A.R.; et al. Oral Azole Antifungal Medications and Risk of Acute Liver Injury, Overall and by Chronic Liver Disease Status. Am. J. Med. 2015, 129, 283–291.e5. [Google Scholar] [CrossRef] [PubMed]
- Ben-Rhouma, K.; Ben-Salah, S.F.; Boulehmi, N.; Bouratbine, A. Phenotypic and Genotypic Characterization of Intestinal Candida spp. in Tunisia. Jundishapur J. Microbiol. 2021, 14, e113800. [Google Scholar] [CrossRef]
- Malinovská, Z.; Čonková, E.; Váczi, P. Biofilm Formation in Medically Important Candida Species. J. Fungi 2023, 9, 955. [Google Scholar] [CrossRef]
- Sundstrom, P. Adhesion in Candida spp. Cell Microbiol. 2002, 4, 461–469. [Google Scholar] [CrossRef]
- Nett, J.E.; Andes, D.R. Contributions of the Biofilm Matrix to Candida Pathogenesis. J. Fungi 2020, 6, 21. [Google Scholar] [CrossRef]
- Baillie, G.S.; Douglas, L.J. Matrix polymers of Candida biofilms and their possible role in biofilm resistance to antifungal agents. J. Antimicrob. Chemother. 2000, 46, 397–403. [Google Scholar] [CrossRef]
- Droney, M.; Reed, E.; Sarwar, S.; Coe, K.; Tran, N. Fluconazole step-down therapy versus echinocandins for the treatment of Candida glabrata invasive candidiasis with candidaemia. J. Antimicrob. Chemother. 2025, 80, 996–1000. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xia, C.; Liu, R.; Zhang, S.; Shen, J.; Wang, Z. Fluconazole-induced changes in azole resistance and biofilm production in Candida glabrata in vitro. Diagn. Microbiol. Infect. Dis. 2025, 111, 116683. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Pan, L.; Zhang, H.; Xie, L.; Wang, X.; Shou, J.; Qi, Y.; Yan, X. Recent Developments on Using Nanomaterials to Combat Candida albicans. Front. Chem. 2021, 9, 813973. [Google Scholar] [CrossRef]
- Varma, A.; Warghane, A.; Dhiman, N.K.; Paserkar, N.; Upadhye, V.; Modi, A.; Saini, R. The role of nanocomposites against biofilm infections in humans. Front. Cell. Infect. Microbiol. 2023, 13, 1104615. [Google Scholar] [CrossRef]
- Karunakaran, G.; Sudha, K.G.; Ali, S.; Cho, E.-B. Biosynthesis of Nanoparticles from Various Biological Sources and Its Biomedical Applications. Molecules 2023, 28, 4527. [Google Scholar] [CrossRef]
- Singh, H.; Desimone, M.F.; Pandya, S.; Jasani, S.; George, N.; Adnan, M.; Aldarhami, A.; Bazaid, A.S.; A Alderhami, S. Revisiting the Green Synthesis of Nanoparticles: Uncovering Influences of Plant Extracts as Reducing Agents for Enhanced Synthesis Efficiency and Its Biomedical Applications. Int. J. Nanomed. 2023, 18, 4727–4750. [Google Scholar] [CrossRef]
- D’souza, S.P.; Chavannavar, S.V.; Kanchanashri, B.; Niveditha, S.B. Pharmaceutical Perspectives of Spices and Condiments as Alternative Antimicrobial Remedy. J. Evid. Based Complement. Altern. Med. 2017, 22, 1002–1010. [Google Scholar] [CrossRef]
- Modan, E.M.; Schiopu, A.-G.; Moga, S.G.; Negrea, D.A.; Istrate, D.; Ciuca, I.; Oproescu, M. Advanced Copper Oxide Chemical and Green Synthesis: Characterization and Antibacterial Evaluation. Crystals 2024, 15, 7. [Google Scholar] [CrossRef]
- Islam, S.U.; Bairagi, S.; Kamali, M.R. Review on green biomass-synthesized metallic nanoparticles and composites and their photocatalytic water purification applications: Progress and perspectives. Chem. Eng. J. Adv. 2023, 14, 100460. [Google Scholar] [CrossRef]
- Ben Nasr, F.; Mnif, S.; Guermazi, H.; Duponchel, B.; Leroy, G.; Aifa, S.; Guermazi, S. Synthesis, Characterization of (Fe, Sn) Doped and Co-Doped Copper Oxide Nanoparticles and Evaluation of their Antibacterial Activities. J. Clust. Sci. 2024, 35, 1827–1843. [Google Scholar] [CrossRef]
- Khlifi, N.; Mnif, S.; Ben Nasr, F.; Fourati, N.; Zerrouki, C.; Chehimi, M.M.; Guermazi, H.; Aifa, S.; Guermazi, S. Non-doped and transition metal-doped CuO nano-powders: Structure-physical properties and anti-adhesion activity relationship. RSC Adv. 2022, 12, 23527–23543. [Google Scholar] [CrossRef] [PubMed]
- Tunell, G.; Posnjak, E.; Ksanda, C.J. Geometrical and optical properties, and crystal structure of tenorite. Z. Krist. Krist. Krist. 1935, 90, 120–142. [Google Scholar] [CrossRef]
- Ku, B.K.; Kulkarni, P. Comparison of diffusion charging and mobility-based methods for measurement of aerosol agglomerate surface area. J. Aerosol Sci. 2012, 47, 100–110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Hola, V.; Bonaventura, G.D.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Tudela, J.; Barchiesi, F.; Bille, J.; Chryssanthou, E.; Cuenca-Estrella, M.; Denning, D.; Donnelly, J.; Dupont, B.; Fegeler, W.; Moore, C.; et al. Method for the determination of minimum inhibitory concentration (MIC) by broth dilution of fermentative yeasts. Clin. Microbiol. Infect. 2003, 9, i–viii. [Google Scholar] [CrossRef]
- Olawuwo, O.S.; Famuyide, I.M.; McGaw, L.J. Antibacterial and Antibiofilm Activity of Selected Medicinal Plant Leaf Extracts Against Pathogens Implicated in Poultry Diseases. Front. Veter. Sci. 2022, 9, 820304. [Google Scholar] [CrossRef]
- Raul, P.K.; Senapati, S.; Sahoo, A.K.; Umlong, I.M.; Devi, R.R.; Thakur, A.J.; Veer, V. CuO nanorods: A potential and efficient adsorbent in water purification. RSC Adv. 2014, 4, 40580–40587. [Google Scholar] [CrossRef]
- Keabadile, O.P.; Aremu, A.O.; Elugoke, S.E.; Fayemi, O.E. Green and Traditional Synthesis of Copper Oxide Nanoparticles—Comparative Study. Nanomaterials 2020, 10, 2502. [Google Scholar] [CrossRef]
- Alqarni, L.S.; Alghamdi, M.D.; Alshahrani, A.A.; Nassar, A.M.; Singh, A.K. Green Nanotechnology: Recent Research on Bioresource-Based Nanoparticle Synthesis and Applications. J. Chem. 2022, 2022, 1–31. [Google Scholar] [CrossRef]
- Garcia-Marin, L.E.; Juarez-Moreno, K.; Vilchis-Nestor, A.R.; Castro-Longoria, E. Highly Antifungal Activity of Biosynthesized Copper Oxide Nanoparticles against Candida albicans. Nanomaterials 2022, 12, 3856. [Google Scholar] [CrossRef]
- Pereira, R.; Fontenelle, R.O.d.S.; Brito, E.; Morais, S. Biofilm of Candida albicans: Formation, regulation and resistance. J. Appl. Microbiol. 2021, 131, 11–22. [Google Scholar] [CrossRef]
- Taran, M.; Rad, M.; Alavi, M. Antibacterial activity of copper oxide (CuO) nanoparticles biosynthesized by Bacillus sp. FU4: Optimization of experiment design. Pharm. Sci. 2017, 23, 198–206. [Google Scholar] [CrossRef]
- Vidovix, T.B.; Quesada, H.B.; Januário, E.F.D.; Bergamasco, R.; Vieira, A.M.S. Green synthesis of copper oxide nanoparticles using Punica granatum leaf extract applied to the removal of methylene blue. Mater. Lett. 2019, 257, 126685. [Google Scholar] [CrossRef]
- Latif, S.; Abdulaziz, F.; Alanazi, A.M.; Alsehli, A.H.; Alsowayigh, M.M.; Alanazi, A.A. Effect of H2O2 @CuONPs in the UV Light-Induced Removal of Organic Pollutant Congo Red Dye: Investigation into Mechanism with Additional Biomedical Study. Molecules 2023, 28, 410. [Google Scholar] [CrossRef]
- Saied, M.; Hasanin, M.; Abdelghany, T.M.; Amin, B.H.; Hashem, A.H. Anticandidal activity of nanocomposite based on nanochitosan, nanostarch and mycosynthesized copper oxide nanoparticles against multidrug-resistant Candida. Int. J. Biol. Macromol. 2023, 242 Pt 1, 124709. [Google Scholar] [CrossRef]
- Muñoz-Escobar, A.; Reyes-López, S.Y.; Mukherjee, A. Antifungal susceptibility of Candida species to copper oxide nanoparticles on polycaprolactone fibers (PCL-CuONPs). PLoS ONE 2020, 15, e0228864. [Google Scholar] [CrossRef] [PubMed]
- Sasarom, M.; Wanachantararak, P.; Chaijareenont, P.; Okonogi, S. Biosynthesis of copper oxide nanoparticles using Caesalpinia sappan extract: In vitro evaluation of antifungal and antibiofilm activities against Candida albicans. Drug Discov. Ther. 2023, 17, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Mlinarić, N.M.; Zore, A.; Veselinovic, V.; Trtić, N.; Dolić, O.; Štukelj, R.; Abram, A.; Učakar, A.; Adamović, T.; Vidrih, R.; et al. Antimicrobial Activity of Poly(methyl methacrylate) Doped with CuO and ZnO Nanoparticles. ACS Omega 2025, 10, 13060–13072. [Google Scholar] [CrossRef]
- Wang, D.; Zeng, N.; Li, C.; Li, Z.; Zhang, N.; Li, B. Fungal biofilm formation and its regulatory mechanism. Heliyon 2024, 10, e32766. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


| Sample | Grain Size (µm) | Area (µm2) |
|---|---|---|
| CuONPs (green synthesis) | ||
| CuONPs (chemical synthesis) [19] |
| Sample | D (nm) | ε (10−4) | ρ (g.cm−3) | SSA (m2. g−1) |
|---|---|---|---|---|
| CuO (green synthesis) | 37.07 | −6.59 | 6.64 | 24.38 |
| CuO (Chemical synthesis) [19] | 51.93 | 5.85 | 6.71 | 17.20 |
| Compound | Concentration of MIC (µg/mL) for Candida spp. | |||
|---|---|---|---|---|
| C. glabrata | C. tropicalis | C. luistaniae | C. albicans | |
| Nano 0 | 125 | 125 | 125 | 125 |
| Nano G | 125 | 125 | 125 | 62.5 |
| Nano 1 | 12.5 | 25 | 12.5 | 50 |
| Nano 2 | 50 | 25 | 50 | 100 |
| Nano 3 | n.a | 50 | 50 | 25 |
| Fluconazole | 8 | 32 | 8 | 16 |
| Nanoparticle Type | Antibiofilm Activity % | |||
|---|---|---|---|---|
| C. glabrata | C. luistaniae | C. albicans | C. tropicalis | |
| Nano 0 | 50.2 | 51.3 | 24.7 | 52.2 |
| Nano G | 51.3 | 52.1 | 26.5 | 54.4 |
| Nano 1 | 4.6 | 9 | 10 | 46.5 |
| Nano 2 | −0.4 | 4.9 | 12.7 | 43 |
| Nano 3 | 5 | 7 | 15.9 | 45 |
| Fluconazole | −1.5 | −2.8 | 5 | 37.6 |
| Materials | Biofilm Eradication Percentage % Concentration (100 μg/mL) | |||
|---|---|---|---|---|
| C. glabrata | C. lusitaniae | C. albicans | C.tropicalis | |
| Nano 0 | 66.66 | 47.08 | 59.87 | 66.29 |
| Nano G | 50.81 | 57.67 | 68.51 | 61.32 |
| Nano 1 | 67.21 | 47.51 | 58.02 | 65.19 |
| Nano 2 | 65.57 | 60.77 | 55.55 | 62.98 |
| Nano 3 | 68.30 | 62.98 | 67.90 | 67.95 |
| Fluconazole | 63.38 | 66.13 | 66.66 | 66.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalaf, H.Y.; Nasr, F.B.; Noomi, B.S.; Mnif, S.; Aifa, S. Assessing the Efficacy of Chemical and Green-Synthesized CuO Nanoparticles in Combatting Clinical Candida Species: A Comparative Study. Microbiol. Res. 2025, 16, 178. https://doi.org/10.3390/microbiolres16080178
Khalaf HY, Nasr FB, Noomi BS, Mnif S, Aifa S. Assessing the Efficacy of Chemical and Green-Synthesized CuO Nanoparticles in Combatting Clinical Candida Species: A Comparative Study. Microbiology Research. 2025; 16(8):178. https://doi.org/10.3390/microbiolres16080178
Chicago/Turabian StyleKhalaf, Hiba Younis, Ferid Ben Nasr, Bashar Sadeq Noomi, Sami Mnif, and Sami Aifa. 2025. "Assessing the Efficacy of Chemical and Green-Synthesized CuO Nanoparticles in Combatting Clinical Candida Species: A Comparative Study" Microbiology Research 16, no. 8: 178. https://doi.org/10.3390/microbiolres16080178
APA StyleKhalaf, H. Y., Nasr, F. B., Noomi, B. S., Mnif, S., & Aifa, S. (2025). Assessing the Efficacy of Chemical and Green-Synthesized CuO Nanoparticles in Combatting Clinical Candida Species: A Comparative Study. Microbiology Research, 16(8), 178. https://doi.org/10.3390/microbiolres16080178






