Microbial Communities’ Composition of Supralittoral and Intertidal Sediments in Two East African Beaches (Djibouti Republic)
Abstract
1. Introduction
2. Materials and Methods
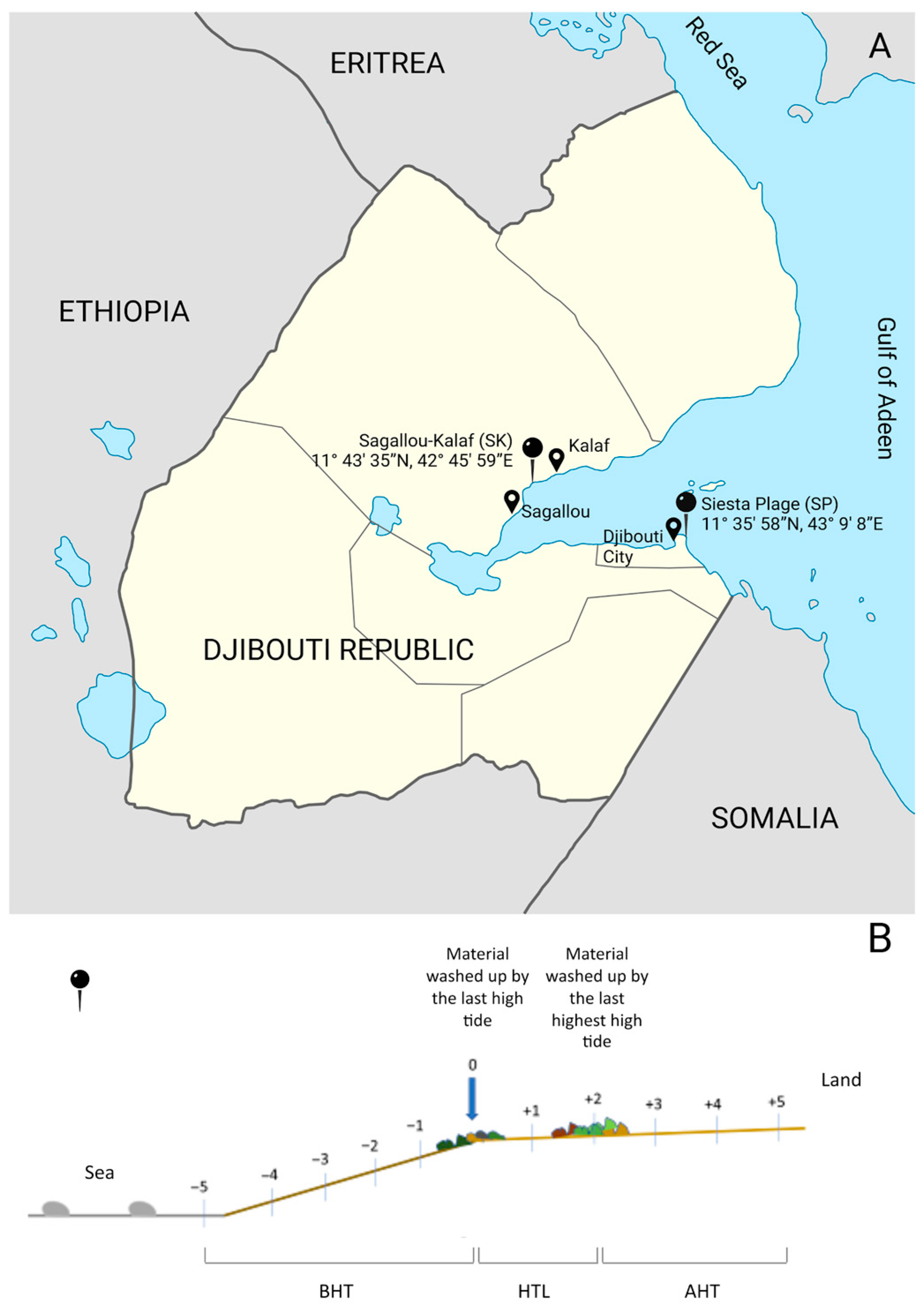
2.1. Sampling
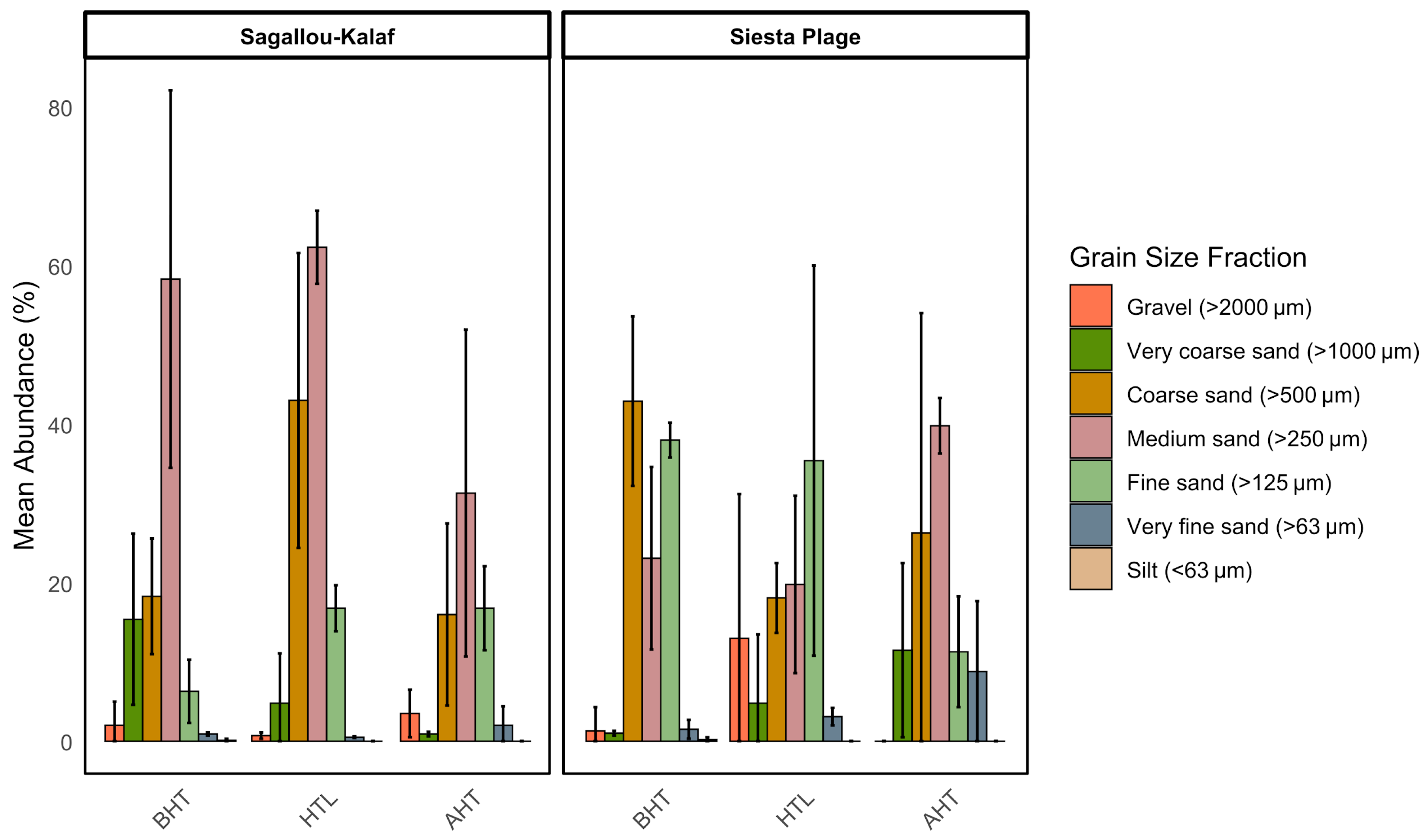
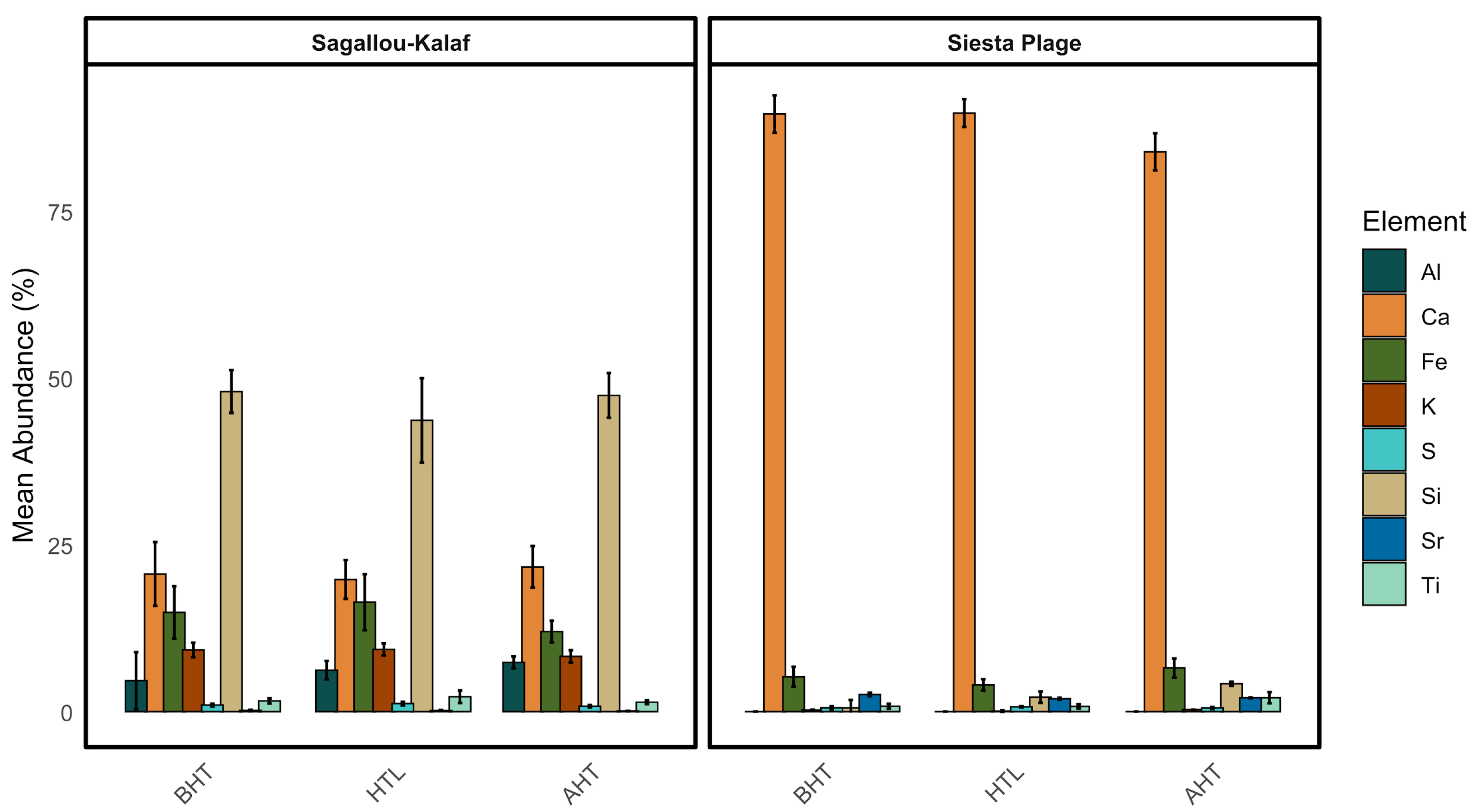
2.2. Sediment Characterization: Granulometry and Mineralogical Composition
2.3. Sediment-Associated Microbial Community Analyses
2.3.1. Genomic DNA Extraction and Microbial Taxonomic Marker Sequencing
2.3.2. Sequence Processing, Taxonomic Assignment, and Community Analysis
3. Results
3.1. Sediment Analysis
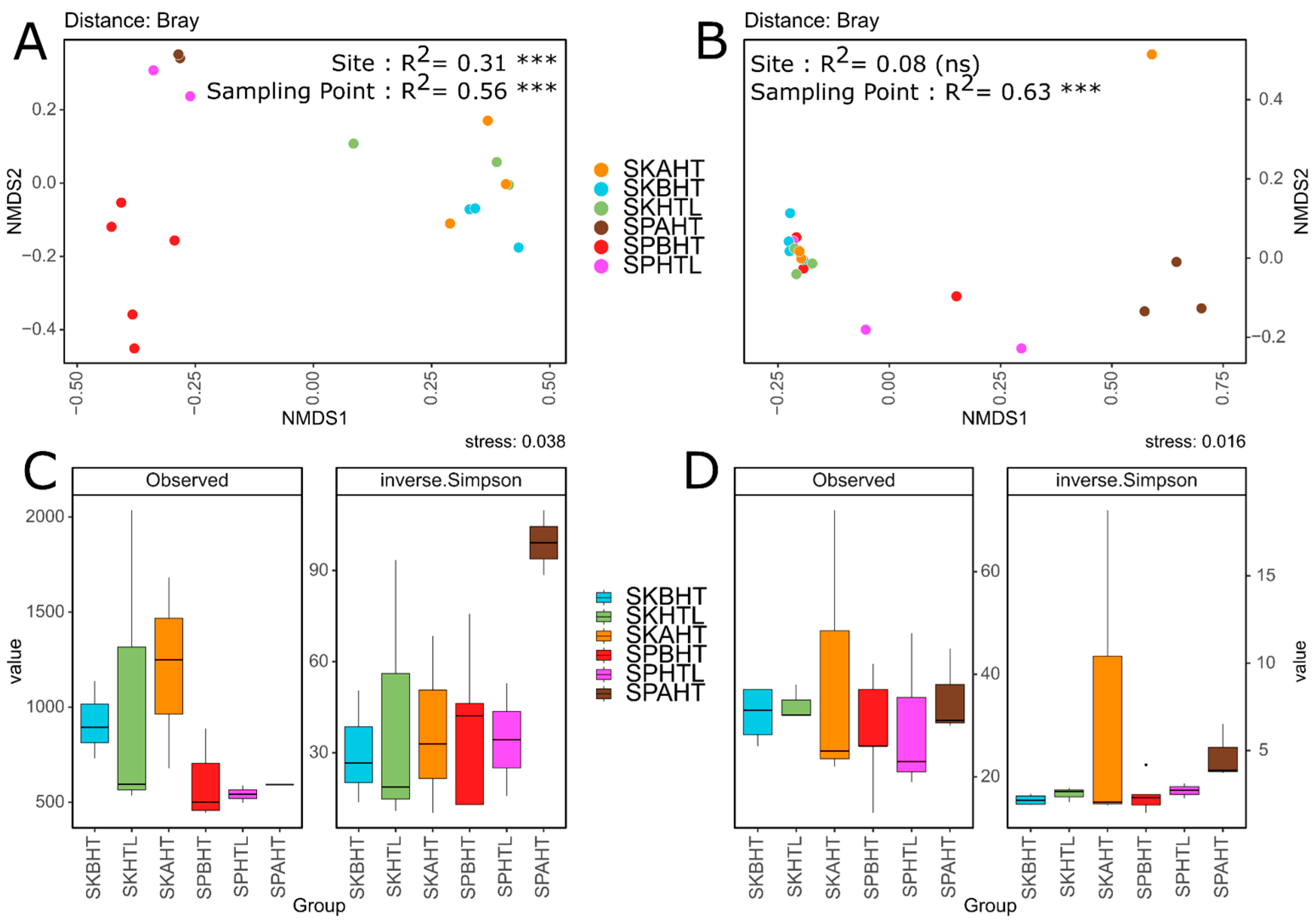
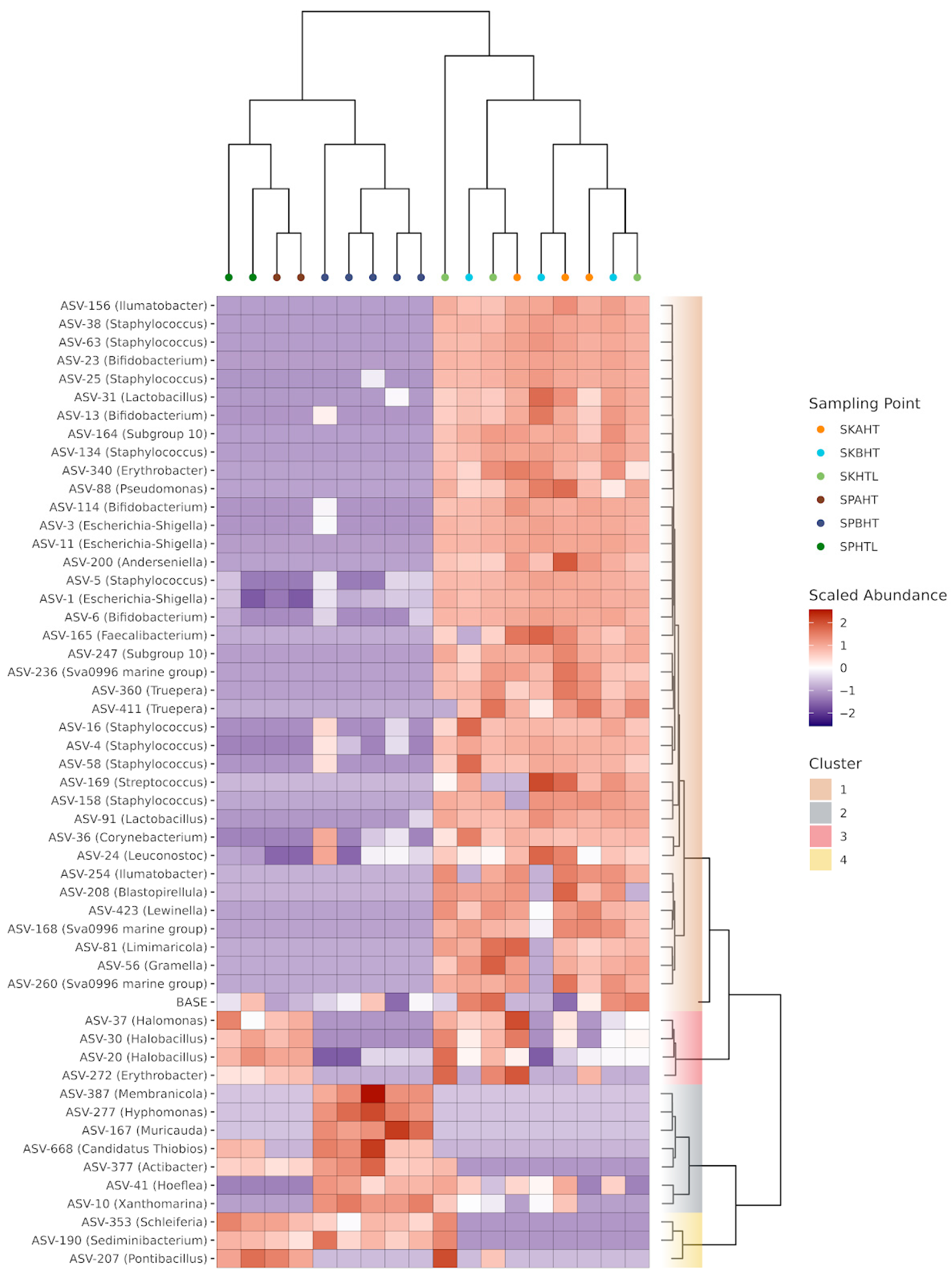
3.2. Microbial Community Structure and Diversity Patterns
3.3. Influence of the Distance from the High Tide Line on the Distribution of Bacterial Taxa
4. Discussion
4.1. Site-Specific Microbial Signatures: Rural vs. Urban Beaches
4.2. Microbial Distribution Alongside the Transect
4.3. Sediment Composition and Microbial Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SK | Sagallou-Kalaf |
| SP | Siesta Plage |
| BHT | Below High Tide |
| HTL | High Tide Line |
| AHT | Above High Tide |
References
- McLachlan, A.; Brown, A.C. The Ecology of Sandy Shores, 2nd ed.; Academic Press: Cambridge, MA, USA, 2006. [Google Scholar] [CrossRef]
- Vassallo, P.; Paoli, C.; Fabiano, M. Ecosystem level analysis of sandy beaches using thermodynamic and network analyses: A study case in the NW Mediterranean Sea. Ecol. Indic. 2012, 15, 10–17. [Google Scholar] [CrossRef]
- Defeo, O.; McLachlan, A.; Schoeman, D.S.; Schlacher, T.A.; Dugan, J.; Jones, A.; Lastra, M.; Scapini, F. Threats to sandy beach ecosystems: A review. Estuar. Coast. Shelf Sci. 2009, 81, 1–12. [Google Scholar] [CrossRef]
- Santmire, J.A.; Leff, L.G. The effect of sediment grain size on bacterial communities in streams. J. N. Am. Benthol. Soc. 2007, 26, 601–610. [Google Scholar] [CrossRef]
- Arboleda-Baena, C.; Freilich, M.; Pareja, C.B.; Logares, R.; De la Iglesia, R.; Navarrete, S.A. Microbial community and network responses across strong environmental gradients: How do they compare with macroorganisms? FEMS Microbiol. Ecol. 2024, 100, fiae017. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Goto, D.; Yan, T. Effects of autochthonous microbial community on the die-off of fecal indicators in tropical beach sand. FEMS Microbiol. Ecol. 2010, 74, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Whitman, R.L.; Harwood, V.J.; Edge, T.A.; Nevers, M.B.; Byappanahalli, M.N.; Vijayavel, K.; Brandão, J.; Sadowsky, M.J.; Alm, E.W.; Crowe, A.; et al. Microbes in Beach Sands: Integrating Environment, Ecology and Public Health. Rev. Environ. Sci. Bio. Technol. 2014, 13, 329–368. [Google Scholar] [CrossRef] [PubMed]
- Sabino, R.; Rodrigues, R.; Costa, I.; Carneiro, C.; Cunha, M.; Duarte, A.; Faria, N.; Ferreira, F.; Gargaté, M.; Júlio, C.; et al. Routine screening of harmful microorganisms in beach sands: Implications to public health. Sci. Total. Environ. 2014, 472, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Solo-Gabriele, H.M.; Harwood, V.J.; Kay, D.; Fujioka, R.S.; Sadowsky, M.J.; Whitman, R.L.; Wither, A.; Caniça, M.; da Fonseca, R.C.; Duarte, A.; et al. Beach sand and the potential for infectious disease transmission: Observations and recommendations. J. Mar. Biol. Assoc. UK 2016, 96, 101–120. [Google Scholar] [CrossRef]
- Horel, A.; Mortazavi, B.; Sobecky, P.A. Responses of microbial community from northern Gulf of Mexico sandy sediments following exposure to deepwater horizon crude oil. Environ. Toxicol. Chem. 2012, 31, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.; Mucha, A.P.; Teixeira, C.; Bordalo, A.A.; Almeida, C.M.R. Biodegradation of petroleum hydrocarbons in estuarine sediments: Metal influence. Biodegradation 2013, 24, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Newton, R.J.; Huse, S.M.; Morrison, H.G.; Peake, C.S.; Sogin, M.L.; McLellan, S.L.; Gilbert, J.A. Shifts in the microbial community composition of Gulf Coast beaches following beach oiling. PLoS ONE 2013, 8, e74265. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.S.; Gupta, A.A.; Kellogg, C.A. Regime shift in sandy beach microbial communities following Deepwater Horizon oil spill remediation efforts. PLoS ONE 2014, 9, e102934. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, V.; Almeida, C.; Almeida, T.; Teixeira, C.; Mucha, A.P. Indigenous microbial communities along the NW Portuguese Coast: Potential for hydrocarbons degradation and relation with sediment contamination. Mar. Pollut. Bull. 2018, 131 Pt A, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-R, L.M.; Overholt, W.A.; Hagan, C.; Huettel, M.; Kostka, J.E.; Konstantinidis, K.T. Microbial community successional patterns in beach sands impacted by the Deepwater Horizon oil spill. ISME J. 2015, 9, 1928–1940. [Google Scholar] [CrossRef] [PubMed]
- Heritier-Robbins, P.; Karthikeyan, S.; Hatt, J.K.; Kim, M.; Huettel, M.; Kostka, J.E.; Konstantinidis, K.T.; Rodriguez-R, L.M. Beach sand oil spills select for generalist microbial populations. ISME J. 2021, 15, 3418–3422. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; LokaBharathi, P.A. Heterotrophic Bacterial Populations in Tropical Sandy Beaches. 1980. Available online: https://drs.nio.res.in/drs/handle/2264/6833 (accessed on 3 June 2025).
- Shet, S.A.; Garg, S. Prokaryotic diversity of tropical coastal sand dunes ecosystem using metagenomics. 3 Biotech 2021, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Behera, S.; Dash, P.K. Potential of Microbial Diversity of Coastal Sand Dunes: Need for Exploration in Odisha Coast of India. Sci. World J. 2019, 2019, 2758501. [Google Scholar] [CrossRef] [PubMed]
- Palit, K.; Rath, S.; Chatterjee, S.; Das, S. Microbial diversity and ecological interactions of microorganisms in the mangrove ecosystem: Threats, vulnerability, and adaptations. Environ. Sci. Pollut. Res. 2022, 29, 32467–32512. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Solo-Gabriele, H.M.; Fleming, L.E.; Elmir, S. Monitoring marine recreational water quality using multiple microbial indicators in an urban tropical environment. Water Res. 2004, 38, 3119–3131. [Google Scholar] [CrossRef] [PubMed]
- Piggot, A.M.; Klaus, J.S.; Johnson, S.; Phillips, M.C.; Solo-Gabriele, H.M. Relationship between Enterococcal Levels and Sediment Biofilms at Recreational Beaches in South Florida. Appl. Environ. Microbiol. 2012, 78, 5973–5982. [Google Scholar] [CrossRef] [PubMed]
- Yamahara, K.M.; Walters, S.P.; Boehm, A.B. Growth of Enterococci in Unaltered, Unseeded Beach Sands Subjected to Tidal Wetting. Appl. Environ. Microbiol. 2009, 75, 1517–1524. [Google Scholar] [CrossRef]
- Samarasekera, K.; Abeygunawardena, S.I. Microbiology of Seawater and Sand in a Selected Bathing Site of Sri Lanka—A Study Towards Microbial Quality Assessment. Front. Environ. Microbiol. 2017, 3, 1. [Google Scholar] [CrossRef]
- Jones, E.G. Are there more marine fungi to be described? Bot. Mar. 2011, 54, 343–354. [Google Scholar] [CrossRef]
- Yarden, O. Fungal association with sessile marine invertebrates. Front. Microbiol. 2014, 5, 228. [Google Scholar] [CrossRef] [PubMed]
- Godinho, V.M.; de Paula, M.T.R.; Silva, D.A.S.; Paresque, K.; Martins, A.P.; Colepicolo, P.; Rosa, C.A.; Rosa, L.H. Diversity and distribution of hidden cultivable fungi associated with marine animals of Antarctica. Fungal Biol. 2019, 123, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Amend, A.; Burgaud, G.; Cunliffe, M.; Edgcomb, V.P.; Ettinger, C.L.; Gutiérrez, M.H.; Heitman, J.; Hom, E.F.Y.; Ianiri, G.; Jones, A.C.; et al. Fungi in the Marine Environment: Open Questions and Unsolved Problems. mBio 2019, 10, e01189-18. [Google Scholar] [CrossRef] [PubMed]
- Stal, L.J.; Cretoiu, M.S. The Marine Microbiome. In The Microbiomes of Humans, Animals, Plants, and the Environment; Springer Nature: Dordrecht, The Netherlands, 2022; Volume 3. [Google Scholar]
- Overy, D.P.; Rämä, T.; Oosterhuis, R.; Walker, A.K.; Pang, K.-L. The Neglected Marine Fungi, Sensu stricto, and Their Isolation for Natural Products’ Discovery. Mar. Drugs 2019, 17, 42. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Bradbury, H.J.; Antler, G.; Burdige, D.J.; Bennett, T.D.; Li, S.; Turchyn, A.V. Sediment mineralogy influences the rate of microbial sulfate reduction in marine sediments. Earth Planet. Sci. Lett. 2022, 598, 117841. [Google Scholar] [CrossRef]
- de Boer, W.; Mao, Y.; Hagenaars, G.; de Vries, S.; Slinger, J.; Vellinga, T. Mapping the Sandy Beach Evolution Around Seaports at the Scale of the African Continent. J. Mar. Sci. Eng. 2019, 7, 151. [Google Scholar] [CrossRef]
- Curren, E.; Leong, S.C.Y. Profiles of bacterial assemblages from microplastics of tropical coastal environments. Sci. Total. Environ. 2019, 655, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Wentworth, C.K. A Scale of Grade and Class Terms for Clastic Sediments. J. Geol. 1922, 30, 377–392. [Google Scholar] [CrossRef]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M.; Bourtzis, K. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; Volume 18, pp. 315–322. [Google Scholar] [CrossRef]
- Renzi, S.; Nenciarini, S.; Bacci, G.; Cavalieri, D. Yeast metagenomics: Analytical challenges in the analysis of the eukaryotic microbiome. Microbiome Res. Rep. 2024, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Erik, S.W. Using DECIPHER v2.0 to Analyze Big Biological Sequence Data in R. R J. 2016, 8, 352. [Google Scholar] [CrossRef]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef] [PubMed]
- Abarenkov, K.; Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; May, T.W.; Frøslev, T.G.; Pawlowska, J.; Lindahl, B.; Põldmaa, K.; Truong, C.; et al. The UNITE database for molecular identification and taxonomic communication of fungi and other eukaryotes: Sequences, taxa and classifications reconsidered. Nucleic Acids Res. 2024, 52, D791–D797. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Lahti, L.; Shetty, S. Microbiome R Package, version 3.21; Bioconductor: Boston, MA, USA, 2017. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’HAra, R.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package_. R Package Version 2.6–4. 2022. Available online: https://cir.nii.ac.jp/crid/1370584340724217483 (accessed on 3 June 2025).
- Lin, H.; Das Peddada, S. Analysis of compositions of microbiomes with bias correction. Nat. Commun. 2020, 11, 3514. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, R.A.M.; Chen, Z.J. ggplot2: Elegant Graphics for Data Analysis (2nd ed.). Meas. Interdiscip. Res. Perspect. 2019, 17, 160–167. [Google Scholar] [CrossRef]
- Huuki, H.; Vilkki, J.; Vanhatalo, A.; Tapio, I. Fecal microbiota colonization dynamics in dairy heifers associated with early-life rumen microbiota modulation and gut health. Front. Microbiol. 2024, 15, 1353874. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.; Schwarzkopf, S.; Kinoshita, A.; Tröscher-Mußotter, J.; Dänicke, S.; Camarinha-Silva, A.; Huber, K.; Frahm, J.; Seifert, J. Evolution of rumen and oral microbiota in calves is influenced by age and time of weaning. Anim. Microbiome 2021, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, K.D.; McNay, M.; Cao, Y.; Ebentier, D.; Madison, M.; Griffith, J.F. A multi-beach study of Staphylococcus aureus, MRSA, and enterococci in seawater and beach sand. Water Res. 2012, 46, 4195–4207. [Google Scholar] [CrossRef] [PubMed]
- Sibanda, T.; Ramganesh, S. Taxonomic and functional analyses reveal existence of virulence and antibiotic resistance genes in beach sand bacterial populations. Arch. Microbiol. 2021, 203, 1753–1766. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Benmessaoud, Y.; Hamdail, B.R.; Ezziyyani, M. Intelligent System for Forecasting Climate Deterioration and Assessing the Potential Impact of Pollutants. In International Conference on Advanced Intelligent Systems for Sustainable Development (AI2SD’2023); Ezziyyani, M., Kacprzyk, J., Balas, V.E., Eds.; AI2SD 2023. Lecture Notes in Networks and Systems; Springer: Cham, Switzerland, 2024; Volume 930. [Google Scholar] [CrossRef]
- Liu, L.; Zou, L.; Gao, D.M. Spatiotemporal Distribution of Microorganism in Mudflats Environment of the Yellow River Estuary Intertidal Area. Adv. Mater. Res. 2014, 1030–1032, 603–608. [Google Scholar] [CrossRef]
- Wang, Z.; Fuad, T.I.; Liu, J.; Lin, K.; Liu, L.; Gao, C.; Wang, W.; Liu, X. Spatial Patterns of Microbial Communities in Intertidal Sediments of the Yellow River Estuary, China. Microb. Ecol. 2025, 87, 173. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lin, Z.; Kuang, B.; Ni, Z.; Chen, X.; Guo, B.; Zhu, G.; Bai, S. Electroactive algae-bacteria wetlands for the treatment of micro-polluted aquaculture wastewater: Pilot-scale verification. Biochem. Eng. J. 2022, 184, 108471. [Google Scholar] [CrossRef]
- Rinke, C.; Schmitz-Esser, S.; Stoecker, K.; Nussbaumer, A.D.; Molnár, D.A.; Vanura, K.; Wagner, M.; Horn, M.; Ott, J.A.; Bright, M. “Candidatus Thiobios zoothamnicoli,” an Ectosymbiotic Bacterium Covering the Giant Marine Ciliate Zoothamnium niveum. Appl. Environ. Microbiol. 2006, 72, 2014–2021. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Yin, Q.; Jiang, Z.; Yan, H.; Nian, Y. Pollution characteristics and microbial community succession of a rural informal landfill in an arid climate. Ecotoxicol. Environ. Saf. 2023, 262, 115295. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, L.; Yin, X.; Zhang, T. Polycyclic aromatic hydrocarbon (PAH) biodegradation capacity revealed by a genome-function relationship approach. Environ. Microbiome 2023, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Rahul, K.; Azmatunnisa, M.; Sasikala, C.; Ramana, C.V. Hoeflea olei sp. nov., a diesel-oil-degrading, anoxygenic, phototrophic bacterium isolated from backwaters and emended description of the genus Hoeflea. Int. J. Syst. Evol. Microbiol. 2015, 65, 2403–2409. [Google Scholar] [CrossRef] [PubMed]
- Atalla, M.M.; Zainab, H.K.; Eman, R.H.; Youssry, A.; Aty, A.A.E. Screening of some marine-derived fungal isolates for lignin degrading enzymes (LDEs) production. Agric. Biol. J. North Am. 2010, 1, 591–599. [Google Scholar]
- Hodhod, M.S.E.-D.; Gaafar, A.-R.Z.; Alshameri, A.; Qahtan, A.A.; Noor, A.; Abdel-Wahab, M. Molecular characterization and bioactive potential of newly identified strains of the extremophilic black yeast Hortaea werneckii isolated from Red Sea mangrove. Biotechnol. Biotechnol. Equip. 2020, 34, 1288–1298. [Google Scholar] [CrossRef]
- Poli, A.; Varese, G.C.; Garzoli, L.; Prigione, V. Seagrasses, seaweeds and plant debris: An extraordinary reservoir of fungal diversity in the Mediterranean Sea. Fungal Ecol. 2022, 60, 101156. [Google Scholar] [CrossRef]
- Ianiri, G.; LeibundGut-Landmann, S.; Dawson, T.L. Malassezia: A Commensal, Pathogen, and Mutualist of Human and Animal Skin. Annu. Rev. Microbiol. 2022, 76, 757–782. [Google Scholar] [CrossRef] [PubMed]
- Nenciarini, S.; Renzi, S.; di Paola, M.; Meriggi, N.; Cavalieri, D. The yeast–human coevolution: Fungal transition from passengers, colonizers, and invaders. WIREs Mech. Dis. 2024, 16, e1639. [Google Scholar] [CrossRef] [PubMed]
- Saxton, K.E.; Rawls, W.J. Soil Water Characteristic Estimates by Texture and Organic Matter for Hydrologic Solutions. Soil Sci. Soc. Am. J. 2006, 70, 1569–1578. [Google Scholar] [CrossRef]
- Ning, Z.; Yu, K.; Wang, Y.; Huang, X.; Han, M.; Zhang, J. Carbon and nutrient dynamics of permeable carbonate and silicate sands adjacent to coral reefs around Weizhou Island in the northern South China Sea Estuarine Coast. Shelf Sci. 2019, 225, 106229. [Google Scholar] [CrossRef]
- Boyadzhieva, I.; Berberov, K.; Atanasova, N.; Krumov, N.; Kabaivanova, L. Isolation, Purification and In Vitro Characterization of a Newly Isolated Alkalophilic Phytase Produced by the Halophile Cobetia marina Strain 439 for Use as Animal Food Supplement. Fermentation 2025, 11, 39. [Google Scholar] [CrossRef]
- Romano, I.; Finore, I.; Nicolaus, G.; Huertas, F.J.; Lama, L.; Nicolaus, B.; Poli, A. Halobacillus alkaliphilus sp. nov., a halophilic bacterium isolated from a salt lake in Fuente de Piedra, southern Spain. Int. J. Syst. Evol. Microbiol. 2008, 58 Pt 4, 886–890. [Google Scholar] [CrossRef] [PubMed]
- D’entremont, T.W.; López-Gutiérrez, J.C.; Walker, A.K.; Taylor, I.E. Inoculating rhizome-propagated Sporobolus pumilus with a native mycorrhizal fungus increases salt marsh plant growth and survival. FACETS 2021, 6, 1134–1145. [Google Scholar] [CrossRef]
- Lin, W.; Liu, X.; Gong, L.; Liu, R.; Ling, M.; Guo, C.; Meng, H.; Luo, Z.; Du, X.; Guo, Y.; et al. Impact of environmental factors on diversity of fungi in sediments from the Shenzhen River Estuary. Arch. Microbiol. 2023, 205, 96. [Google Scholar] [CrossRef] [PubMed]
- Lobo, L.Q.; Izabel-Shen, D.; Albertsson, J.; Raymond, C.; Gunnarsson, J.S.; Broman, E.; Nascimento, F.J.A. Salinity and resource availability as drivers of Baltic benthic fungal diversity. Environ. DNA 2024, 6, e526. [Google Scholar] [CrossRef]
- Floudas, D.; Binder, M.; Riley, R.; Barry, K.; Blanchette, R.A.; Henrissat, B.; Martínez, A.T.; Otillar, R.; Spatafora, J.W.; Yadav, J.S.; et al. The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 2012, 336, 1715–1719. [Google Scholar] [CrossRef] [PubMed]
- Vrijmoed, L.L.P.; Hodgkiss, I.J. Wood DegradIng Activity of some Lignicolous Marine Fungi. In Biodeterioration 7; Houghton, D.R.J., Smith, R.N., Eggins, H.O.W., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 1988; pp. 768–773. [Google Scholar] [CrossRef]
- Olguin, J.; Velez, P.; Solís-Weiss, V.; Barrios, A.; Walker, A.K.; Ponce-Vélez, G.; González, M.C.; Figueroa, M.; Botello, A. An overview of fungal taxonomic, functional, and genetic diversity in coastal and oceanic biomes in megadiverse Mexico. Bot. Mar. 2023, 66, 471–490. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renzi, S.; Russo, A.; D’Alessandro, A.; Ciattini, S.; Soliman, S.C.; Nistri, A.; Pretti, C.; Cavalieri, D.; Ugolini, A. Microbial Communities’ Composition of Supralittoral and Intertidal Sediments in Two East African Beaches (Djibouti Republic). Microbiol. Res. 2025, 16, 173. https://doi.org/10.3390/microbiolres16080173
Renzi S, Russo A, D’Alessandro A, Ciattini S, Soliman SC, Nistri A, Pretti C, Cavalieri D, Ugolini A. Microbial Communities’ Composition of Supralittoral and Intertidal Sediments in Two East African Beaches (Djibouti Republic). Microbiology Research. 2025; 16(8):173. https://doi.org/10.3390/microbiolres16080173
Chicago/Turabian StyleRenzi, Sonia, Alessandro Russo, Aldo D’Alessandro, Samuele Ciattini, Saida Chideh Soliman, Annamaria Nistri, Carlo Pretti, Duccio Cavalieri, and Alberto Ugolini. 2025. "Microbial Communities’ Composition of Supralittoral and Intertidal Sediments in Two East African Beaches (Djibouti Republic)" Microbiology Research 16, no. 8: 173. https://doi.org/10.3390/microbiolres16080173
APA StyleRenzi, S., Russo, A., D’Alessandro, A., Ciattini, S., Soliman, S. C., Nistri, A., Pretti, C., Cavalieri, D., & Ugolini, A. (2025). Microbial Communities’ Composition of Supralittoral and Intertidal Sediments in Two East African Beaches (Djibouti Republic). Microbiology Research, 16(8), 173. https://doi.org/10.3390/microbiolres16080173






