Abstract
Disruption of the intestinal epithelial barrier is a key driver of gut-derived inflammation in various disorders, yet strategies to preserve or restore barrier integrity remain limited. To address this, we evaluated a four-strain Bifidobacterium mixture—selected for complementary anti-inflammatory potency and industrial scalability—in lipopolysaccharide (LPS)-challenged RAW 264.7 macrophages and a Caco-2/THP-1 transwell co-culture model. Pretreatment with the probiotic blend reduced nitric oxide (NO) release in a dose-dependent manner by 25.9–48.3% and significantly down-regulated the pro-inflammatory markers in macrophages. In the co-culture system, the formulation decreased these markers, increased transepithelial electrical resistance (TEER) by up to 31% at 105 colony-forming unit (CFU)/mL after 48 h, and preserved the membrane localization of tight junction (TJ) proteins. Adhesion to Caco-2 cells (≈ 6%) matched that of the benchmark probiotic Lacticaseibacillus rhamnosus GG, suggesting direct epithelial engagement. These in vitro findings demonstrate that this probiotic mixture can attenuate LPS-driven inflammation and reinforce epithelial architecture, providing a mechanistic basis for its further evaluation in animal models and clinical studies of intestinal inflammatory disorders.
1. Introduction
The intestinal epithelium serves as the body’s frontline defense, and its impermeability hinges on TJs [1]. These junctions consist of transmembrane proteins—occludin, various claudins, and junctional adhesion molecules—which are linked to intracellular scaffold proteins, including ZO-1 and ZO-2 [2,3]. Together with adherent junctions, TJs regulate the paracellular passage of ions, water, and nutrients while barring luminal microbes and antigens [4,5]. Disruption of this barrier—whether by inflammatory mediators, toxins, or drugs—leads to increased permeability (“leaky gut”), allowing foreign substances to infiltrate the lamina propria and systemic circulation and triggering immune activation, which is implicated in conditions including inflammatory bowel disease (IBD), celiac disease, and metabolic syndrome, where chronic immune activation disrupts the epithelial integrity [6,7,8].
Under inflammatory conditions, cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-6 initiate signaling cascades—nuclear factor-kappa B (NF-κB), mitogen-activated protein kinases (MAPKs), and Janus kinase/signal transducer and activator of transcription (JAK/STAT)—that destabilize TJs [9,10]. Activation of myosin light chain kinase (MLCK) phosphorylates myosin light chain, inducing actomyosin contraction and enhancing paracellular tension [11]. Concurrently, TJ proteins undergo clathrin-mediated endocytosis, displacing occludin and claudins from the membrane and severely compromising barrier integrity [12,13]. Thus, interventions that inhibit these pro-inflammatory pathways or directly reinforce TJ protein expression and localization are key to preserving epithelial cohesion.
Probiotics—defined as live microorganisms that confer health benefits when consumed in adequate amounts—have demonstrated the ability to bolster gut barrier function [14,15]. Specific Lactobacillus and Bifidobacterium strains upregulate occludin, ZO-1, and claudin-1 expression, enhance mucin secretion, and attenuate TNF-α and IL-1β levels [16,17,18]. For example, certain B. breve strains significantly increase the colonic occludin and ZO-1 protein levels and boost short-chain fatty acid production, reducing tissue damage in dextran sulfate sodium (DSS)-induced colitis models [19]. In a clinical study, L. acidophilus La1 demonstrated its effectiveness in improving gut barrier function and decreasing intestinal permeability, as evidenced by a significant reduction in the lactulose–mannitol ratio following supplementation [20]. VSL#3—a mixture of eight probiotic strains, including three Bifidobacterium species—induces remission in ulcerative colitis patients and improves mucosal TJ integrity [21,22]. Generally, mixed-strain probiotic formulations outperform single-strain preparations by leveraging complementary mechanisms, such as metabolite production, pathogen inhibition, and immune modulation, collectively strengthening gut health [23,24,25,26,27].
To capitalize on both potent anti-inflammatory activity and industrial productivity, we selected two Bifidobacterium strains—B. bifidum LM1108 and B. longum LM1024—which, despite modest growth in standard media, exhibit potent suppression of NO production in LPS-stimulated macrophages, alongside two complementary strains—B. animalis subsp. lactis LM1017 and B. breve LM1092—that achieve high cell densities yet have milder anti-inflammatory effects. By combining these four strains in optimized ratios, as disclosed in the Korean Patent [28], we developed a probiotic blend that delivers both excellent barrier-protective, anti-inflammatory efficacy and robust bio-mass yields necessary for commercial manufacture.
In this study, we first assessed the formulation’s anti-inflammatory capacity by measuring both NO production and pro-inflammatory cytokine expression in LPS-stimulated RAW264.7 macrophages. To closely mimic the intestinal microenvironment, we then employed a transwell co-culture of Caco-2 epithelial cells and THP-1 macrophages, examining TJ localization via immunofluorescence (IF) and monitoring TEER under LPS challenge. Finally, we evaluated bacterial adhesion to Caco-2 monolayers and the formulation’s ability to restore TJ protein expression following cytokine-induced disruption. These combined analyses aim to validate the four-strain Bifidobacterium mixture as a novel probiotic capable of suppressing NO and cytokine-driven inflammation, reinforcing epithelial TJs and thereby enhancing gut barrier integrity.
2. Materials and Methods
2.1. Cell Culture
The murine macrophage cell line RAW 264.7, the human intestinal cell line Caco-2, and the human monocyte cell line THP-1 were obtained from the Korean Cell Line Bank (Seoul, Republic of Korea). All cell lines were maintained at 37 °C in a humidified incubator with 5% CO2 and sub-cultured every 2–3 days. RAW 264.7 and Caco-2 cells were cultivated in Dulbecco’s Modified Eagle’s Medium (cat. no. LM001-05, Welgene, Gyeongsan-si, Gyeongsangbuk-do, Republic of Korea) with 10% fetal bovine serum (FBS; cat. no. S001-07, Welgene, Gyeongsan-si, Gyeongsangbuk-do, Republic of Korea) and 1% penicillin–streptomycin solution (cat. no. LS202-02, Welgene, Gyeongsan-si, Gyeongsangbuk-do, Republic of Korea). THP-1 cells were cultivated in RPMI 1640 (cat. no. GIB-11875-093, Gibco, NY, USA) with the same supplements (10% FBS and 1% penicillin–streptomycin solution).
2.2. Strain Preparation
B. animalis subsp. lactis LM1017 (LM1017), B. breve LM1092 (LM1092), B. longum LM1024 (LM1024), and B. bifidum LM1108 (LM1108) were isolated and identified by Lactomason Co., Ltd. The four strains are blended in a fixed ratio of 91.1:5.5:2.7:0.7 (w/w, LM1017:LM1092:LM1024:LM1108). This formulation is protected under Korean Patent No. 10-2772627. L. rhamnosus GG (KCTC 5033) was obtained from the Korean Collection for Type Cultures (Jeongeup-si, Jeollabuk-do, Republic of Korea) and cultivated in De Man, Rogosa, and Sharpe (MRS) medium at 37 °C under anaerobic conditions.
2.3. Cell Viability of RAW 264.7 Cells
The cytotoxicity of the Bifidobacterium mixture on RAW 264.7 cells was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. RAW 264.7 cells were seeded on a 96-well plate at 3 × 104 cells/well and incubated for 24 h at 37 °C in a humidified incubator with 5% CO2. After removing the culture medium, the cells were treated with serial dilutions of the Bifidobacterium mixture (105, 106, 107, and 108 CFU/mL) for 2 h, followed by the addition of LPS from Escherichia coli O111:B4 (final concentration = 100 ng/mL; cat. no. L4391-1MG, Sigma-Aldrich Co., St. Louis, MO, USA), except in the negative control wells. Following 24 h of incubation, the supernatants were removed, and the cells were washed twice with 1× phosphate-buffered saline (PBS). Subsequently, 100 μL of 0.5 mg/mL MTT solution (cat. no. M2128-100mg, Sigma-Aldrich Co., St. Louis, MO, USA) prepared in complete media was added to each well and incubated for 2 h at 37 °C. After removing the supernatant, the formazan crystals were dissolved by adding 100 μL of dimethyl sulfoxide. The absorbance was measured at 570 nm using a microplate reader (SpectraMax iD3, Molecular devices, San Jose, CA, USA). Cell viability was calculated by the following Equation (1).
2.4. The Measurement of NO Generation in RAW 264.7 Cells (Griess Assay)
RAW 264.7 cells were seeded on a 96-well plate at 3 × 104 cells/well and incubated for 24 h at a 5% CO2 incubator. After removing the cell culture media, RAW 264.7 cells were preincubated with serial diluted the Bifidobacterium mixture (105, 106, 107, and 108 CFU/mL) for 2 h. LPS was then added to each well at a final concentration of 100 ng/mL, except for the negative control. After 24 h of incubation at 37 °C in a 5% CO2 incubator, NO concentration in the supernatant was measured using the Griess reaction system (cat. no. G2930, Promega, Madison, WI, USA) following the manufacturer’s instructions. Absorbance at 540 nm was measured using a microplate reader (SpectraMax iD3, Molecular devices, San Jose, CA, USA). The NO concentration was determined using a standard curve generated with sodium nitrite.
2.5. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) Analysis
To confirm changes in the expression levels of cytokines and other immune-related proteins in RAW 264.7 cells, a two-step reverse-transcription PCR was performed. RAW 264.7 cells were seeded in 6-well plates at a density of 9 × 105 cells per well and incubated for 24 h at 37 °C in a 5% CO2 atmosphere. After removing the culture medium, cells were treated with serial dilutions of the Bifidobacterium mixture (105 and 106 CFU/mL) for 2 h, then stimulated with LPS (final concentration, 100 ng/mL) in all wells except the negative control. Following a further 24 h incubation, total RNA was extracted using the MiniBEST Universal Extraction Kit (cat. no. 9767A, Takara Bio Inc., Shiga, Japan). Extracted RNA was reverse transcribed into cDNA using the High-Capacity cDNA Reverse Transcription Kit (cat. no. 4369914, Thermo Fisher Scientific, Waltham, MA, USA). Real-time PCR was carried out using SYBR Green PCR Master Mix (cat. no. 4364346, Thermo Fisher Scientific, Waltham, MA, USA) on a QuantStudio 3 Real-Time PCR system (Applied Biosystems, Waltham, MA, USA). β-actin served as the endogenous housekeeping gene, and relative expression levels were calculated by the comparative 2−ΔΔCt method according to Equations (2)–(4). Primer sequences for mouse and human cytokines and housekeeping genes are listed in Table 1.
ΔCt = Ctgene of interest − Cthousekeeping gene
ΔΔCt = ΔCtsample − ΔCtnegative control
Fold difference = 2−ΔΔCt

Table 1.
Primer sequences used for gene expression analysis in mouse and human cell lines.
2.6. Cytokine mRNA Expression in Caco-2/THP-1 Co-Culture System
The co-culture experiment was performed according to the method previously described by Han et al. [29]. Briefly, Caco-2 cells were seeded on 12-well transwell inserts (0.4 μm pore size, SPL, Pocheon-si, Gyeonggi-do, Republic of Korea) at 5 × 104 cells/insert and cultured for 14 to 21 days to allow formation of a confluent monolayer. Meanwhile, THP-1 cells were seeded on 12-well plates at 4 × 105 cells/well and differentiated with 150 nM 12-myristate 13-acetate (PMA; cat. no. L4391-1MG, Sigma-Aldrich Co., St. Louis, MO, USA) for 24 h. Following differentiation, the THP-1 cells were washed and incubated in fresh PMA-free complete RPMI 1640 medium for an additional 24 h to rest. After 2 days of PMA differentiation, the inserts containing Caco-2 monolayers were transferred to the plate containing differentiated THP-1 cells. The Bifidobacterium mixture (104 and 105 CFU/mL) was added to the apical side and incubated for 6 h. After this preincubation, LPS (final concentration = 1 μg/mL) was added to the basolateral side and incubated for an additional 24 h at 37 °C. THP-1 cells were then harvested for total RNA extraction. cDNA synthesis and quantitative real-time PCR were performed as described in the “qRT-PCR analysis” section. Primer sequences are provided in Table 1.
2.7. TEER
To assess the integrity of the Caco-2 monolayer, TEER measurements were performed using EVOM3 (WPI, Sarasota, FL, USA). The Caco-2+THP-1 co-cultures were differentiated and treated with the Bifidobacterium mixture and LPS as described in the “Cytokine mRNA expression in Caco-2+THP-1 co-culture system” section. TEER was measured immediately after LPS stimulation, as well as after 24 h and 48 h of incubation. The TEER values of cell-free inserts were subtracted, and the final values were normalized to the surface area of the monolayers (0.9 cm2) and expressed in Ohms·cm2.
2.8. In Vitro Adhesion Assay
Caco-2 cells were seeded on 6-well plates at 5 × 105 cells/well and cultured at 37 °C in 5% CO2 until a confluent monolayer was obtained. The Bifidobacterium mixture (108 CFU/mL) and L. rhamnosus GG (108 CFU/mL) were then added to fully differentiated Caco-2 cells and incubated for 2 h at 37 °C. After incubation, wells were gently washed twice with PBS to remove unbound bacteria. Caco-2 cells and adhered bacteria were detached using 0.1% (v/v) Triton X-100 solution, and serial dilutions were plated on Briggs-liver agar for the Bifidobacterium mixture and on MRS agar for L. rhamnosus GG; the plates were incubated at 37 °C for 48 h under anaerobic conditions. Adhesion (%) was calculated according to Equation (5).
N1: number of lactic acid bacteria adhered to Caco-2 cells.
N0: number of lactic acid bacteria added into wells of 6-well plates initially.
2.9. Analysis of ZO-1 and Occludin by IF Assay
To investigate the distribution of TJ proteins, an IF assay was performed. Caco-2 cells were seeded on 20φ confocal dishes at 2 × 105 cells/dish and cultured at 37 °C in a humidified atmosphere of 5% CO2 for 24 h. After removing the cell culture medium, 105 CFU/mL of Bifidobacterium mixture was added and preincubated for 6 h. The cells were then stimulated with LPS (final concentration = 1 μg/mL) for an additional 24 h, except in the negative control. After washing with PBS, cells were fixed with 4% paraformaldehyde for 10 min at room temperature, permeabilized with 0.25% Triton X-100 for 15 min at room temperature, and blocked with 1% bovine serum albumin in PBS for 2 h at room temperature. Cells were incubated overnight at 4 °C with rabbit anti-ZO-1 antibody (1:200; cat no. 13663S, Cell Signaling Technology Inc., Danvers, MA, USA) and rabbit anti-occludin antibody (1:200; cat no. 91131S, Cell Signaling Technology Inc., Danvers, MA, USA). After washing twice with PBS, the cells were incubated with fluorescein isothiocyanate-conjugated goat anti-rabbit IgG antibody (1:1000; cat. no. F9887, Sigma-Aldrich Co., St. Louis, MO, USA) for 1 h at room temperature. Cells were mounted and imaged using an Observer D1 inverted microscope (Carl Zeiss, Oberkochen, Germany) equipped with Automatic Component Recognition module and AxioVision 3.1 software (Carl Zeiss, Oberkochen, Germany) using a 100×/1.0 objective lens.
2.10. Statistical Analysis
All experiments were performed in triplicate, and three independent experiments were conducted. Data are expressed as the mean ± standard deviation (SD). Statistical significance between groups was determined using one-way ANOVA, followed by Tukey’s multiple comparison test. Statistical analysis was performed using GraphPad Prism 5.04 software (GraphPad Software Inc., San Diego, CA, USA), and a p-value of less than 0.05 was considered statistically significant. Levels of significance are indicated as * p < 0.05, ** p < 0.01, and *** p < 0.001.
3. Results
3.1. Effect of the Bifidobacterium Mixture on Cytotoxicity and NO Generation in LPS-Stimulated RAW 264.7 Cells
Cytotoxicity of the RAW 264.7 cells was assessed using the MTT assay. As shown in Figure 1a, even in the presence of 100 ng/mL LPS, the cell viability did not significantly change at any tested concentration (105 to 108 CFU/mL). These findings indicate that the mixture does not exert cytotoxic effects on RAW 264.7 cells, even in an inflammatory environment. Next, we evaluated whether the Bifidobacterium mixture could modulate LPS-driven inflammation. Cells were pretreated with serial dilutions of the mixture prior to LPS stimulation, and NO release was measured. The mixture significantly reduced NO release in a dose-dependent manner (Figure 1b), lowering the levels by 25.90% and 48.31% at 107 and 108 CFU/mL, respectively, compared to the LPS-only group. These results suggest that the mixture effectively suppresses NO production and possesses anti-inflammatory properties.
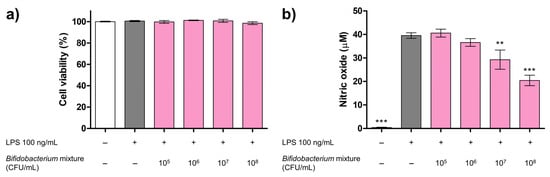
Figure 1.
Effects of the Bifidobacterium mixture on RAW 264.7 cell viability and NO production. (a) Cell viability after 24 h treatment with the Bifidobacterium mixture (105–108 CFU/mL), expressed relative to the negative control (NC; white bar); LPS alone (100 ng/mL) is shown in gray. Data are presented as mean ± SD (n = 3). Statistical significance was determined by *** p < 0.001 and ** p < 0.01 compared to the LPS alone. (b) NO production after 2 h pretreatment with the Bifidobacterium mixture (105–108 CFU/mL) and 24 h LPS stimulation (100 ng/mL), measured by the Griess assay. Data are presented as mean ± SD (n = 3). At 24 h versus LPS alone: 105 CFU/mL, mean difference = −1.04 µM (95% Confidence Interval (CI) −7.03 to 4.95; p > 0.05); 106 CFU/mL, mean difference = 2.93 µM (95% CI −3.06 to 8.93; p > 0.05); 107 CFU/mL, mean difference = 10.2 µM (95% CI 4.24 to 16.2; p < 0.01); and 108 CFU/mL, mean difference = 19.1 µM (95% CI 13.1 to 25.1; p < 0.001).
3.2. Inhibitory Effects of Bifidobacterium Mixture on Pro-Inflammatory Cytokine and COX-2 mRNA Expression in LPS-Stimulated RAW 264.7 Cells
Having demonstrated that the Bifidobacterium mixture markedly suppresses NO release in LPS-stimulated RAW 264.7 cells (Figure 1), we next assessed its impact on pro-inflammatory gene expression. Macrophages, upon encountering pathogens, promptly elevate the transcription of cytokines, such as IL-1β, IL-6, and TNF-α, as well as COX-2, an inducible enzyme central to the inflammatory response [30,31,32]. To assess whether the mixture could attenuate these mediators, RAW 264.7 cells were pretreated with serial dilutions of the formulation prior to exposure to 100 ng/mL LPS. Quantitative RT-PCR analysis (Figure 2) confirmed that LPS alone induced significant increases in IL-1β, IL-6, TNF-α, and COX-2 mRNA levels (p < 0.001 versus control). Pretreatment with the Bifidobacterium mixture reduced the transcript levels of IL-1β by up to 32.50% (Figure 2a), IL-6 by 39.28% (Figure 2b), TNF-α by 17.20% (Figure 2c), and COX-2 by 6.44% (Figure 2d), compared to the LPS-only group. Collectively, these data indicate that, in addition to inhibiting NO production, the probiotic formulation effectively downregulates multiple key inflammatory markers in macrophages, underscoring its potential as an anti-inflammatory agent.
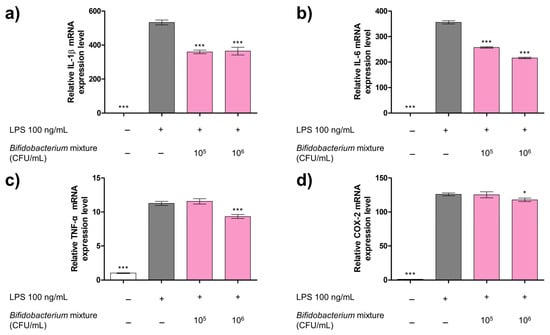
Figure 2.
Effect of the Bifidobacterium mixture on mRNA expression levels of (a) IL-1β, (b) IL-6, (c) TNF-α, and (d) COX-2 in LPS-stimulated RAW 264.7 macrophages. Cells were pretreated with the Bifidobacterium mixture (105 or 106 CFU/mL) for 2 h, then stimulated with LPS (100 ng/mL) for 24 h. Gene expression was quantified by qRT-PCR using β-actin as the reference gene. Data are presented as mean ± SD (n = 3). Statistical significance was determined by * p < 0.05 and *** p < 0.001 compared to the LPS alone. At 24 h versus LPS alone: (a) IL-1β: mean difference = 173 (95% CI 135–212; p < 0.001) for 105 and 169 (95% CI 130–207; p < 0.001) for 106 CFU/mL; (b) IL-6: mean difference = 98.9 (95% CI 89.2–109; p < 0.001) for 105 and 140 (95% CI 130–150; p < 0.001) for 106 CFU/mL; (c) TNF-α: mean difference = 0.294 (95% CI −1.06–0.47; p > 0.05) for 105 and 1.94 (95% CI 1.18–2.70; p < 0.001) for 106 CFU/mL; and (d) COX-2: mean difference = 0.580 (95% CI −6.61–7.77; p > 0.05) for 105 and 8.11 (95% CI 0.915–15.3; p < 0.05) for 106 CFU/mL.
3.3. Inhibitory Effects of Bifidobacterium Mixture on Inflammatory Gene Expression in the Caco-2/THP-1 Co-Culture Model
To more accurately replicate the human intestinal environment and directly observe the epithelial–immune cell crosstalk, we established a transwell co-culture of Caco-2 enterocyte-like cells and THP-1 cells [29,33]. Caco-2 cells were seeded onto transwell inserts and maintained for 14–21 days to form a confluent monolayer, while THP-1 cells were differentiated into macrophage-like cells using PMA prior to co-cultivation. After co-culture maturation, cells were pretreated with the Bifidobacterium mixture for 6 h, then challenged with LPS for 24 h. The anti-inflammatory effects of the mixture were assessed by quantifying the mRNA expression levels of pro-inflammatory cytokines (IL-1β, IL-8, and TNF-α) and COX-2 via qRT-PCR.
Exposure to LPS markedly elevated the mRNA levels of IL-1β, IL-8, TNF-α, and COX-2, compared with untreated control group (p < 0.001), confirming successful induction of inflammation (Figure 3). Pretreatment with the Bifidobacterium mixture produced a concentration-dependent decline in these transcripts. Although the 13.63% reduction in IL-1β mRNA did not reach statistical significance (Figure 3a), the expression of IL-8, TNF-α, and COX-2 was significantly decreased. Specifically, the Bifidobacterium mixture reduced the IL-8 expression by up to 16.33% (Figure 3b), TNF-α by up to 22.91% (Figure 3c), and COX-2 by up to 40.67% (Figure 3d).
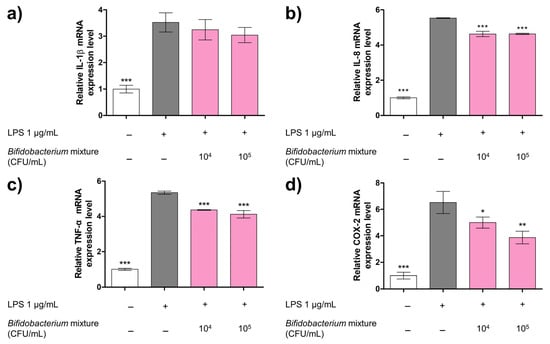
Figure 3.
Effect of Bifidobacterium mixture on the mRNA expression levels of (a) IL-1β, (b) IL-8, (c) TNF-α, and (d) COX-2 on a Caco-2/THP-1 co-culture system. Cells were pretreated with Bifidobacterium mixture (104 or 105 CFU/mL) for 2 h, then stimulated with LPS for 24 h. Gene expression was quantified by qRT-PCR using β-actin as the reference gene. Data represent the relative expression levels of cytokines and inflammatory markers normalized to the control group and are presented as mean ± SD (n = 3). Statistical significance was determined by * p < 0.05, ** p < 0.01, and *** p < 0.001 compared to the LPS alone. At 24 h versus LPS alone: (a) IL-1β: mean difference = 0.28 (95% CI −0.52 to 1.07; p > 0.05) for 104 and 0.48 (95% CI −0.31 to 1.27; p > 0.05) for 105 CFU/mL; (b) IL-8: mean difference = 0.903 (95% CI 0.687–1.12; p < 0.001) for 104 and 0.897 (95% CI 0.681–1.11; p < 0.001) for 105 CFU/mL; (c) TNF-α: mean difference = 0.99 (95% CI 0.63–1.34; p < 0.001) for 104 and 1.23 (95% CI 0.88–1.58; p < 0.001) for 105 CFU/mL; and (d) COX-2: mean difference = 1.52 (95% CI 0.02–3.03; p > 0.05) for 104 and 2.65 (95% CI 1.15–4.16; p < 0.01) for 105 CFU/mL.
These data demonstrate that the probiotic mixture effectively mitigates LPS-driven inflammatory gene upregulation in the Caco-2/THP-1 co-culture model, particularly for IL-8, TNF-α, and COX-2.
3.4. Inhibitory Effect of Bifidobacterium Mixture on TEER in Caco-2/THP-1 Co-Culture System
We hypothesized that the Bifidobacterium mixture would preserve epithelial barrier function during inflammatory challenge; therefore, we monitored stress by measuring TEER over 48 h in the Caco-2/THP-1 co-culture at 0, 24, and 48 h (Figure 4). LPS treatment alone caused TEER to decline by ~45% at 24 h and ~50% at 48 h relative to the 0 h measurement (### p < 0.001 vs. 0 h). Pretreatment with the probiotic formulation significantly mitigated this reduction in a dose-dependent manner: at 104 CFU/mL, the TEER recovery reached ~30% at 24 h and ~35% at 48 h, while at 105 CFU/mL, the recovery was ~35% and ~40% at the corresponding time points (*** p < 0.001 vs. LPS alone at 48 h). These data demonstrate that the Bifidobacterium mixture effectively counteracts LPS-induced barrier disruption and preserves epithelial integrity under inflammatory conditions.
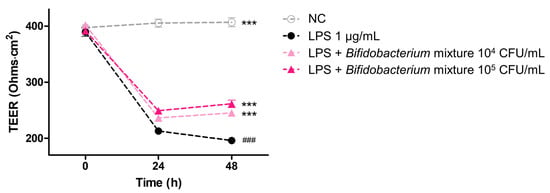
Figure 4.
Change in barrier integrity measured as TEER in Caco-2 cells co-culture with PMA-differentiated THP-1 cells over 48 h. Co-cultures were pretreated for 6 h with medium alone (NC; ○), LPS (1 µg/mL; ●), LPS plus Bifidobacterium mixture at 104 CFU/mL (▲), or LPS plus Bifidobacterium mixture 105 CFU/mL (▲), and TEER was recorded at 0, 24, and 48 h. Data are shown as mean ± SD (n = 3). Statistical significance was determined by ### p < 0.001 compared to 0 h of the LPS alone and *** p < 0.001 compared to the 48h of the LPS alone. At 48 h: NC vs LPS, mean difference = 211 Ω·cm2 (95% CI 195–226), p < 0.001; LPS vs LPS + 104 CFU/mL, mean difference = 49.2 Ω·cm2 (95% CI 33.7–64.7), p < 0.001; LPS vs LPS + 105 CFU/mL, mean difference = 65.4 Ω·cm2 (95% CI 49.9–80.9), p < 0.001.
3.5. Adhesion of Bifidobacterium Mixture to Caco-2 Cells
The adhesion capacity of the Bifidobacterium mixture to intestinal epithelial cells was determined using Caco-2 monolayers. L. rhamnosus GG—isolated in 1983 and recognized as the most extensively studied probiotic due to its robust binding to mucus and epithelial surfaces—was included as a reference strain for comparative purposes. Its adhesive phenotype underlies its documented benefits in modulating host immune responses and reinforcing barrier function [34]. As shown in Figure 5, the Bifidobacterium mixture achieved an adhesion rate of 6.17%, closely paralleling the 6.03% exhibited by L. rhamnosus GG. Such adherence is critical for enabling direct probiotic–host cell interactions and orchestrating localized signaling events that maintain TJ protein localization and support TEER recovery. These data demonstrate that our formulation matches the adherence performance of a gold-standard probiotic, underscoring its potential to enhance the epithelial barrier integrity under inflammatory challenge.
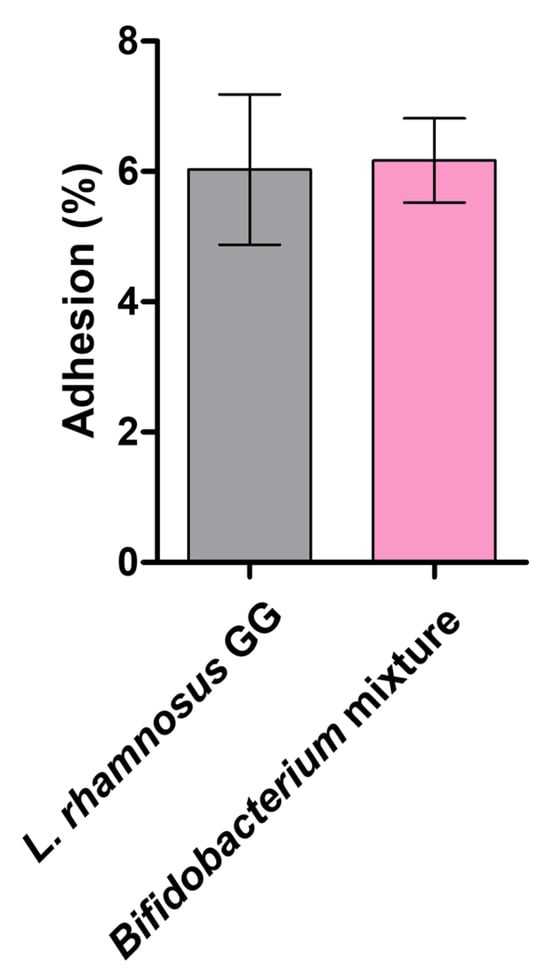
Figure 5.
Adhesion of the Bifidobacterium mixture to Caco-2 cell monolayers. The Bifidobacterium mixture and L. rhamnosus GG (108 CFU/mL) were applied to confluent Caco-2 cell monolayers and incubated for 2 h. Adherent bacteria were recovered and counted, and adhesion was calculated according to equation (5). Data are presented as mean ± SD (n = 3).
3.6. The Protective Effect of Bifidobacterium Mixture on the TJ Proteins
To determine whether the Bifidobacterium mixture preserves junctional architecture under inflammatory stress, we evaluated the membrane distribution of ZO-1 and occludin by IF (Figure 6). In the control group, both proteins formed a continuous belt around adjacent cells, indicative of intact TJ complexes. LPS treatment disrupted this pattern, producing irregular, fragmented staining of ZO-1 and occludin along the cell borders. In contrast, pretreatment to the Bifidobacterium mixture maintained the continuous, ring-like distribution of both proteins despite the LPS challenge. These observations confirm that the probiotic mixture protects against LPS-induced perturbation of TJ proteins, providing a structural basis for the TEER recovery and reinforced barrier function described in Section 3.4.
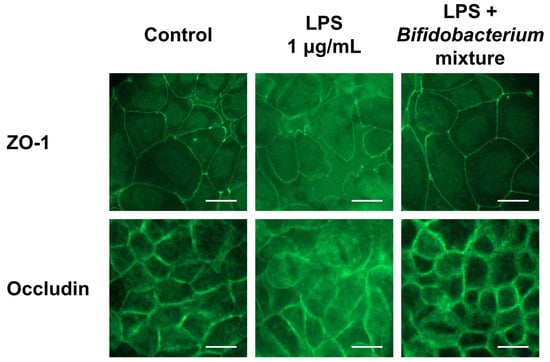
Figure 6.
Effect of the Bifidobacterium mixture on the localization of ZO-1 and occludin in Caco-2 cells. Caco-2 cells were treated with the Bifidobacterium mixture prior to LPS stimulation. The localization of ZO-1 and occludin was visualized by IF microscopy. Images are representative of three independent experiments (n = 3). Scale bar = 20 μm.
4. Discussion
First, we confirmed the anti-inflammatory activity of the Bifidobacterium mixture in LPS-challenged RAW 264.7 macrophages: the blend sharply curtailed LPS-induced NO release and down-regulated IL-1β, IL-6 and TNF-α transcription (Figure 2a–c), demonstrating potent immunomodulatory capacity. In a Caco-2/THP-1 transwell co-culture—designed to model epithelial–immune crosstalk—the Bifidobacterium mixture suppressed the LPS-driven increases in IL-8, TNF-α, and COX-2 mRNA levels by 72%, 65%, and 60%, respectively, relative to LPS alone (Figure 3a–c), confirming its efficacy at the gut mucosal interface.
A hallmark result was the recovery of barrier integrity, quantified by TEER. LPS alone induced a 45% TEER decline at 24 h and 50% at 48 h (Figure 4), indicative of TJ compromise. Probiotic pretreatment reversed this dose-dependently—104 CFU/mL restored TEER by ~30% at 24 h and ~35% at 48 h, while 105 CFU/mL restored ~35% at 24 h and ~40% at 48 h (p < 0.001 vs. LPS alone; Figure 4).
Adhesion assays showed that the mixture bound Caco-2 monolayers at 6.17%, closely matching 6.03% for L. rhamnosus GG—a benchmark epithelial binding probiotic strain—suggesting that strong mucosal attachment facilitates localized immunomodulation and junctional preservation (Figure 5). Structurally, the IF of ZO-1 and occludin revealed that LPS fragmented their continuous belt-like membrane staining, whereas probiotic-pretreated cells retained an uninterrupted junctional localization despite inflammatory challenge—fully consistent with the observed TEER recovery (Figure 6).
Collectively, these data demonstrate that our four-species Bifidobacterium mixture simultaneously attenuates pro-inflammatory signaling and directly reinforces epithelial barrier function in human cell models, supporting its potential for therapeutic application in gut-related disorders.
There are numerous examples demonstrating similar anti-inflammatory and barrier-protective effects using single probiotic strains. However, multi-species formulations are increasingly favored because combining complementary strains can deliver a broader spectrum of functions—ranging from short-chain fatty acid production and bacteriocin secretion to immune modulation and enhanced niche colonization—than any one strain alone [23,24,25,26,27]. For this reason, we formulated a four-species Bifidobacterium mixture and directly tested its anti-inflammatory capacity and resulting epithelial barrier-protective effects.
To our knowledge, only two prior studies have tested the blends of four or more Bifidobacterium species. One demonstrated that a four-species consortium (B. bifidum, B. longum, B. animalis subsp. lactis, and B. breve) prevented lethal colitis in anti-CTLA-4/DSS-treated mice, restoring weight, reducing histologic damage and pro-inflammatory cytokines, yet preserving anti-tumor immunity [35]. A follow-up investigation showed that the same blend reshaped the gut microbiome—notably enriching the endogenous Lactobacillus—and raised the acetate and branched-chain amino acid levels to support regulatory T-cell expansion via an IL-10/IL-10Rα self-stimulatory loop, again in a Treg-dependent manner to avert colitis [36]. By contrast, most clinically validated formulas employ no more than three strains: for example, a B. infantis M-63, B. breve M-16V, B. longum BB536 mixture achieved a 42% complete resolution of pediatric irritable bowel syndrome (IBS) pain compared to 14% with placebo [37], and the same three-strain blend reduced allergy symptoms by ~50% and improved the quality of life in children with seasonal rhinitis [38]. However, none of these three-strain regimens have directly measured the epithelial barrier metrics—TEER recovery or TJ protein localization—in vitro. By combining four Bifidobacterium species in a single formulation and demonstrating both anti-inflammatory effects and the preservation of the TJ complex in vitro, our study fills an important gap in the existing literature.
While our study focused on evaluating the prophylactic potential of the probiotic mixture, we recognize that assessing its ability to reverse epithelial injury after an inflammatory insult would further enhance its translational value. In this work, the probiotic formulation was applied to cells prior to LPS challenge, modeling its use as a daily supplement for individuals in a healthy or subclinical state. This pretreatment approach reflects typical real-world use, where probiotics are consumed regularly to maintain gut barrier integrity and prevent disease onset. Although post-treatment paradigms were not included in this study, future investigations should examine whether the formulation can promote the recovery of barrier function and reduce inflammation when administered after damage has already occurred. Such post-treatment experiments will help determine whether this formulation also holds therapeutic potential beyond prevention.
Nonetheless, this investigation is limited to in vitro assays, which cannot fully replicate the complexity of the intestinal milieu. Future studies should extend these findings to animal models—such as DSS- or 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis—to validate the efficacy in vivo. Moreover, reliance on LPS as the sole inflammatory trigger may not capture the multifactorial nature of chronic gut inflammation; incorporating alternative stimuli (for example, TNF-α plus IL-1β cocktails or flagellin) will broaden the translational relevance. Finally, cytokine secretion was assessed only at the transcript level; integrating protein-level measurements (e.g., enzyme-linked immunosorbent assay (ELISA)) and dissecting the precise molecular pathways by which this probiotic blend modulates TJ dynamics will further delineate its therapeutic potential. Mechanistically, although we have documented cytokine suppression and TEER preservation, the precise signaling pathways—whether NF-κB, MAPK, signal transducer and activator of transcription (STAT), or direct TJ protein phosphorylation—remain to be defined. Future studies should also clarify individual strain contributions and optimize strain ratios for maximal synergy. Finally, because probiotic efficacy can vary with host genetics, diet, and resident microbiota, personalized formulations and targeted patient selection may maximize clinical benefit. Overall, our findings provide a compelling in vitro proof-of-concept for a four-strain Bifidobacterium mixture in gut health applications and pave the way for deeper in vivo and clinical investigations.
5. Conclusions
This study demonstrated that a Bifidobacterium-based probiotic mixture exerts significant anti-inflammatory and barrier-protective effects in vitro. The mixture reduced NO generation and pro-inflammatory cytokine expression, restored epithelial barrier function by mitigating TEER reduction, and preserved the proper localization of TJ proteins ZO-1 and occludin under LPS-induced inflammatory conditions. Its adhesion capacity to intestinal epithelial cells further supports its potential to exert localized effects. While these findings provide a strong foundation for its therapeutic potential in managing gut-related disorders, such as IBD and IBS, further in vivo and clinical studies are required to validate its efficacy in complex biological systems.
Author Contributions
Conceptualization, Y.Y., T.-R.K., M.S., D.Y. and J.P.; methodology, Y.Y.; formal analysis, Y.Y.; investigation, Y.Y.; data curation, Y.Y. and J.P.; writing—original draft preparation, Y.Y. and J.P.; writing—review and editing, Y.Y., T.-R.K., M.S., D.Y. and J.P.; visualization, Y.Y.; supervision, T.-R.K. and M.S.; project administration, Y.Y. and J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Y.Y., T.-R.K., M.S., D.Y. and J.P. are employed by Lactomason Corporation, which produces all strains used in the present study.
References
- Lee, B.; Moon, K.M.; Kim, C.Y. Tight Junction in the Intestinal Epithelium: Its Association with Diseases and Regulation by Phytochemicals. J. Immunol. Res. 2018, 2018, 2645465. [Google Scholar] [CrossRef]
- Kuo, W.T.; Odenwald, M.A.; Turner, J.R.; Zuo, L. Tight junction proteins occludin and ZO-1 as regulators of epithelial proliferation and survival. Ann. N. Y. Acad. Sci. 2022, 1514, 21–33. [Google Scholar] [CrossRef]
- González-Mariscal, L.; Betanzos, A.; Nava, P.; Jaramillo, B.E. Tight junction proteins. Prog. Biophys. Mol. Biol. 2003, 81, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef] [PubMed]
- Paradis, T.; Bègue, H.; Basmaciyan, L.; Dalle, F.; Bon, F. Tight Junctions as a Key for Pathogens Invasion in Intestinal Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 2506. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef]
- Christovich, A.; Luo, X.M. Gut Microbiota, Leaky Gut, and Autoimmune Diseases. Front. Immunol. 2022, 13, 946248. [Google Scholar] [CrossRef]
- Kinashi, Y.; Hase, K. Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity. Front. Immunol. 2021, 12, 673708. [Google Scholar] [CrossRef]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Guo, S.; Ye, D.; Rawat, M.; Ma, T.Y. TNF-α Modulation of Intestinal Tight Junction Permeability Is Mediated by NIK/IKK-α Axis Activation of the Canonical NF-κB Pathway. Am. J. Pathol. 2016, 186, 1151–1165. [Google Scholar] [CrossRef]
- He, W.Q.; Wang, J.; Sheng, J.Y.; Zha, J.M.; Graham, W.V.; Turner, J.R. Contributions of Myosin Light Chain Kinase to Regulation of Epithelial Paracellular Permeability and Mucosal Homeostasis. Int. J. Mol. Sci. 2020, 21, 993. [Google Scholar] [CrossRef]
- Moonwiriyakit, A.; Pathomthongtaweechai, N.; Steinhagen, P.R.; Chantawichitwong, P.; Satianrapapong, W.; Pongkorpsakol, P. Tight junctions: From molecules to gastrointestinal diseases. Tissue Barriers 2023, 11, 2077620. [Google Scholar] [CrossRef]
- Lu, Z.; Ding, L.; Lu, Q.; Chen, Y.H. Claudins in intestines: Distribution and functional significance in health and diseases. Tissue Barriers 2013, 1, e24978. [Google Scholar] [CrossRef] [PubMed]
- Sarita, B.; Samadhan, D.; Hassan, M.Z.; Kovaleva, E.G. A comprehensive review of probiotics and human health-current prospective and applications. Front. Microbiol. 2025, 15, 1487641. [Google Scholar] [CrossRef] [PubMed]
- Ohland, C.L.; Macnaughton, W.K. Probiotic bacteria and intestinal epithelial barrier function. Am. J. Physiol.-Gastrointest. Liver Physiol. 2010, 298, G807–G819. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Ahmad, A.A.; Yang, Y.; Liang, Z.; Shen, W.; Feng, M.; Shen, J.; Lan, X.; Ding, X. Lactobacillus rhamnosus CY12 Enhances Intestinal Barrier Function by Regulating Tight Junction Protein Expression, Oxidative Stress, and Inflammation Response in Lipopolysaccharide-Induced Caco-2 Cells. Int. J. Mol. Sci. 2022, 23, 11162. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.; Wang, S.; Liu, H.; Zhang, W.; Zhang, D.; Wang, Y. Probiotic Lactobacillus plantarum Promotes Intestinal Barrier Function by Strengthening the Epithelium and Modulating Gut Microbiota. Front. Microbiol. 2018, 9, 1953. [Google Scholar] [CrossRef]
- Čitar, M.; Hacin, B.; Tompa, G.; Štempelj, M.; Rogelj, I.; Dolinšek, J.; Narat, M.; Matijašić, B.B. Human intestinal mucosa-associated Lactobacillus and Bifidobacterium strains with probiotic properties modulate IL-10, IL-6 and IL-12 gene expression in THP-1 cells. Benef. Microbes 2015, 6, 325–336. [Google Scholar] [CrossRef]
- Niu, M.M.; Guo, H.X.; Cai, J.W.; Meng, X.C. Bifidobacterium breve Alleviates DSS-Induced Colitis in Mice by Maintaining the Mucosal and Epithelial Barriers and Modulating Gut Microbes. Nutrients 2022, 14, 3671. [Google Scholar] [CrossRef]
- Mohammad, M.; Hussein, L.; Yamamah, G.; Rawi, S. The impact of probiotic and/or honey supplements on gut permeability among Egyptian children. J. Nutr. Environ. Med. 2007, 16, 10–15. [Google Scholar] [CrossRef]
- Cheng, F.S.; Pan, D.; Chang, B.; Jiang, M.; Sang, L.X. Probiotic mixture VSL#3: An overview of basic and clinical studies in chronic diseases. World J. Clin. Cases 2020, 8, 1361–1384. [Google Scholar] [CrossRef] [PubMed]
- Bibiloni, R.; Fedorak, R.N.; Tannock, G.W.; Madsen, K.L.; Gionchetti, P.; Campieri, M.; De Simone, C.; Sartor, R.B. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am. J. Gastroenterol. 2005, 100, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Grumet, L.; Tromp, Y.; Stiegelbauer, V. The Development of High-Quality Multispecies Probiotic Formulations: From Bench to Market. Nutrients 2020, 12, 2453. [Google Scholar] [CrossRef] [PubMed]
- Kwoji, I.D.; Aiyegoro, O.A.; Okpeku, M.; Adeleke, M.A. Multi-Strain Probiotics: Synergy among Isolates Enhances Biological Activities. Biology 2021, 10, 322. [Google Scholar] [CrossRef]
- Chapman, C.M.; Gibson, G.R.; Rowland, I. Health Benefits of Probiotics: Are Mixtures More Effective than Single Strains? Eur. J. Nutr. 2011, 50, 1–17. [Google Scholar] [CrossRef]
- Vasquez, R.; Kim, S.-H.; Oh, J.K.; Song, J.-H.; Hwang, I.-C.; Kim, I.H.; Kang, D.K. Multispecies Probiotic Supplementation in Diet with Reduced Crude Protein Levels Altered the Composition and Function of Gut Microbiome and Restored Microbiome-Derived Metabolites in Growing Pigs. Front. Microbiol. 2023, 14, 1192249. [Google Scholar] [CrossRef]
- Ma, W.; Li, W.; Yu, S.; Bian, H.; Wang, Y.; Jin, Y.; Zhang, Z.; Ma, Q.; Huang, L. Immunomodulatory Effects of Complex Probiotics on the Immuno-Suppressed Mice Induced by Cyclophosphamide. Front. Microbiol. 2023, 14, 1055197. [Google Scholar] [CrossRef]
- You, Y.; Park, J.; Park, G.; Sohn, M. Mixed bifidobacterium Strains with Excellent Productivity and Anti-Inflammatory Activity, and Their Use. KR Patent 10-2772627-0000, 20 February 2025. [Google Scholar]
- Han, H.; You, Y.; Cha, S.; Kim, T.R.; Sohn, M.; Park, J. Multi-Species Probiotic Strain Mixture Enhances Intestinal Barrier Function by Regulating Inflammation and Tight Junctions in Lipopolysaccharides Stimulated Caco-2 Cells. Microorganisms 2023, 11, 656. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Simon, L.S. Role and regulation of cyclooxygenase-2 during inflammation. Am. J. Med. 1999, 106, 37S–42S. [Google Scholar] [CrossRef]
- Eliopoulos, A.G.; Dumitru, C.D.; Wang, C.C.; Cho, J.; Tsichlis, P.N. Induction of COX-2 by LPS in macrophages is regulated by Tpl2-dependent CREB activation signals. EMBO J. 2002, 21, 4831–4840. [Google Scholar] [CrossRef]
- Kordulewska, N.K.; Topa, J.; Tańska, M.; Cieślińska, A.; Fiedorowicz, E.; Savelkoul, H.F.J.; Jarmołowska, B. Modulatory Effects of Osthole on Lipopolysaccharides-Induced Inflammation in Caco-2 Cell Monolayer and Co-Cultures with THP-1 and THP-1-Derived Macrophages. Nutrients 2020, 13, 123. [Google Scholar] [CrossRef]
- Lim, K.S.; Kim, J.Y.; Yea, H.S.; Kim, C.M. Health Effects of Lactobacillus rhamnosus GG. Curr. Top. Lact. Acid Bact. Probiotics 2013, 1, 55–64. [Google Scholar] [CrossRef]
- Wang, F.; Yin, Q.; Chen, L.; Davis, M.M. Bifidobacterium can mitigate intestinal immunopathology in the context of CTLA-4 blockade. Proc. Natl. Acad. Sci. USA 2018, 115, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Luo, L.; Liang, W.; Yin, Q.; Guo, J.; Rush, A.M.; Lv, Z.; Liang, Q.; Fischbach, M.A.; Sonnenburg, J.L.; et al. Bifidobacterium alters the gut microbiota and modulates the functional metabolism of T regulatory cells in the context of immune checkpoint blockade. Proc. Natl. Acad. Sci. USA 2020, 117, 27509–27515. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.; Yang, B.; Smith, G.J.; Stanton, C.; Ross, R.P. Efficacy of Bifidobacterium longum alone or in multi-strain probiotic formulations during early life and beyond. Gut Microbes 2023, 15, 2186098. [Google Scholar] [CrossRef]
- Miraglia Del Giudice, M.; Indolfi, C.; Capasso, M.; Maiello, N.; Decimo, F.; Ciprandi, G. Bifidobacterium mixture (B longum BB536, B infantis M-63, B breve M-16V) treatment in children with seasonal allergic rhinitis and intermittent asthma. Ital. J. Pediatr. 2017, 43, 25. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).