Abstract
Meloidogyne enterolobii, a highly virulent and broad-host-range plant-parasitic nematode, poses an increasing threat to global agricultural production. By inducing the formation of nutrient-rich giant cells in host roots and deploying a diverse array of effector proteins to modulate plant immune responses, this nematode achieves efficient colonization and invasion, resulting in impaired crop growth and significant economic losses. In recent years, global climate warming combined with the rapid development of protected agriculture has broken the traditional geographical limits of tropical and subtropical regions, thereby increasing the risk of M. enterolobii occurrence in temperate and high-latitude areas. Concurrently, conventional chemical control methods are increasingly limited by environmental pollution and the development of resistance, steering research toward green control strategies. This review systematically summarizes the latest research progress of M. enterolobii in terms of ecological diffusion trends, pathogenic mechanisms, and green control, and explored the feasibility of integrating multidisciplinary technologies to construct an efficient and precise control system. The ultimate aim is to provide theoretical support and technical supports for green and sustainable development of global agriculture.
1. Introduction
Plant-parasitic nematodes (PPNs) are significant pathogens with a global distribution, remarkable adaptability, and wide host range, resulting in annual economic losses of up to USD 157 billion [1]. Among these, root-knot nematodes (RKNs) are classic representatives of obligate endoparasitic nematodes, infecting over 5500 plant species. They pose a particular threat to solanaceous, leguminous, and cucurbit crops, accounting for annual losses exceeding USD 8 billion [2]. Currently, over 100 species of RKNs have been documented, with Meloidogyne incognita, M. hapla, M. arenaria, and M. javanica being the most prevalent [3]. Recently, however, M. enterolobii has emerged as a major species in both protected agriculture and tropical crop regions due to its high virulence, broad host range, and ability to overcome conventional resistance genes [4,5].
Originally discovered in 1983 on the Pacara earpod tree in Danzhou, Hainan, China [6], M. enterolobii was later misidentified as M. mayaguensis on eggplant in Puerto Rico [7] until definitive confirmation was achieved through morphological and molecular evidence in 2004 [8]. Initially confined to tropical and subtropical regions, its distribution has since expanded to mid-latitude areas as a result of global warming and ongoing development of protected agriculture [9]. In China, populations have rapidly migrated northward from southern provinces such as Hainan and Guangdong, reaching regions like Heilongjiang and Jilin, which has jeopardized the safe production of greenhouse vegetables and staple crops [10].
Meloidogyne enterolobii is distinguished by its exceptionally wide host range and a unique set of effector proteins. These proteins enable the nematode to circumvent key resistance genes, facilitating the establishment of high-density infections across a broad spectrum of crops [11,12]. This nematode, characterized by its extremely high infection efficiency and high adaptability, can significantly impair the root function of crops with a single infection [13]. By repeatedly probing host tissues and secreting multiple effectors, the nematode induces the formation of giant cells, which disrupt the normal transport of water and nutrients [14]. In light of the environmental pollution and resistance risks associated with conventional chemical controls, the development of a green control system based on an integrated “monitoring–prevention–interruption–remediation” strategy has become a research focus [15]. Approaches that combine agricultural management practices, biological control agents, and eco-friendly pesticides have shown promising potential by either inducing systemic acquired resistance in host plants or directly inhibiting nematode activity.
This review focuses on the ecological diffusion trend, molecular pathogenic mechanisms, and environmentally sustainable control technologies of M. enterolobii. It analyzes the molecular interactions between the nematode and its hosts, discusses the challenges in breeding for resistance, and provides an outlook on integrated, multi-disciplinary control strategies in the context of climate change. These insights aim to contribute to effective management strategies for this nematode.
2. Global Distribution Characteristics
The geographic distribution of M. enterolobii is significantly temperature-dependent, initially concentrated in tropical and subtropical regions (Figure 1). Early reports documented populations established in European greenhouse systems, notably on Martinique, an overseas region of France and in Sicily (Italy) [16,17]. Subsequently, areas with high incidence emerged in the Niger Delta and the Kenyan Rift Valley, reflecting enhanced ecological adaptability in natural systems [18,19]. Between 2020 and 2023, severe outbreaks were also reported along the Ganges Plain in India and in the Chao Phraya River basin in Thailand [20,21]. In the Americas, aside from occurrences in the Brazilian Amazon, new epidemic regions have been detected in the California Central Valley [12,22], indicating a northward spread into mid-latitude regions.
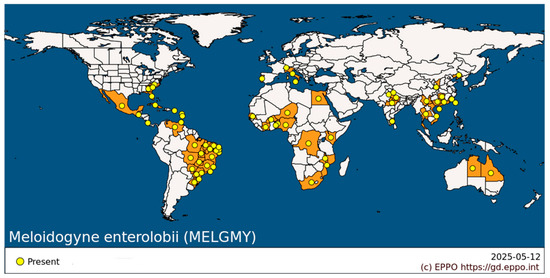
Figure 1.
Geographic distribution of Meloidogyne enterolobii across the world. (For detailed mapping information, refer to https://gd.eppo.int/taxon/MELGMY/datasheet), accessed on 12 May 2025.
In China, M. enterolobii is primarily distributed in open-field vegetable production areas in the south and in protected cultivation zones in the north. Hainan Island (18°–20° N), a representative tropical region, has an annual effective accumulated temperature—defined as the cumulative daily mean temperature above 10 °C, expressed in degree-days, °C·d—of approximately 9000 °C·d, with a vegetable infection rate exceeding 60% [23]. As the distribution of M. enterolobii extends northward along the southeastern coast to provinces such as Guangdong (23° N) and Fujian (26° N), the annual effective accumulated temperature (≥10 °C) ranges from 6000 to 8000 °C·d. This temperature range overlaps with the nematode’s optimal developmental range of 15–35 °C. Additionally, its overwintering survival threshold is approximately 10 °C [24,25]. North of the Qinling–Huaihe climatic boundary (32°–34° N), outdoor environments experience mean annual temperatures below the overwintering threshold of M. enterolobii (10 °C). However, protected agriculture has mitigated this limitation; for instance, in Yangling, Shaanxi (34° N), winter temperatures in greenhouse soils remain above 15 °C [26], and in Shenyang, Liaoning (41° N), the duration of temperatures in solar greenhouses extends to ≥15 °C for up 200 days [27]. Recently, M. enterolobii was detected in a greenhouse in Daqing, Heilongjiang (46° N), marking a significant breakthrough of its northern limit into the temperate zone [28]. Therefore, it is urgently necessary to strengthen the monitoring and control of this nematode to mitigate its impact on agriculture.
3. Damage and Host Range
Meloidogyne enterolobii damages host plants by inducing gall formation, which interferes with water and nutrient absorption, thereby inhibiting plant growth [29]. Its feeding behavior is notably aggressive—during the establishment of feeding sites, the nematode repeatedly punctures host cortical tissues with its stylet, exacerbating cell damage [30,31]. Moreover, infection by M. enterolobii alters the chemical composition of root exudates—such as the levels of amino acids, carbohydrates, and sucrose in root tissues—creating favorable conditions for pathogenic fungi including Fusarium, Pythium, and Rhizoctonia, which further exacerbate crop damage [32,33]. The damage caused by root-knot nematodes is closely linked to their population density, with conventional threshold levels set at 0.5–2 s-stage juveniles (J2) per gram of soil [34]. In contrast, the damage threshold for M. enterolobii is as low as 0.25 J2 per gram, which is lower than the damage thresholds reported for other Meloidogyne species [35]. Under identical inoculation conditions, the infection efficiency of M. enterolobii is 30–50% higher than that of common RKN species [36].
Furthermore, while the optimum temperature for many RKNs, such as M. incognita and M. javanica, ranges from 25 to 30 °C, their growth and reproduction are inhibited above 35 °C. In contrast, M. enterolobii can survive at temperatures as high as 44 °C, demonstrating remarkable heat tolerance and superior environmental adaptability. In addition, M. enterolobii has been documented to infect more than 61 plant species across 24 families [37]. Due to its high infection capability and broad host adaptability, M. enterolobii has emerged as a dominant pathogen in tropical and subtropical agricultural ecosystems.
4. Molecular Mechanisms in the Interaction Between M. enterolobii and Host Plants
4.1. Life Cycle of M. enterolobii
The life cycle of M. enterolobii is similar to that of other root-knot nematodes. It begins with the egg stage, from which the first-stage juvenile (J1) hatches and subsequently molts into the second-stage juvenile (J2), the only infective stage. Attracted by root exudates, J2s penetrate the elongation zone of young roots and migrate intercellularly until they reach the vascular tissues, where they establish a feeding site. Using their stylet, the nematodes repeatedly pierce plant cell walls and inject secretions from their esophageal glands, which reprogram host cells to induce the formation of multinucleated giant cells. These giant cells are formed through multiple rounds of nuclear division without cytokinesis and serve as permanent feeding sites, supplying nutrients essential for nematode development. After establishing feeding sites, J2s resume development and undergo three successive molts to become third-stage juvenile (J3) and fourth-stage juveniles (J4), eventually maturing into adults. The females become swollen and assume a pear-shaped morphology, producing gelatinous egg masses containing several hundred eggs, which are deposited on the root surface or released into the surrounding soil. Notably, M. enterolobii reproduces through obligatory mitotic parthenogenesis (apomixis), enabling females to produce offspring without fertilization [31]. Under favorable environmental conditions, M. enterolobii can complete a full life cycle within 25 to 35 days [37].
4.2. Formation of Feeding Sites
Following the initiation of infection, M. enterolobii establishes highly specialized feeding sites within the vascular cylinder of host roots [38]. These sites are characterized by the presence of multinucleated giant cells, which are induced through the injection of esophageal gland secretions that reprogram host cellular processes [39,40]. Unlike the feeding sites formed by other common RKNs, such as M. incognita, the giant cells induced by M. enterolobii in tomatoes exhibit distinctive structural and metabolic features, including thinner cell walls, denser cytoplasmic content, and enhanced metabolic activity [41,42]. These cellular modifications facilitate a more efficient nutrient flow toward the nematode, promoting faster growth and reproduction. Additionally, surrounding parenchyma cells often proliferate and become hypertrophied, contributing to the formation of root galls. The superior functionality of these feeding sites is believed to underpin the high virulence and reproductive capacity of M. enterolobii across a wide range of host plants.
4.3. Functions of Effector Proteins
Effector proteins—small molecules secreted by the nematode—play crucial roles during host invasion, feeding site establishment, and the suppression of plant defense responses. Although transcriptomic and genomic studies on M. enterolobii began relatively recently [4], the few reported effectors (summarized in Table 1) already suggest distinctive features in their composition and regulatory functions. These effectors contribute to: (1) Host adaptability—they facilitate infection in a wide range of crops, including those that typically exhibit strong resistance, such as tomatoes and peppers, by suppressing host defenses and breaching resistance barriers [43]. (2) Suppression of plant immunity—certain effectors, such as MeTCTP, are highly expressed in the dorsal esophageal gland and help suppress host programmed cell death, thereby aiding nematode parasitism [44]. (3) Temperature adaptability—the stability and functionality of specific effector proteins (MeHsp70) may underlie the ability of the nematode’s ability to maintain virulence under high-temperature conditions typical of tropical and subtropical environments.

Table 1.
Identified effectors of Meloidogyne enterolobii and their functions.
Although research on M. enterolobii effectors is still in its early stages, evidence suggests that these proteins are involved not only in fundamental parasitic processes, such as cell wall degradation and immune regulation, but also in conferring broad-spectrum host infectivity through unique structural and expression patterns. More research should further elucidate their molecular mechanisms to support the development of novel control strategies.
4.4. Host Resistance Genes Against RKNs
Host recognition and defense against RKNs largely depend on two layers of immunity: pattern-triggered immunity (PTI) and effector-triggered immunity (ETI) [56]. In these processes, resistance (R) genes play a crucial role by directly or indirectly recognizing nematode effectors and triggering downstream defense signaling cascades [57]. Most cloned and validated RKN resistance genes encode nucleotide-binding leucine-rich repeat (NB-LRR) proteins, such as Mi-1.2, Mi-9, Hero, and Me [58,59]. These genes typically possess a conserved nucleotide-binding (NB) domain and leucine-rich repeat (LRR) domains; their high variability enhances the recognition of a diverse array of nematode effectors [60,61].
In many plant genomes, R genes are organized into multigene clusters, where gene duplication and tandem arrangement provide the raw material for the evolution of novel recognition specificities [62]. For instance, the Mi gene family in tomatoes consists of clusters containing three to four members, which, together, constitute a multilayered defense network [63]. Mechanisms such as gene duplication, domain shuffling, and alternative splicing have significantly contributed to the dynamic evolution of the host immune system during its arms race with nematodes [64,65].
In contrast to other RKNs, M. enterolobii possesses the ability to overcome the Mi-1 resistance gene [66,67]. This phenomenon indicates the presence of intraspecific virulence variation within the nematode population, suggesting that during prolonged coevolution with resistant hosts, M. enterolobii has gradually developed adaptive strategies to circumvent defense mechanisms. Similarly to other plant pathogens, the repeated deployment of the same resistance gene can accelerate the breakdown of resistance, thereby presenting significant challenges for both breeding programs and integrated disease management.
5. Rapid Detection Techniques for M. enterolobii
Due to the high pathogenicity of M. enterolobii, establishing rapid, sensitive, and specific detection methods is critical for its integrated management. In recent years, various detection techniques have been developed, including real-time quantitative PCR (qPCR), droplet digital PCR (ddPCR), locked nucleic acid (LNA)-modified probes, loop-mediated isothermal amplification (LAMP), and multiplex PCR. Novel qPCR and ddPCR methods utilizing TaqMan probes, with species-specific primer and probe combinations, enable precise detection of M. enterolobii in soil samples. The absolute quantification capability of ddPCR significantly enhances detection sensitivity, providing a powerful tool for early diagnosis [68,69].
In the context of complex DNA backgrounds, LNA-modified real-time PCR assays that incorporate high-affinity LNA probes effectively reduce non-specific amplification, thereby enhancing the accuracy of field sample detection [70]. Additionally, closed-tube colorimetric and fluorescence LAMP assays, which benefit from ease of operation, low equipment requirements, and simplified nucleic acid extraction protocols, enable rapid on-site identification of nematodes [71,72]. A novel isothermal detection method based on enzyme-mediated dual exponential amplification (EmDEA) has recently been developed, offering sensitivity and specificity comparable to that of qPCR. By integrating a portable constant-temperature module with a rapid DNA extraction protocol, this method facilitates on-site detection of M. enterolobii, significantly enhancing diagnostic efficiency in the field. Additionally, it provides advantages in sample handling and result interpretation [73].
Under laboratory conditions, PCR amplification techniques utilizing ribosomal DNA (rDNA) and mitochondrial DNA (mtDNA) significantly enhance interspecies discrimination [74]. The specific primer sequences for common root-knot nematodes are presented in Table 2. With the growing availability of genomic data, targeting species-specific sequences has emerged as an effective strategy for rapid diagnosis [75,76].

Table 2.
The species-specific primers for root-knot nematodes.
6. Green Control Strategies for M. enterolobii
Currently, the management of M. enterolobii follows a “prevention-first, integrated control” strategy, combining cultural practices, environmentally friendly pesticides, biological control, and the breeding of resistant cultivars to achieve sustainable nematode suppression. As illustrated in Figure 2, this integrated approach emphasizes green and eco-friendly management practices, including optimized cultivation techniques, targeted pesticide application, and the use of biocontrol agents and resistant crop varieties tailored to regional conditions and cropping systems.
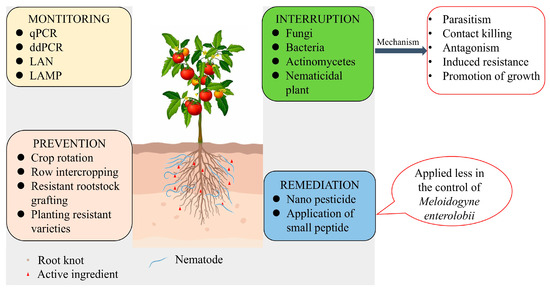
Figure 2.
Green control strategies for Meloidogyne enterolobii.
6.1. Cultural Control
The optimal temperature for the development of M. enterolobii is 25 °C and 30 °C, and its activity patterns are closely related to temperature [36]. Consequently, control measures must be tailored to regional climatic differences. In the high-temperature regions of Southern China, winter soil temperatures often remain above 10 °C, facilitating the continuous survival of eggs and J2 in crop residues or shallow soil layers, which results in overlapping generations throughout the year. Recommended practices include the post-harvest removal of crop residues, deep tillage, and subsequent solarization. Planting marigolds in winter also can diminish nematode populations by secreting nematicidal compounds such as α-thiophene [80]. In colder northern regions, low temperatures naturally limit nematode survival. However, the implementation of greenhouse cultivation has created suitable conditions for M. enterolobii. Therefore, early removal of greenhouse coverings in spring to take advantage of transient cold spells can reduce the nematode population [5]. Crop rotation is another effective measure, Rashidifard et al. [81] demonstrated that rotating cowpea with maize significantly reduced M. enterolobii damage to maize.
6.2. Precision Chemical Control
In recent years, the research on chemical control of RKN has mainly focused on the combined application of traditional synthetic pesticides and new biological source preparations. Traditional pesticides such as organophosphorus, carbamate, and other antagonistic compounds can effectively inhibit the activity of nematodes in the short term, however, their environmental residues, toxicities, and the risks of resistance limit their long-term application, while green pesticides have broad application prospects. Recent studies on the chemical control of RKNs have prioritized the integration of conventional pesticides with biobased strategies, such as nano-formulations and peptide biopesticides, through mechanism-driven approaches. This integration enhances efficacy while reducing environmental residues and the risks of resistance [82,83]. For instance, Peng et al. [84] developed a nano-nematicide by combining fluopyram with nano-scale cationic star polymers, which improved both the efficacy and residual activity of the compound, resulting in significant yield enhancements and cost reduction.
Moreover, studies on the specific effector proteins of M. enterolobii have elucidated crucial molecular interactions between the host and pathogen, thereby suggesting novel targets for drug design. In particular, small peptides have garnered significant attention due to their low molecular weight, specific modes of action, low toxicity, and ease of engineering [85]. Zhao et al. [86] delineated the jasmonate-signaling hub where the transcription factor MYC2 is regulated by the E3 ligase, PUB21, and subsequently employed AI-driven screening to identify the antiproteolysis peptide APP3-14. This peptide achieved 80% disease control in greenhouse and field trials, effectively disrupting pathogen colonization and spread. This research confirmed the high specificity and efficacy of small peptides in plant protection, establishing a comprehensive paradigm that spans from molecular target discovery to mechanistic elucidation and AI-guided biopesticide development. Drawing on advancements in citrus Huanglongbing research, the potential of peptide technology for controlling M. enterolobii appears equally promising. Future studies should integrate AI to accelerate peptide screening and explore their synergistic and potentiating effects under field conditions.
6.3. Biological Control
The biological control of M. enterolobii primarily relies on microbial agents and their natural metabolites, which suppress nematodes through mechanisms such as antagonism, parasitism, induction of plant resistance, and growth promotion (Table 3) [87]. Bacillus species secrete lipopeptides that disrupt the osmotic regulation of nematode cuticles, while strains of Pseudomonas act by parasitizing egg masses and inducing systemic resistance in the host plant. Parasitic fungi degrade the chitinous layers of egg shells, leading to direct nematode mortality, from which predatory fungi capture J2 or adult nematodes through specialized trapping networks. Trichoderma and Aspergillus spp. also inhibit nematode growth through niche competition and the secretion of antagonistic metabolites [88,89]. Actinomycetes produce compounds, such as avermectins and terpenes, that specifically interfere with nematode neural transmission and development [90]. Furthermore, plant-derived bioactive compounds, including terpenoids (which act as repellents and inhibit egg hatch), phenolics, alkaloids, and flavonoids (which disrupt metabolism and activate plant defense responses), have been shown to reduce nematode survival and reproduction [91,92].
In recent years, natural organic polymers have garnered significant attention for their potential to regulate soil microecology. Pasche et al. [93] demonstrated that collagen and chitosan additives significantly reduce M. enterolobii egg counts and enhance plant growth in both natural and agricultural soils. These additives also enrich populations of potential nematode-antagonistic microbes, including Streptomyces, Bacillus, and Phialemonium. Through combined microbiome and metabolome analyses, the authors revealed that these materials enhance plant resistance by reshaping microbial networks and metabolite profiles, thereby unveiling a novel approach for indirect nematode control via soil microecological modulation. Furthermore, the study identified key disease-suppressive microbes, such as Paradevosia shaoguanensis, providing theoretical support for the future development of precise biocontrol agents based on synthetic microbial consortia (SynComs).
Recent efforts have also investigated the synergistic effects of combining multiple biocontrol agents and optimizing application techniques across various crop systems and soil conditions [94]. Although challenges persist regarding the field stability, persistence, and elucidation of the modes of action of microbial biocontrol agents, advances in molecular biology, fermentation technology, and gene regulation indicate that microbial-based products for controlling M. enterolobii are expected to become increasingly efficient, precise, and sustainable.

Table 3.
Biological control resources for root-knot nematodes.
Table 3.
Biological control resources for root-knot nematodes.
| Representative Category | Agent Type | Representative Species/Compounds | Mechanism of Action | References |
|---|---|---|---|---|
| Bacteria | Antagonistic bacteria | Bacillus subtilis, B. thuringiensis | Secretion of secondary metabolites that directly inhibit nematode development | [95] |
| Parasitic bacteria | Brevibacillus laterosporus | Direct parasitism of eggs or juveniles, extracting nutrients | [96] | |
| Induced systemic resistance (ISR)-eliciting bacteria | Lactobacillus spp., Pseudomonas spp. | Induction of plant systemic defenses | [97,98,99] | |
| Fungi | Parasitic fungi | Purpureocillium lilacinum, Metarhizium anisopliae | Penetration and degradation of egg shells or cuticle of juveniles | [100] |
| Predatory fungi | Arthrobotrys oligospora, Dactylella spp. | Formation of trapping structures to actively capture and kill nematode juveniles or adults | [101,102] | |
| Antagonistic fungi | Trichoderma viride, T. harzianum, T. harzianum | Secretion of antifungal/antinematodal compounds, competition for nutrients, and induction of plant defenses | [103,104] | |
| Actinomycetes | Parasitic actinomycetes | Streptomyces griseus, S. aureofaciens | Inhibition of egg hatching and direct killing of nematodes | [105] |
| Antagonistic actinomycetes | S. roseoflavus, S. hydrogenans | Production of antagonistic metabolites and competition for space and nutrients | [106] | |
| Nematicidal Plants | Terpenoids and essential oils | Azadirachtin, cucurbitacin, ursolic acid | Release of volatile oils that repel nematodes and inhibit egg hatching | [107,108] |
| Phenolics and flavonoids | Phenolic acids, flavonoids, gossypol | Disruption of nematode metabolism and neural transmission, reducing survival and reproduction | [109] | |
| Alkaloids | Nicotine, lupanine, sanguinarine | Disruption of nematode cell membranes and nervous system | [110,111] |
6.4. Breeding for Resistance
Due to its unique pathogenicity and ability to overcome traditional resistance genes, M. enterolobii has emerged as a significant barrier in the breeding for nematode resistance. Traditionally, the Mi-1 gene has been the most commercially utilized resistance gene against RKNs, providing substantial resistance to species such as M. incognita, M. arenaria, and M. javanica. However, M. enterolobii can overcome Mi-1 mediated resistance [112], underscoring the limitations of depending on a single source of resistance.
In recent years, several R genes, including Mi-2 to Mi-9 and Mi-HT, have been identified from wild tomato species. Among these, only Mi-3, Mi-5, Mi-9, and Mi-HT demonstrate significant thermostability, indicating their functionality under high-temperature conditions [113]. However, many of these genes remain in the validation phase, and their commercial application is still a distant prospect. Distinctive R genes, such as Me and N in peppers, Mj in cucurbits, and SacMi in eggplants, have been reported in crops like tomatoes, peppers, cucurbits, eggplants, and carrot (Table 4). Nevertheless, in practical applications, such as those in Brazilian greenhouses, even rootstocks containing resistance alleles like Me1 and Me3/Me7 have occasionally succumbed to M. enterolobii infection [11], suggesting that certain resistance genes may not be sufficiently robust against this nematode.
To address the breakdown of resistance, researchers are actively screening for novel resistance resources. Silva et al. [43] identified several resistant lines among both commercial and wild tomato varieties through pathogenicity assays. Rutter et al. [12] inoculated 93 sweet potato accessions with two physiological races of M. enterolobii that differ in virulence and evaluated their resistance by counting galls and egg masses. Ultimately, 19 accessions were identified as resistant to both physiological races.
Conventional breeding typically spans over a decade, and in the absence of breeding of resistant cultivars, this process is notably sluggish. Recent advances in PCR-based molecular markers and marker-assisted selection (MAS) have significantly enhanced breeding efficiency; however, their application in breeding for resistance against M. enterolobii remains limited [114]. Accelerating the molecular identification and functional validation of resistance genes, along with integrating these genes into adapted cultivars through transgenic or molecular breeding approaches, will be crucial for future progress. Furthermore, the use of resistant rootstock grafting has been validated as an alternative strategy in certain crops when suitable resistant cultivars are unavailable [115,116].

Table 4.
Major crop resistance genes and their resistance characteristics.
Table 4.
Major crop resistance genes and their resistance characteristics.
| Crop | Germplasm Accession | Resistance Gene | Key Features | References |
|---|---|---|---|---|
| Tomato | Solanum peruvianum and S. pimpinellifolium | Mi-1 | Confers resistance to Meloidogyne incognita, M. arenaria and M. javanica; located at the distal end of the short arm of chromosome 6; temperature-sensitive | [117,118] |
| Other Mi alleles | Confer resistance to either M. incognita or M. javanica; heat-stable | [119] | ||
| Mi-9 | Homologous to Mi-1, at the distal end of chromosome 6; heat-stable; broad-spectrum resistance to M. incognita, M. arenaria, M. javanica | [120,121] | ||
| Mi-HT | At the same locus on chromosome 6; heat-stable, broad-spectrum resistance | [112] | ||
| Hero | Located on chromosome 4; confers resistance to root-knot nematodes and potato cyst nematode | [122] | ||
| Pepper | Nemaheart, Carolina wonder | N | Resistance to M. incognita, M. arenaria, M. javanica; susceptibility to M. hapla; resistance breaks down above 28 °C | [123] |
| PI 322719, PI 201234, CM334 | Me1, Me3 | Resistance to M. incognita, M. arenaria, M. javanica; no resistance to M. enterolobii; mapped to the distal 28 cM region of chromosome P9 | [13] | |
| Cucurbits | LI 90430 | Mj | Resistance to M. incognita, M. arenaria, M. javanica, M. hapla; used in cultivars ‘Lucia’, ‘Manteo’, ‘Shelby | [124] |
| Cucumis metuliferus | EVM0025394 EVM0006042 | Resistance to M. incognita; not yet deployed in commercial breeding | [125] | |
| Cucumis hystrix | Csa5M608240.1 Csa5M610420.1 Csa5M623410.1 Csa5M610370.1 | Resistance to M. incognita; not yet deployed in commercial breeding | [126] | |
| Eggplant | Solanum aculeatissimum | SacMi | Resistance to M. incognita; member of the NBS-LRR gene family | [127] |
| Carrot | Brasilia, PI 652188 | Mj-1, Mj-2 | Confer resistance to M. incognita and M. javanica; both loci mapped on chromosome 8, separated by 41 cM | [128,129] |
7. Challenges and Future Perspectives
Despite significant progress in recent years in understanding the pathogenic biology, host interaction mechanisms, and green control strategies against M. enterolobii, many key challenges remain unresolved. To construct an efficient, precise, and sustainable integrated management system, future research should delve into the specific directions highlighted below.
7.1. Deciphering Temperature Adaptation Mechanisms Using High-Throughput Omics and Gene Editing Technologies
Traditional studies have predominantly emphasized physiological and pathological data, thereby lacking a systematic elucidation of the underlying molecular regulatory mechanisms. Future research should leverage high-throughput approaches—such as transcriptomics, proteomics, and metabolomics—to characterize the molecular responses and adaptive regulatory networks of M. enterolobii under varying temperature conditions. Concurrently, gene editing tools, including CRISPR-Cas9, can be utilized to functionally validate candidate regulatory genes, thereby uncovering the critical molecular nodes that govern nematode growth and reproduction in response to heat stress.
Furthermore, RNA interference (RNAi) serves as a complementary and readily accessible tool for functional gene analysis. Due to its simplicity, cost-effectiveness, and ability to target specific genes, RNAi has been successfully applied in various nematode systems to study stress response mechanisms. Incorporating RNAi into thermal adaptation research will facilitate targeted gene silencing of candidates involved in temperature sensing, signal transduction, and downstream defense regulation [130,131]. This approach will advance the functional elucidation of the molecular basis underlying heat tolerance in M. enterolobii, especially when integrated with omics-based gene discovery and gene-editing validation workflows.
7.2. Developing Dynamic Predictive Models Based on GIS and Machine Learning
Currently, the prediction of M. enterolobii distribution risk primarily relies on regional statistical data derived from experiments, which lacks a dynamically updated global risk assessment. Future efforts should aim to integrate climate, soil, and crop cultivation data using Geographic Information Systems (GIS) and combine these with machine learning algorithms to construct dynamic predictive models that can monitor and forecast the distribution risk of M. enterolobii in real time. Such models will facilitate early warning systems and control measures across regions and national borders, thereby guiding agricultural management decisions.
7.3. Integrating Multimodal Control Strategies with Intelligent Controlled-Release Systems
Green control measures should transition from singular approaches to a synergistic, multimodal strategy. Future research should focus on the following areas:
Synergy between chemical and biological control: investigating the synergistic effects between traditional chemical controls and innovative biobased formulations (such as small peptides and nano-controlled-release agents) to optimize dosage regimens that ensure efficacy while minimizing environmental risks.
Intelligent controlled-release technologies: Utilizing nanotechnology and micro-control technologies-such as nanocarriers and stimuli-responsive polymers to develop intelligent controlled-release systems that facilitate targeted application. These systems could automatically adjust the release rate based on environmental cues such as soil temperature and moisture, thereby enhancing the durability and stability of field control measures.
7.4. Potential Links Between Microbiome Manipulation and the Pathogenic Mechanisms of M. enterolobii
Recent studies have demonstrated that root-knot nematodes (RKNs) are not merely passive parasites, they actively modulate the rhizosphere microbiome to create a favorable environment for infection [132]. For instance, M. incognita can induce restructuring of rhizosphere communities, enriching them with microbes such as Pseudomonas and Bacillus; this alteration indirectly interferes with plant immunity, thereby promoting nematode colonization [133]. Certain effectors, such as MiMIF-2, have also been shown to directly alter microbial ecosystems, further enhancing parasitic capabilities [134]. Although direct evidence for such mechanisms in M. enterolobii remains limited, its high virulence and broad host range suggest that it may employ similar microbiome modulation strategies. Notably, M. enterolobii may secrete specific effectors that recruit microbial communities distinct from those associated with other RKNs, exhibiting a unique pattern of microbiome regulation. Moreover, due to its wider host spectrum, M. enterolobii is likely to induce more complex and diverse microbiome shifts across different hosts, further complicating the elucidation of its pathogenic mechanisms.
Future research should focus on whether M. enterolobii influences the composition of rhizospheric and endophytic microbiomes through the secretion of specific effectors, and how such modulation interacts with host immune responses. Additionally, investigating microbiome responses in host genotypes with varying resistance levels to M. enterolobii may provide critical clues for identifying disease-suppressive microbial consortia. Dynamic tracking of microbiome succession during infection, coupled with the identification of core functional microbes and the dissection of their regulatory mechanisms, will offer novel insights into the pathogenic ecology of M. enterolobii and lay the foundation for developing microbiome-based biocontrol strategies.
7.5. Promoting International Collaboration and the Construction of Data-Sharing Platforms
Given that M. enterolobii crosses national boundaries and impacts global agriculture, establishing an international joint monitoring and data-sharing platform is of paramount importance. Future efforts should focus on developing standardized molecular identification protocols, such as COI barcoding and SNP markers, by compiling databases of resistance and effector proteins to ensure information interoperability. This database sharing will provide reliable data support for control decisions across various countries. Additionally, transnational collaborative initiatives will facilitate the rapid integration of resources and enable a swift response to emerging disease challenges from a global perspective.
By pursuing these multifaceted research directions and promoting interdisciplinary collaboration, it will be possible to construct a comprehensive management system that spans from molecular analysis to field application. Such an integrated approach will not only provide theoretical and technical support for the effective control of M. enterolobii, but also establish a solid foundation for the green, safe, and sustainable development of global agriculture.
8. Conclusions
This review highlights that the range of M. enterolobii is rapidly expanding beyond tropical regions due to climate change and the practices of protected agriculture. The high virulence, broad host spectrum, and unique effector repertoire of this nematode have rendered conventional resistant cultivars ineffective, as evidenced in tomatoes and other major crops. Sustainable management approaches, including crop rotation, green pesticides, and biological agents, show considerable promise but require further field validation and optimization. Future research must develop dynamic predictive models based on GIS and machine learning to anticipate distribution shifts and inform targeted interventions. High-throughput omics and gene editing techniques, such as CRISPR/Cas9, should be employed to dissect the molecular basis of thermal tolerance and pathogenicity, enabling the identification of novel control targets. Integrating advanced nanocarrier systems with peptide biopesticides, guided by AI-driven design algorithms, offers a transformative avenue for precise and low-dose nematode suppression. Establishing international data-sharing platforms, standardized diagnostic protocols (including qPCR, ddPCR, and LAMP), and collaborative networks will be essential for coordinated global management of this emerging threat.
Author Contributions
Conceptualization, M.S. and H.L.; methodology, M.S. and R.L.; software, D.U.N.M. and Y.L.; validation, N.L. and W.G.; formal analysis, H.L. and N.L.; investigation, M.S. and Z.M.; resources, Z.M. and H.L.; data curation, D.U.N.M. and W.G.; writing—original draft preparation, M.S. and R.L.; writing—review and editing, M.S., R.L. and H.L.; visualization, H.L.; supervision, J.Z. and Z.M.; project administration, H.L. and Z.M.; funding acquisition, J.Z. and H.L. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by National Natural Science Foundation of China (32260654), the National Key R&D program (2023YFD1400400) and the Gansu Province Key R&D program (24YFWA015).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge Solomon Boamah (College of Plant Protection, Gansu Agricultural University, China) for professional English language polishing.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abad, P.; Gouzy, J.; Aury, J.M.; Castagnone-Sereno, P.; Danchin, E.G.; Deleury, E.; Perfus-Barbeoch, L.; Anthouard, V.; Artiguenave, F.; Blok, V.C.; et al. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 2008, 26, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Mantelin, S.; Bellafiore, S.; Kyndt, T. Meloidogyne graminicola: A major threat to rice agriculture. Mol. Plant Pathol. 2017, 18, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.; Gaur, H.S.; Helder, J.; Jones, M.G.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Koutsovoulos, G.D.; Poullet, M.; Elashry, A.; Kozlowski, D.K.L.; Sallet, E.; Da Rocha, M.; Perfus-Barbeoch, L.; Martin-Jimenez, C.; Frey, J.E.; Ahrens, C.H.; et al. Genome assembly and annotation of Meloidogyne enterolobii, an emerging parthenogenetic root-knot nematode. Sci. Data 2020, 7, 324. [Google Scholar] [CrossRef] [PubMed]
- Jin, N.; Chen, Y.P.; Liu, Q.; Jian, H. Research progresses in occurrence, diagnoses, pathogenic mechanisms and integrated management of vegetable root-knot nematodes in China. J. Plant Prot. 2022, 49, 424–438. [Google Scholar]
- Yang, B.J.; Eisenback, J.D. Meloidogyne enterolobii n. sp. (Meloidogynidae), a root-knot nematode parasitizing pacara earpod tree in China. J. Nematol. 1983, 15, 381–391. [Google Scholar] [PubMed]
- Rammah, A.; Hirschmann, H. Meloidogyne mayaguensis n. sp. (Meloidogynidae), a Root-knot nematode from Puerto Rico. J. Nematol. 1988, 20, 58–69. [Google Scholar] [PubMed]
- Xu, J.H.; Liu, P.L.; Meng, Q.P.; Long, H.B. Characterisation of Meloidogyne species from China using isozyme phenotypes and amplified Mitochondrial DNA restriction fragment length polymorphism. Eur. J. Plant Pathol. 2004, 110, 309–315. [Google Scholar] [CrossRef]
- Santos, D.; Abrantes, I.; Maleita, C. The quarantine root-knot nematode Meloidogyne enterolobii—A potential threat to Portugal and Europe. Plant Pathol. 2019, 68, 1607–1615. [Google Scholar] [CrossRef]
- Yang, F.; Xu, X.; Guo, R.; Yu, W.J.; Peng, Y.L.; Ji, H.L.; Zhuo, F.Y. Identification of root-knot nematode species from paddy field and surrounding environment in northern China. J. Northwest A&F Univ. (Nat. Sci. Ed.) 2024, 52, 98–108. [Google Scholar]
- Pinheiro, J.B.; Boiteux, L.S.; Almeida, M.R.A.; Pereira, R.B.; Galhardo, L.C.S.; Gomes Carneiro, R.M.D. First report of Meloidogyne enterolobii in capsicum rootstocks carrying the Me1 and Me3/Me7 genes in central Brazil. Nematropica 2015, 45, 184–188. [Google Scholar]
- Rutter, W.B.; Wadl, P.A.; Mueller, J.D.; Agudelo, P. Identification of sweet potato germplasm resistant to pathotypically distinct Isolates of Meloidogyne enterolobii from the Carolinas. Plant Dis. 2021, 105, 3147–3153. [Google Scholar] [CrossRef] [PubMed]
- Djian-Caporalino, C.; Fazari, A.; Arguel, M.J.; Vernie, T.; VandeCasteele, C.; Faure, I.; Brunoud, G.; Pijarowski, L.; Palloix, A.; Lefebvre, V.; et al. Root-Knot Nematode (Meloidogyne spp.) Me resistance genes in pepper (Capsicum annuum L.) are clustered on the P9 chromosome. Theor. Appl. Genet. 2007, 114, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Yang, D.; Niu, J.H.; Zhao, J.L.; Jian, H. De novo analysis of the transcriptome of Meloidogyne enterolobii to uncover potential target genes for biological control. Int. J. Mol. Sci. 2016, 17, 1442. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, G.; Khan, A.; Khan, A.A.; Ali, A.; Mohhamad, H.I. Biological control: A novel strategy for the control of the plant parasitic nematodes. Antonie Van Leeuwenhoek 2021, 114, 885–912. [Google Scholar] [CrossRef] [PubMed]
- Kiewnick, S.; Karssen, G.; Brito, J.A.; Oggenfuss, M.; Frey, J.E. First report of root-knot nematode Meloidogyne enterolobii on tomato and cucumber in Switzerland. Plant Dis. 2008, 92, 1370. [Google Scholar] [CrossRef] [PubMed]
- Quénéhervé, P.; Godefroid, M.; Mège, P.; Marie-Luce, S. Diversity of Meloidogyne spp. parasitizing plants in Martinique Island, French West Indies. Nematropica 2011, 41, 191–199. [Google Scholar]
- Karuri, H.W.; Olago, D.; Neilson, R.; Mararo, E.; Villinger, J. A survey of root knot nematodes and resistance to Meloidogyne incognita in sweet potato varieties from Kenyan fields. Crop Pro. 2017, 92, 114–121. [Google Scholar] [CrossRef]
- Olajide, E.; Kolombia, Y.A.; Amah, D.; Couvreur, M.; Swennen, R.; Coyne, D.; Cortada, L.; Bert, W. First report of the root-knot nematode Meloidogyne enterolobii parasitizing Plantain (Musa spp. AAB) in Nigeria. Plant Dis. 2023, 107, 970. [Google Scholar] [CrossRef]
- Ghule, T.M.; Phani, V.; Somvanshi, V.S.; Patil, M.; Bhattacharyya, S.; Khan, M.R. Further observations on Meloidogyne enterolobii (Nematoda: Meloidogynidae) infecting guava (Psidium guajava) in India. J. Nematol. 2020, 52, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jindapunnapat, K.; Chinnasri, B.; Beesa, N.; Chomphuphuang, N. Molecular phylogeny and morphological studies reveal a 30-year-old rain tree (Samanea saman) maintains populations of Meloidogyne enterolobii, a new host plant in Thailand. J. Phytopathol. 2023, 171, 409–420. [Google Scholar] [CrossRef]
- Riva, G.; Brito, J.A.; de Oliveira, C.; Marin, M.; Gu, M.; Bui, H.X.; Desaeger, J. Identification, distribution, and hosts of Meloidogyne spp. infecting horticultural crops in Florida, USA with focus on Meloidogyne enterolobii. J. Nematol. 2024, 56, 20240042. [Google Scholar] [CrossRef] [PubMed]
- Long, H.B.; Bai, C.; Peng, J.; Zeng, F.Y. First report of the root-knot nematode Meloidogyne enterolobii infecting Jujube in China. Plant Dis. 2014, 98, 1451. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Wang, R.Y.; Chen, S.L.; Li, X.H.; Ma, J. First report of root-knot nematode Meloidogyne enterolobii on sweet potato in China. Plant Dis. 2014, 98, 702. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Hou, X.Y.; Cheng, M.; Deng, M.X.; Cheng, X.; Liu, G.K. First report of Meloidogyne enterolobii on Ginger (Zingiber officinale) in China. Plant Dis. 2018, 102, 684. [Google Scholar] [CrossRef]
- Pan, S.; Wang, Q.L.; Wei, P.Y.; Song, Q.L.; Liu, C.; Chen, Z.J.; Li, Y.M. First report of root-knot nematode Meloidogyne enterolobii infecting coriander in Shaanxi, Northern China. Plant Dis. 2024, 108, 1118. [Google Scholar] [CrossRef]
- Niu, J.H.; Jian, H.; Guo, Q.X.; Chen, C.L.; Wang, X.Y.; Liu, Q.; Guo, Y.D. Evaluation of loop-mediated isothermal amplification (LAMP) assays based on 5S rDNA-IGS2 regions for detecting Meloidogyne enterolobii. Plant Pathol. 2012, 61, 809–819. [Google Scholar] [CrossRef]
- Ji, Y.R.; Xiao, X.; Zhang, Z.H.; Li, G.W.; Dong, Y.; Yang, Q.L.; Gao, Y.; Nie, D.; Shi, J. Pathogenic identification of cucumber root-knot nematodes in Daqing greenhouse. J. Anhui Agric. Sci. 2022, 50, 144–146. [Google Scholar]
- Siddique, S.; Coomer, A.; Baum, T.; Williamson, V.M. Recognition and response in plant-nematode interactions. Annu. Rev. Phytopathol. 2022, 60, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Trudgill, D.L.; Blok, V.C. Apomictic, polyphagous root-knot nematodes: Exceptionally successful and damaging biotrophic root pathogens. Annu. Rev. Phytopathol. 2001, 39, 53–77. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.N.; Moens, M.; Starr, J.L. Root-Knot Nematodes; CABI: Cambridge, MA, USA, 2009. [Google Scholar]
- Gomes, V.M.; Souza, R.M.; Almeida, A.M.; Dolinski, C. Relationships between M. enterolobii and F. solani: Spatial and temporal dynamics in the occurrence of guava decline. Nematoda 2014, 1, e01014. [Google Scholar] [CrossRef]
- Souza, R.M.; Oliveira, D.F.; Gomes, V.M.; Viana, A.J.S.; Silva, G.H.; Machado, A.R.T. Meloidogyne enterolobii-induced changes in guava root exudates are associated with root rotting caused by Neocosmospora falciformis. J. Nematol. 2023, 55, 20230055. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.B.; Sikora, R.A.; Greco, N.; Vito, M.D.; Caubel, G. Screening techniques and sources of resistance to nematodes in cool season food legumes. Euphytica 1993, 73, 59–66. [Google Scholar] [CrossRef]
- Long, H.B.; Sun, Y.F.; Zeng, F.Y.; Bai, C. Effect of initial population density of Meloidogyne enterolobii on cucumber growth in greenhouse. Chin. J. Trop. Agric. 2015, 35, 61–64. [Google Scholar]
- Velloso, J.A.; Maquilan, M.A.D.; Campos, V.P.; Brito, J.A.; Dickson, D.W. Temperature effects on development of Meloidogyne enterolobii and M. floridensis. J. Nematol. 2022, 54, 20220013. [Google Scholar] [CrossRef] [PubMed]
- Philbrick, A.N.; Adhikari, T.B.; Louws, F.J.; Gorny, A.M. Meloidogyne enterolobii, a major threat to tomato production: Current status and future prospects for its management. Front. Plant Sci. 2020, 16, 606395. [Google Scholar] [CrossRef] [PubMed]
- Holbein, J.; Franke, R.B.; Marhavý, P.; Fujita, S.; Górecka, M.; Sobczak, M.; Geldner, N.; Schreiber, L.; Grundler, F.M.W.; Siddique, S. Root endodermal barrier system contributes to defence against plant-parasitic cyst and root-knot nematodes. Plant J. 2019, 100, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Bellafiore, S.; Shen, Z.; Rosso, M.N.; Abad, P.; Shih, P.; Briggs, S.P. Direct identification of the Meloidogyne incognita secretome reveals proteins with host cell reprogramming potential. PLoS Pathog. 2008, 4, e1000192. [Google Scholar] [CrossRef] [PubMed]
- Jagdale, S.; Rao, U.; Giri, A.P. Effectors of root-knot nematodes: An arsenal for successful parasitism. Front. Plant Sci. 2021, 12, 800030. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.G.K.; Goto, D.B. Root-knot nematodes and giant cells. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Jones, J., Gheysen, G., Fenoll, C., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 83–100. [Google Scholar]
- Collett, R.L.; Rashidifard, M.; Marais, M.F. Hendrika, Insights into the life-cycle development of Meloidogyne enterolobii, M. incognita and M. javanica on tomato, soybean and maize. Eur. J. Plant Pathol. 2024, 168, 137–146. [Google Scholar] [CrossRef]
- Silva, A.J.D.; Oliveira, G.H.D.; Pastoriza, R.J.; Maranho, E.H.; Carvalho Filho, J.L.S.D. Search for sources of resistance to Meloidogyne enterolobii in commercial and wild tomatoes. Hortic. Bras. 2019, 37, 188–198. [Google Scholar] [CrossRef]
- Zhuo, K.; Chen, J.S.; Lin, B.R.; Wang, J.; Sun, F.X.; Hu, L.L.; Liao, J.L. A novel Meloidogyne enterolobii effector MeTCTP promotes parasitism by suppressing programmed cell death in host plants. Mol. Plant Pathol. 2017, 18, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, K.; Chi, Y.L.; Hu, L.L.; Luo, M.; Liao, J.L. Cloning and RNA interference analysis of a pectate lyase gene of Meloidogyne enterolobii. Acta Phytopathol. Sin. 2011, 41, 473–481. [Google Scholar]
- Long, H.B.; Sun, Y.F.; Zeng, F.Y.; Peng, J.; Bai, C. Identification and developmental analysis of a new pectate lyase gene Me-pel2 in the root-knot nematode Meloidogyne enterolobii. Chin. J. Trop. Crops. 2016, 37, 92–98. [Google Scholar]
- Long, H.B.; Wei, L.S.Z.; Zhao, Z.X.; Chen, M.C. Cloning and functional analysis of a cellulose binding protein gene Me-cbp-1 from the Root-Knot Nematode Meloidogyne enterolobii. J. Agric. Biotechnol. 2017, 25, 196–204. [Google Scholar]
- Pei, J.; Feng, T.Z.; Long, H.B.; Chen, Y.; Pei, Y.L.; Sun, Y.F. Molecular characterization and virus-induced gene silencing of a collagen gene, Me-col-1, in root-knot nematode Meloidogyne enterolobii. Life 2022, 12, 2103. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.R. Cloning and RNAi Effect Analysis of the Effector Gene Me-cm-1 from Meloidogyne enterolobii. Master’s Thesis, Hainan University, Haikou, China, 2021. [Google Scholar]
- Wei, L.S.Z.; Long, H.B.; Chen, M.C.; Zhao, Z.X. Cloning and RNAi of Me-mapk1 gene from the root-knot nematode Meloidogyne enterolobii. Chin. J. Trop. Crops 2016, 37, 1980–1985. [Google Scholar]
- Lian, D.M.; Wang, H.F.; Zhao, Z.X.; Chen, M.C. Cloning and prokaryotic expression of Hsp70 from Meloidogyne enterolobii. Acta Phytopathol. Sin. 2015, 45, 7–13. [Google Scholar]
- Jin, J. Identification and Functional Analysis of Secreted Effector, MeCPI, from Meloidogyne enterolobii. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2016. [Google Scholar]
- Feng, T.Z.; Long, H.B.; Pei, Y.L.; Sun, Y.F.; Wei, L.S.Z. Cloning and functional analysis of a novel effector gene Me-3C06 from Meloidogyne enterolobii. In Green Ecological Sustainable Development and Plant Protection—Proceedings of the 12th National Congress and Academic Annual Meeting of the Chinese Society of Plant Protection, Changsha, China, 8 November, 2017; Chinese Society of Plant Protection: Changsha, China, 2017; p. 1. [Google Scholar]
- Feng, T.Z.; Chen, Y.; Li, Z.R.; Pei, J.; Peng, D.L.; Peng, H.; Long, H.B. A novel chorismate mutase effector secreted from root-knot nematode Meloidogyne enterolobii manipulates plant immunity to promote parasitism. J. Integr. Agric. 2024, 23, 4107–4119. [Google Scholar] [CrossRef]
- Chen, Y.P.; Liu, Q.; Sun, X.Q.; Liu, L.; Zhao, J.L.; Yang, S.S.; Wang, X.F.; Quentin, M.; Abad, P.; Favery, B.; et al. Meloidogyne enterolobii MeMSP1 effector targets the glutathione-S-transferase phi GSTF family in Arabidopsis to manipulate host metabolism and promote nematode parasitism. New Phytol. 2023, 240, 2468–2483. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, S.T.; Coaker, G.; Day, B.; Staskawicz, B.J. Host-microbe interactions: Shaping the evolution of the plant immune response. Cell 2006, 124, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Vos, P.; Simons, G.; Jesse, T.; Wijbrandi, J.; Heinen, L.; Hogers, R.; Frijters, A.; Groenendijk, J.; Diergaarde, P.; Reijans, M.; et al. The tomato Mi-1 gene confers resistance to both root-knot nematodes and potato aphids. Nat. Biotechnol. 1998, 16, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- Schaff, J.E.; Nielsen, D.M.; Smith, C.P.; Scholl, E.H.; Bird, D.M. Comprehensive transcriptome profiling in tomato reveals a role for glycosyltransferase in Mi-mediated nematode resistance. Plant Physiol. 2007, 144, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Jablonska, B.; Ammiraju, J.S.; Bhattarai, K.K.; Mantelin, S.; Martinez de Ilarduya, O.; Roberts, P.A.; Kaloshian, I. The Mi-9 gene from Solanum arcanum conferring heat-stable resistance to root-knot nematodes is a homolog of Mi-1. Plant Physiol. 2007, 143, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, G.J.; Anderson, P.A.; Dodds, P.N.; Ellis, J.G. Relationships between rust resistance genes at the M locus in flax. Mol. Plant Pathol. 2010, 11, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Radwan, O.; Gandhi, S.; Heesacker, A.; Whitaker, B.; Taylor, C.; Plocik, A.; Kesseli, R.; Kozik, A.; Michelmore, R.W.; Knapp, S.J. Genetic diversity and genomic distribution of homologs encoding NBS-LRR disease resistance proteins in sunflower. Mol. Genet. Genomics. 2008, 280, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Seah, S.; Telleen, A.C.; Williamson, V.M. Introgressed and endogenous Mi-1 gene clusters in tomato differ by complex rearrangements in flanking sequences and show sequence exchange and diversifying selection among homologues. Theor. Appl. Genet. 2007, 114, 1289–1302. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.; Manley, J.L. Cell signaling and the control of pre-mRNA splicing. Nat. Rev. Mol. Cell Biol. 2004, 5, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Him, N.A.; Gillan, V.; Emes, R.D.; Maitland, K.; Devaney, E. Hsp-90 and the biology of nematodes. BMC Evol. Biol. 2009, 9, 254. [Google Scholar] [CrossRef] [PubMed]
- Kiewnick, S.; Dessimoz, M.; Franck, L. Effects of the Mi-1 and the N root-knot nematode-resistance gene on infection and reproduction of Meloidogyne enterolobii on tomato and pepper cultivars. J. Nematol. 2009, 41, 134–139. [Google Scholar] [PubMed]
- Mantelin, S.; Bhattarai, K.K.; Jhaveri, T.Z.; Kaloshian, I. Mi-1-Mediated resistance to Meloidogyne incognita in tomato may not rely on ethylene but hormone perception through ETR3 participates in limiting nematode infection in a susceptible host. PLoS ONE 2013, 8, e63281. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Long, H.; Feng, T.; Pei, Y.; Sun, Y.; Zhang, X. Development of a novel primer–TaqMan probe set for diagnosis and quantification of Meloidogyne enterolobii in soil using qPCR and droplet digital PCR assays. Int. J. Mol. Sci. 2022, 23, 11185. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, S.; Guo, Y.; Wang, L.; Feng, T.; Zhang, X.; Long, H. Development of the duplex droplet digital PCR for quantitative monitoring of mixed infections of Meloidogyne incognita and M. enterolobii. Pest. Manag. Sci. 2025, 81, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- Kiewnick, S.; Frey, J.E.; Braun-Kiewnick, A. Development and validation of LNA-based Quantitative Real-Time PCR assays for detection and identification of the root-knot nematode Meloidogyne enterolobii in complex DNA backgrounds. Phytopathology 2015, 105, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.H.; Guo, Q.X.; Jian, H.; Chen, C.L.; Yang, D.; Liu, Q.; Guo, Y.D. Rapid detection of Meloidogyne spp. by LAMP assay in soil and roots. Crop Prot. 2011, 30, 1063–1069. [Google Scholar] [CrossRef]
- Suwanngam, A.; Schiffer, P.H.; Sasnarukkit, A.; Siripattanapipong, S.; Jindapunnapat, K.; Chinnasri, B.; Ruang-Areerate, T. Development of colorimetric and fluorescent closed tube LAMP assay using simplified extraction for diagnosis of Meloidogyne enterolobii in root tissues. Sci. Rep. 2025, 15, 160. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.X.; Gao, B.; Wang, R.Y.; Chen, S.L.; Li, X.H.; Dong, Y.H.; Ma, J. Development of enzyme-mediated duplex exponential amplification assay for detection and identification of Meloidogyne enterolobii in field. Microorganisms 2025, 13, 1353. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.L.; Maria, M.; Barsalote, E.M.; Castillo, P.; Zheng, J.W. Morphological and molecular characterization of the rice root-knot nematode, Meloidogyne graminicola, Golden and Birchfeild, 1965 occurring in Zhejiang, China. J. Integr. Agric. 2018, 17, 2724–2733. [Google Scholar] [CrossRef]
- Kamran, M.; Javed, N.; Ullah, I.; Nazir, S.; Jamil, S.; Iqbal, M.Z.; Abbas, H.; Khan, S.A.; Ehetisham ul Haq, M. Genetic variability among different populations of root knot nematodes based on their encumbrance response to pasteuria isolates using PCR-RFLP. Plant Pathol. J. 2019, 35, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, T.; Li, C.; Ye, W.; Davis, E. Distribution of Meloidogyne enterolobii in eastern North Carolina and comparison of four isolates. Plant Health Prog. 2020, 21, 91–96. [Google Scholar] [CrossRef]
- Zijlstra, C.; Donkers-Venne, D.T.; Fargette, M. Identification of Meloidogyne incognita, M. javanica and M. arenaria using sequence characterised amplified region (SCAR) based PCR assays. Nematology 2000, 2, 847–853. [Google Scholar] [CrossRef]
- Feng, G.Q.; Dong, L.Y.; Chen, Y.J.; Shang, H.; Liu, Y.Z.; Li, J.R.; Yang, J.Z.; Cui, X.M.; Yang, P.W. PCR detection of nematode isolated from Panax notoginseng. Southwest China J. Agric. Sci. 2008, 2, 100–102. [Google Scholar]
- Long, H.; Liu, H.; Xu, J.H. Development of a PCR diagnostic for the root-knot nematode Meloidogyne enterolobii. Acta Phytopathol. Sin. 2006, 36, 109–115. [Google Scholar]
- Huang, F.Y.; Deng, X.P.; Gao, L.L.; Cai, X.J.; Yan, D.; Cai, Y.Z.; Chen, X.L.; Yang, M.; Tong, W.J.; Yu, L. Effect of marigold (Tagetes erecta L.) on soil microbial communities in continuously cropped tobacco fields. Sci. Rep. 2022, 12, 19632. [Google Scholar] [CrossRef] [PubMed]
- Rashidifard, M.; Ashrafi, S.; Claassens, S.; Thünen, T.; Fourie, H. A pilot approach investigating the potential of crop rotation with sainfoin to reduce Meloidogyne enterolobii infection of Maize under greenhouse conditions. Front. Plant Sci. 2021, 12, 659322. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, C.J.; Bian, X.W.; Zeng, S.Y.; Lin, K.C.; Wu, B.; Zhang, G.A.; Zhang, X. Nematicidal efficacy of isothiocyanates against root-knot nematode Meloidogyne javanica in cucumber. Crop Prot. 2011, 30, 33–37. [Google Scholar] [CrossRef]
- Burns, A.R.; Baker, R.J.; Kitner, M.; Knox, J.; Cooke, B.; Volpatti, J.R.; Vaidya, A.S.; Puumala, E.; Palmeira, B.M.; Redman, E.M.; et al. Selective control of parasitic nematodes using bioactivated nematicides. Nature 2023, 618, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Jian, J.Z.; Long, H.B.; Jiang, Q.H.; Huang, W.K.; Kong, L.A.; Yin, M.Z.; Shen, J.; Su, X.F.; Peng, D.L.; et al. Self-assembled nanonematicide induces adverse effects on oxidative stress, succinate dehydrogenase activity, and ATP generation. ACS Appl. Mater. Interfaces 2023, 15, 31173–31184. [Google Scholar] [CrossRef] [PubMed]
- Frei dit Frey, N.; Favery, B. Plant-parasitic nematode secreted peptides hijack a plant secretory pathway. New Phytol. 2021, 229, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Yang, H.; Sun, Y.; Zhang, J.; Gao, K.; Wu, J.; Zhu, C.; Yin, C.; Chen, X.; Liu, Q.; et al. Targeted MYC2 stabilization confers citrus Huanglongbing resistance. Science 2025, 388, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Forghani, F.; Hajihassani, A. Recent advances in the development of environmentally benign treatments to control root-knot nematodes. Front. Plant Sci. 2020, 11, 1125. [Google Scholar] [CrossRef] [PubMed]
- Sikandar, A.; Gao, F.; Mo, Y.; Chen, Q.; Ullah, R.M.K.; Wu, H. Efficacy of Aspergillus tubingensis GX3’ fermentation against Meloidogyne enterolobii in tomato (Solanum lycopersicum L.). Plants 2023, 12, 2724. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Guo, H.; Zhang, K.; Zhao, M.; Ruan, J.; Chen, J. Trichoderma and its role in biological control of plant fungal and nematode disease. Front. Microbiol. 2023, 14, 1160551. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rodriguez, J.A.; Reyes-Pérez, J.J.; Quiñones-Aguilar, E.E.; Hernandez-Montiel, L.G. Actinomycete potential as biocontrol agent of phytopathogenic fungi: Mechanisms, source, and applications. Plants 2022, 11, 3201. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Song, B.A. Natural nematicidal active compounds: Recent research progress and outlook. J. Integr. Agric. 2021, 20, 2015–2031. [Google Scholar] [CrossRef]
- Li, G.H.; Zhang, K.Q. Natural nematicidal metabolites and advances in their biocontrol capacity on plant parasitic nematodes. Nat. Prod. Rep. 2023, 40, 646–675. [Google Scholar] [CrossRef] [PubMed]
- Pasche, J.M.; Sawlani, R.; Buttrós, V.H.; Desaeger, J.; Garrett, K.A.; Martins, S.J. Underground guardians: How collagen and chitin amendments shape soil microbiome structure and function for Meloidogyne enterolobii control. Microbiome 2025, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- López-Lima, D.; Alarcón-Utrera, D.; Ordáz-Meléndez, J.; Villain, L.; Carrión, G. Metarhizium carneum formulations: A promising new biological control to be incorporated in the integrated management of Meloidogyne enterolobii on tomato plants. Plants 2023, 12, 3431. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Lu, Y.Q.; Liao, K.M.; Ma, X.Q.; Meng, L.L.; Jiang, M.G.; Zhou, Y. A novel tpp-like protein from Bacillus thuringiensis strain GXUN31-2 with nematicidal activity against Meloidogyne enterolobii. Microb. Pathog. 2025, 198, 107191. [Google Scholar] [CrossRef] [PubMed]
- Migunova, V.D.; Sasanelli, N. Bacteria as biocontrol tool against phytoparasitic nematodes. Plants 2021, 10, 389. [Google Scholar] [CrossRef] [PubMed]
- Stucky, T.; Hochstrasser, M.; Meyer, S.; Segessemann, T.; Ruthes, A.C.; Ahrens, C.H.; Pelludat, C.; Dahlin, P. A novel robust screening assay identifies pseudomonas strains as reliable antagonists of the root-knot nematode Meloidogyne incognita. Microorganisms 2023, 11, 2011. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.Q.; Fu, Q.; Zhang, J.; Hao, G.Y.; Liang, C.; Duan, F.M.; Ma, J.; Zhao, H.H.; Song, W.W. Paenibacillus polymyxa J2-4 induces cucumber to enrich rhizospheric Pseudomonas and contributes to Meloidogyne incognita management under field conditions. Pest. Manag. Sci. 2025, 81, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.J.; Park, A.R.; Kim, S.; Yeon, J.; Yu, N.H.; Ha, S.; Chang, J.Y.; Park, H.W.; Kim, J.C. Biological control of root-knot nematodes by organic acid-producing lactobacillus brevis WiKim0069 isolated from kimchi. Plant Pathol. J. 2019, 35, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.D.; Carneiro, R.M.D.G.; Faria, M.; Souza, D.A.; Lopes, R.B. Evaluation of pochonia chlamydosporia and Purpureocillium lilacinum for suppression of Meloidogyne enterolobii on tomato and banana. J. Nematol. 2017, 49, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zou, C.G.; Xu, J.P.; Ji, X.L.; Niu, X.M.; Yang, J.K.; Huang, X.W.; Zhang, K.Q. Molecular mechanisms of nematode-nematophagous microbe interactions: Basis for biological control of plant-parasitic nematodes. Annu. Rev. Phytopathol. 2015, 53, 67–95. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, R.S.; Patil, J.A.; Yadav, S. Prospects of using predatory nematodes in biological control for plant parasitic nematodes—A review. Biological Control. 2021, 160, 104668. [Google Scholar] [CrossRef]
- Ramatsitsi, N.; Dube, Z.P.; Ramachela, K.; Motloba, T. Bio-control efficacy of selected indigenous nematophagous fungi against Meloidogyne enterolobii in vitro and on dry bean (Phaseolus vulgaris L.). Int. Microbiol. 2024, 28, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Pofu, K.M.; Mashela, P.W. Interactive effects of filamentous fungi and cucurbitacin phytonematicide on growth of cowpea and suppression of Meloidogyne enterolobii. Front. Microbiol. 2022, 13, 765051. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Jang, H.J.; Park, J.Y.; Yang, Y.K.; Kim, M.J.; Park, C.S.; Lee, S.; Yun, B.S.; Lee, S.J.; Lee, S.W.; et al. Efficacy evaluation of Streptomyces nigrescens KA-1 against the root-knot nematode Meloidogyne incognita. Biol. Control 2023, 179, 105150. [Google Scholar] [CrossRef]
- Sharma, N.; Manhas, R.K.; Ohri, P. Streptomyces hydrogenans strain DH-16 alleviates negative impact of Meloidogyne incognita stress by modifying physio-biochemical attributes in Solanum lycopersicum plants. Sci. Rep. 2022, 12, 15214. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.G.; Luo, X.D. Meliaceous limonoids: Chemistry and biological activities. Chem. Rev. 2011, 111, 7437–7522. [Google Scholar] [CrossRef] [PubMed]
- Naz, I.; Saifullah; Khan, M.R. Nematicidal activity of nonacosane-10-ol and 23a-homostigmast-5-en-3β-ol isolated from the roots of Fumaria parviflora (Fumariaceae). J. Agric. Food Chem. 2013, 61, 5689–5695. [Google Scholar] [CrossRef] [PubMed]
- Alves, G.C.S.; Ferri, P.H.; Seraphin, J.C.; Fortes, G.A.C.; Rocha, M.R.; Santos, S.C. Principal response curves analysis of polyphenol variation in resistant and susceptible cotton after infection by a root-knot nematode (RKN). Physiol. Mol. Plant Pathol. 2016, 96, 19–28. [Google Scholar] [CrossRef]
- Rattan, R.S. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. 2010, 29, 913–920. [Google Scholar] [CrossRef]
- Jang, J.Y.; Dang, Q.L.; Choi, Y.H.; Choi, G.J.; Jang, K.S.; Cha, B.; Luu, N.H.; Kim, J.C. Nematicidal activities of 4-quinolone alkaloids isolated from the aerial part of Triumfetta grandidens against Meloidogyne incognita. J. Agric. Food Chem. 2015, 63, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Seid, A.; Fininsa, C.; Mekete, T.; Decraemer, W.; Wesemael, W.M.L. Tomato (Solanum lycopersicum) and root-knot nematodes (Meloidogyne spp.)—A century-old battle. Nematology 2015, 17, 995–1009. [Google Scholar] [CrossRef]
- El-Sappah, A.H.; M. M., I.; El-Awady, H.H.; Yan, S.; Qi, S.M.; Liu, J.Y.; Cheng, G.T.; Liang, Y. Tomato natural resistance genes in controlling the Root-Knot Nematode. Genes 2019, 10, 925. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Choudhary, S.; Naaz, N.; Sharma, N.; Laskar, R.A. Recent advancements in molecular marker-assisted selection and applications in plant breeding programmes. J. Genet. Eng. Biotechnol. 2021, 19, 128. [Google Scholar] [CrossRef] [PubMed]
- Phani, V.; Gowda, M.T.; Dutta, T.K. Grafting vegetable crops to manage plant-parasitic nematodes: A review. J. Pest. Sci. 2024, 97, 539–560. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Li, H.; Tian, X.X.; Xie, G.Y.; Chen, Y.L.; Zhu, J.; Zhu, G.P. Analysis of metabolomic discrepancy in response to the infection of Meloidogyne enterolobii between tomato rootstocks with diverse resistance. Chin. J. Trop. Crops 2024, 45, 2407–2415. [Google Scholar]
- Rashid, M.H.; Al-Mamun, M.H.; Uddin, M.N. How durable is root knot nematode resistance in tomato? Plant Breed. Biotech. 2017, 5, 143–162. [Google Scholar] [CrossRef]
- Milligan, S.B.; Bodeau, J.; Yaghoobi, J.; Kaloshian, I.; Zabel, P.; Williamson, V.M. The root knot nematode resistance gene Mi from tomato is a member of the leucine zipper, nucleotide binding, leucine-rich repeat family of plant genes. Plant Cell 1998, 10, 1307–1319. [Google Scholar] [CrossRef] [PubMed]
- Veremis, J.C.; Roberts, P.A. Relationships between Meloidogyne incognita resistance genes in Lycopersicon peruvianum differentiated by heat sensitivity and nematode virulence. Theor. Appl. Genet. 1996, 93, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Ammiraju, J.S.; Veremis, J.C.; Huang, X.; Roberts, P.A.; Kaloshian, I. The heat-stable root-knot nematode resistance gene Mi-9 from Lycopersicon peruvianum is localized on the short arm of chromosome 6. Theor. Appl. Genet. 2003, 106, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.J.; Ling, J.; Zhao, J.L.; Yang, Y.H.; Yang, Y.; Li, Y.; Jiao, Y.; Mao, Z.C.; Wang, Y.; Xie, B.Y. Chromosome-scale genome assembly-assisted identification of Mi-9 gene in Solanum arcanum accession LA2157, conferring heat-stable resistance to Meloidogyne incognita. Plant Biotechnol. J. 2023, 21, 1496–1509. [Google Scholar] [CrossRef] [PubMed]
- Ernst, K.; Kumar, A.; Kriseleit, D.; Kloos, D.U.; Phillips, M.S.; Ganal, M.W. The broad-spectrum potato cyst nematode resistance gene (Hero) from tomato is the only member of a large gene family of NBS-LRR genes with an unusual amino acid repeat in the LRR region. Plant J. 2002, 31, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Fazari, A.; Palloix, A.; Wang, L.; Yan Hua, M.; Sage-Palloix, A.-M.; Zhang, B.X.; Djian-Caporalino, C. The root-knot nematode resistance N-gene co-localizes in the Me-genes cluster on the pepper (Capsicum annuum L.) P9 chromosome. Plant Breed. 2012, 131, 665–673. [Google Scholar] [CrossRef]
- Walters, S.A.; Wehner, T.C. ‘Lucia’, ‘Manteo’, and ‘Shelby’ root-knot nematode-resistant cucumber inbred lines. Hort. Sci. 1997, 32, 1301–1303. [Google Scholar] [CrossRef]
- Xie, X.X.; Ling, J.; Lu, J.R.; Mao, Z.C.; Zhao, J.L.; Zheng, S.J.; Yang, Q.H.; Li, Y.; Visser, R.G.F.; Bai, Y.L.; et al. Genetic dissection of Meloidogyne incognita resistance genes based on VIGS functional analysis in Cucumis metuliferus. BMC Plant Biol. 2024, 24, 964. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Wang, X.; Liu, X.J.; Yang, S.Q.; Yu, X.Q.; Qian, C.T.; Li, J.; Lou, Q.F.; Chen, J.F. Candidate genes underlying the quantitative trait loci for root-knot nematode resistance in a Cucumis hystrix introgression line of cucumber based on population sequencing. J. Plant Res. 2019, 132, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.H.; Liu, J.; Bao, S.Y.; Yang, Y.; Zhuang, Y. Molecular cloning and characterization of a wild eggplant Solanum aculeatissimum NBS-LRR gene, involved in plant resistance to Meloidogyne incognita. Int. J. Mol. Sci. 2018, 19, 583. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Matthews, W.C.; Cavagnaro, P.F.; Iorizzo, M.; Roberts, P.A.; Simon, P.W. Inheritance and mapping of Mj-2, a new source of root-knot nematode (Meloidogyne javanica) resistance in carrot. J. Hered. 2014, 105, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Boiteux, L.S.; Belter, J.G.; Roberts, P.A.; Simon, P.W. RAPD linkage map of the genomic region encompassing the root-knot nematode (Meloidogyne javanica) resistance locus in carrot. Theor. Appl. Genet. 2000, 100, 439–446. [Google Scholar] [CrossRef]
- Rogers, A.K.; Phillips, C.M. RNAi pathways repress reprogramming of C. elegans germ cells during heat stress. Nucleic Acids Res. 2020, 48, 4256–4273. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, Y.; Kawano, K.; Iwasaki, T.; Kawano, T. RNA interference-mediated growth control of the southern root-knot nematode Meloidogyne incognita. Biosci. Biotechnol. Biochem. 2012, 76, 378–380. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.Y.; Cao, Y.; Zhang, K.Q. Metagenomic insights into communities, functions of endophytes, and their associations with infection by root-knot nematode Meloidogyne incognita in tomato roots. Sci. Rep. 2015, 5, 17087. [Google Scholar] [CrossRef] [PubMed]
- Elhady, A.; Adss, S.; Hallmann, J.; Heuer, H. Rhizosphere microbiomes modulated by pre-crops assisted plants in resistance to plant-parasitic nematodes. Front. Microbiol. 2017, 8, 1133. [Google Scholar]
- Liu, R.; Chen, M.F.; Liu, B.L.; Huang, K.W.; Mao, Z.C.; Li, H.X.; Zhao, J.L. A root-knot nematode effector manipulates the rhizosphere microbiome for establishing parasitism relationship with hosts. Front. Microbiol. 2023, 14, 1217863. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).