Beyond Fish Pathogens: A Comprehensive Overview of Aeromonas salmonicida
Abstract
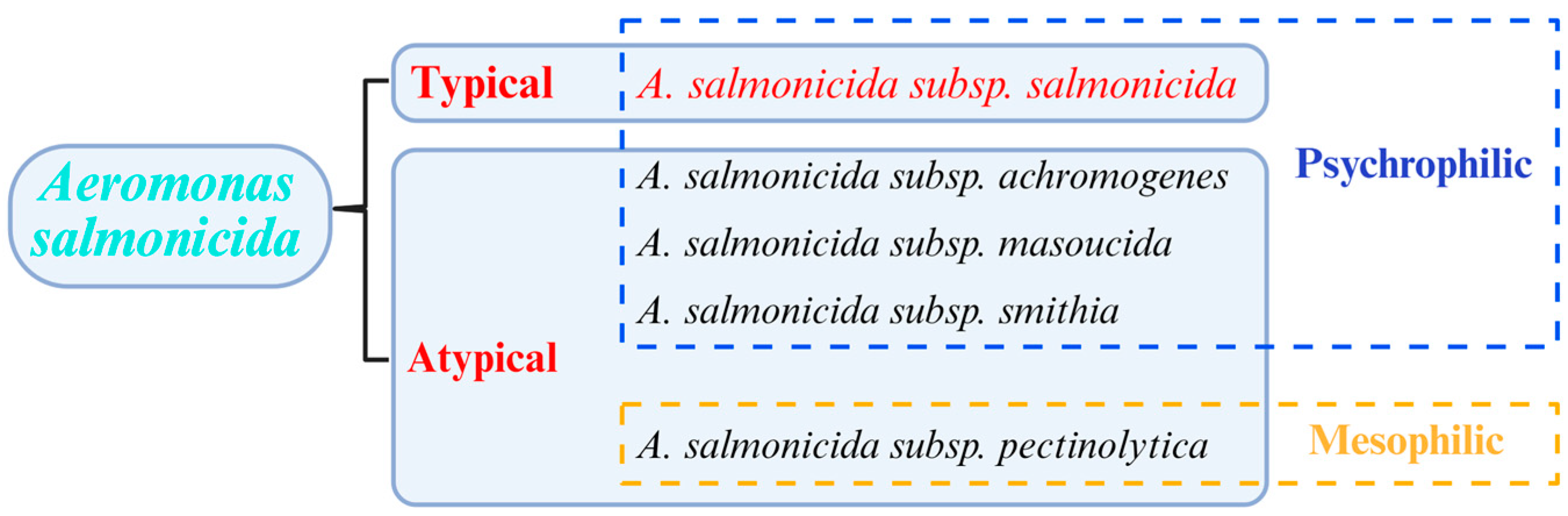
1. The Classification of A. salmonicida
2. Plasmids
3. Virulence Factors and Pathogenic Mechanisms
3.1. Exotoxin
3.2. Extracellular Enzyme
3.3. A-Layer Protein
3.4. Secretory System
| Secretory System | Mechanism of Action | References |
|---|---|---|
| T2SS | Transport virulence factors, such as aerolysin, amylase, and protease. | [14,85,86,87] |
| T3SS | The main pathogenic system of A. salmonicida. | [14,75,76] |
| T4SS | The original genes that undergo genetic transfer among bacteria. | [81] |
| T6SS | Injection of different types of toxin proteins into the host. | [88,89] |
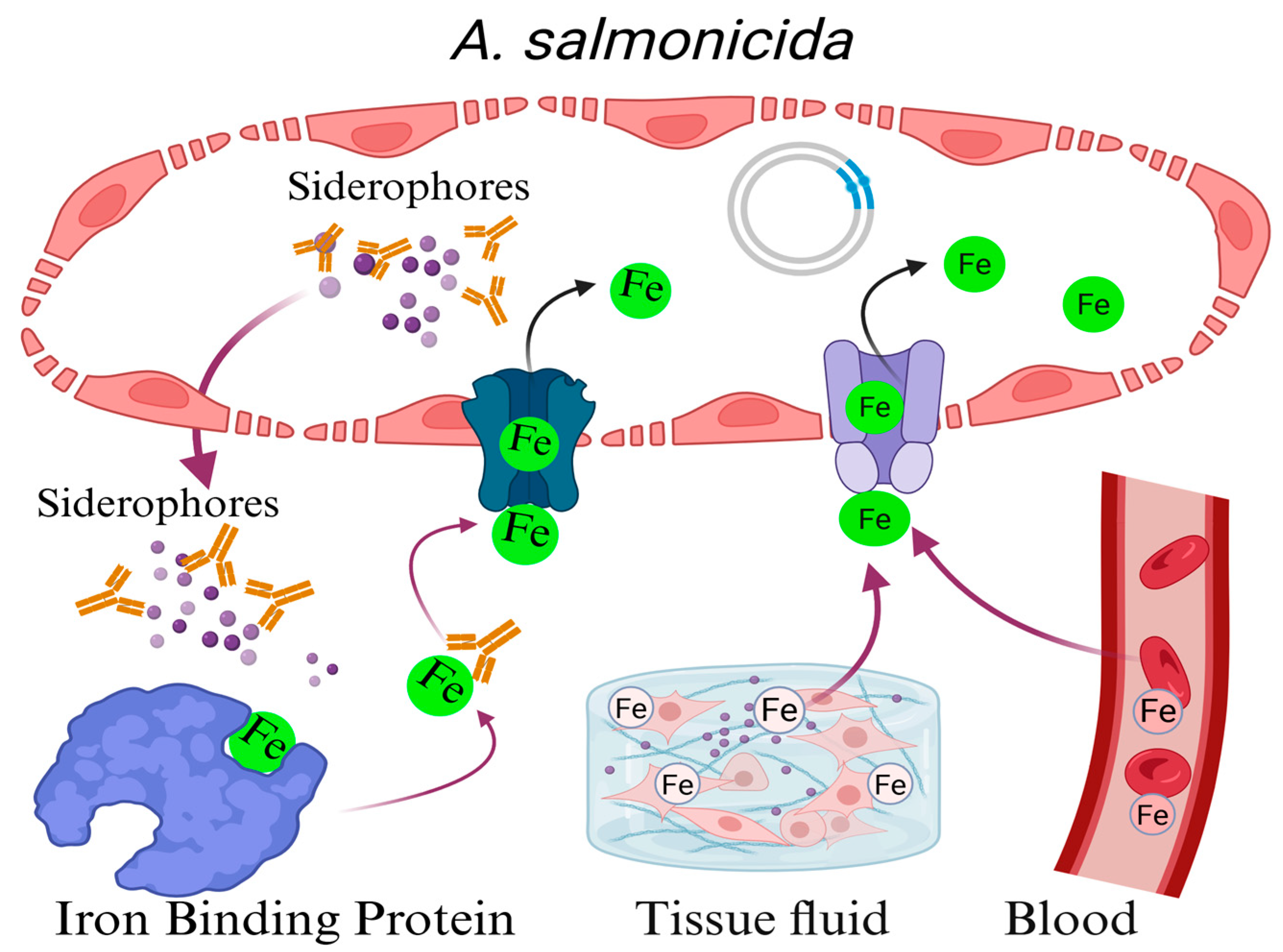
3.5. Iron Ion Uptake System
3.6. Quorum Sensing
4. The Pathogenicity and Clinical Signs of A. salmonicida
4.1. Fish
4.2. Others
5. Prevention and Treatment of A. salmonicida
6. Summary and Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, P.; Li, J.; He, T.T.; Li, N.; Mo, Z.L.; Nie, P.; Xie, H.X. Pathogenic characterization of Aeromonas salmonicida subsp. masoucida turbot isolate from China. J. Fish Dis. 2020, 43, 1145–1154. [Google Scholar] [CrossRef]
- Gudmundsdóttir, B.K.; Hvanndal, I.; Björnsdóttir, B.; Wagner, U. Analysis of exotoxins produced by atypical isolates of Aeromonas salmonicida, by enzymatic and serological methods. J. Fish Dis. 2003, 26, 15–29. [Google Scholar] [CrossRef]
- Pavan, M.E.; Abbott, S.L.; Zorzópulos, J.; Janda, J.M. Aeromonas salmonicida subsp. pectinolytica subsp. nov., a new pectinase-positive subspecies isolated from a heavily polluted river. Int. J. Syst. Evol. Microbiol. 2000, 50 Pt 3, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Tomás, J.M. The Aeromonas salmonicida Lipopolysaccharide Core from Different Subspecies: The Unusual subsp. pectinolytica. Front. Microbiol. 2016, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- Cipriano, R.C.; Bullock, G.L. Furunculosis and Other Diseases Caused by Aeromonas salmonicida; Fish Disease Leaflet; US Fish & Wildlife Publications: Falls Church, VA, USA, 2001.
- Dallaire-Dufresne, S.; Tanaka, K.H.; Trudel, M.V.; Lafaille, A.; Charette, S.J. Virulence, genomic features, and plasticity of Aeromonas salmonicida subsp. salmonicida, the causative agent of fish furunculosis. Vet. Microbiol. 2014, 169, 1–7. [Google Scholar] [CrossRef]
- Wiklund, T.; Dalsgaard, I. Occurrence and significance of atypical Aeromonas salmonicida in non-salmonid and salmonid fish species: A review. Dis. Aquat. Organ. 1998, 32, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Ruppé, E.; Cherkaoui, A.; Wagner, N.; La Scala, G.C.; Beaulieu, J.Y.; Girard, M.; Frey, J.; Lazarevic, V.; Schrenzel, J. In vivo selection of a multidrug-resistant Aeromonas salmonicida during medicinal leech therapy. New Microbes New Infect. 2017, 21, 23–27. [Google Scholar] [CrossRef]
- Vincent, A.T.; Charette, S.J. To Be or Not to Be Mesophilic, That Is the Question for Aeromonas salmonicida. Microorganisms 2022, 10, 240. [Google Scholar] [CrossRef]
- Charette, S.J. Microbe profile: Aeromonas salmonicida: An opportunistic pathogen with multiple personalities. Microbiology 2021, 167, 001052. [Google Scholar] [CrossRef]
- Chu, S.; Noonan, B.; Cavaignac, S.; Trust, T.J. Endogenous mutagenesis by an insertion sequence element identifies Aeromonas salmonicida AbcA as an ATP-binding cassette transport protein required for biogenesis of smooth lipopolysaccharide. Proc. Natl. Acad. Sci. USA 1995, 92, 5754–5758. [Google Scholar] [CrossRef]
- Gulla, S.; Lund, V.; Kristoffersen, A.B.; Sørum, H.; Colquhoun, D.J. vapA (A-layer) typing differentiates Aeromonas salmonicida subspecies and identifies a number of previously undescribed subtypes. J. Fish Dis. 2016, 39, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Lund, V.; Mikkelsen, H. Genetic diversity among A-proteins of atypical strains of Aeromonas salmonicida. Dis. Aquat. Organ. 2004, 61, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Reith, M.E.; Singh, R.K.; Curtis, B.; Boyd, J.M.; Bouevitch, A.; Kimball, J.; Munholland, J.; Murphy, C.; Sarty, D.; Williams, J.; et al. The genome of Aeromonas salmonicida subsp. salmonicida A449: Insights into the evolution of a fish pathogen. BMC Genom. 2008, 9, 427. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.R.; Kim, A.; Lee, Y.; Kim, N.; Roh, H.; Kim, D.H. Complete Genome Sequence of Aeromonas salmonicida subsp. masoucida Strain BR19001YR, Isolated from Diseased Korean Rockfish (Sebastes schlegelii). Microbiol. Resour. Announc. 2021, 10, e01281-20. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, J.; Tu, M.; Gao, L.; Zhang, Y.; Rao, Y.; Rao, L.; Gui, M. Complete genome sequence provides information on quorum sensing related spoilage and virulence of Aeromonas salmonicida GMT3 isolated from spoiled sturgeon. Food Res. Int. 2024, 196, 115039. [Google Scholar] [CrossRef] [PubMed]
- Vaillancourt, K.C.; Paquet, V.E.; Vincent, A.T.; Schneider, A.; Thompson, C.; Laurent, M.; Frenette, M.; Charette, S.J. Draft Genome Sequence of an Aeromonas salmonicida subsp. salmonicida Strain from the Canadian Pacific Coast Bearing a Variant of pRAS1. Microbiol. Resour. Announc. 2021, 10, e00291-21. [Google Scholar] [CrossRef]
- Vincent, A.T.; Le Breton, A.; Bernatchez, A.; Gagné-Thivierge, C.; Paquet, V.E.; Thibault, E.; Charette, S.J.; Gantelet, H. Draft Genome Sequences of Four Aeromonas salmonicida subsp. achromogenes Strains, 23051, 23053, 23055, and 23056, Isolated from Senegalese Sole (Solea senegalensis). Microbiol. Resour. Announc. 2019, 8, e00631-19. [Google Scholar] [CrossRef]
- Ma, J.; Myrsell, V.L.; Dietrich, J.; Cain, K.D. Genome sequence of the virulent Aeromonas salmonicida atypical strain T30 isolated from sablefish with furunculosis. Microbiol. Resour. Announc. 2023, 12, e0053523. [Google Scholar] [CrossRef]
- Vincent, A.T.; Hosseini, N.; Charette, S.J. The Aeromonas salmonicida plasmidome: A model of modular evolution and genetic diversity. Ann. N. Y. Acad. Sci. 2021, 1488, 16–32. [Google Scholar] [CrossRef]
- Vasquez, I.; Hossain, A.; Gnanagobal, H.; Valderrama, K.; Campbell, B.; Ness, M.; Charette, S.J.; Gamperl, A.K.; Cipriano, R.; Segovia, C.; et al. Comparative Genomics of Typical and Atypical Aeromonas salmonicida Complete Genomes Revealed New Insights into Pathogenesis Evolution. Microorganisms 2022, 10, 189. [Google Scholar] [CrossRef]
- Aoki, T.; Egusa, S.; Kimura, T.; Watanabe, T. Detection of R factors in naturally occurring Aeromonas salmonicida strains. Appl. Microbiol. 1971, 22, 716–717. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Kitao, T.; Iemura, N.; Mitoma, Y.; Nomura, T. The Susceptibility of Aeromonas salmonicida Strains Isolated in Cultured and Wild Salmonids to Various Chemotherapeutics. Nippon Suisan Gakkaishi 1983, 49, 17–22. [Google Scholar] [CrossRef]
- Sørum, H.; L’Abée-Lund, T.M.; Solberg, A.; Wold, A. Integron-containing IncU R plasmids pRAS1 and pAr-32 from the fish pathogen Aeromonas salmonicida. Antimicrob. Agents Chemother. 2003, 47, 1285–1290. [Google Scholar] [CrossRef]
- L’Abée-Lund, T.M.; Sørum, H. Functional Tn5393-like transposon in the R plasmid pRAS2 from the fish pathogen Aeromonas salmonicida subspecies salmonicida isolated in Norway. Appl. Environ. Microbiol. 2000, 66, 5533–5535. [Google Scholar] [CrossRef]
- L’Abée-Lund, T.M.; Sørum, H. A global non-conjugative Tet C plasmid, pRAS3, from Aeromonas salmonicida. Plasmid 2002, 47, 172–181. [Google Scholar] [CrossRef]
- Massicotte, M.A.; Vincent, A.T.; Schneider, A.; Paquet, V.E.; Frenette, M.; Charette, S.J. One Aeromonas salmonicida subsp. salmonicida isolate with a pAsa5 variant bearing antibiotic resistance and a pRAS3 variant making a link with a swine pathogen. Sci. Total Environ. 2019, 690, 313–320. [Google Scholar]
- L’Abée-Lund, T.M.; Sørum, H. Class 1 integrons mediate antibiotic resistance in the fish pathogen Aeromonas salmonicida worldwide. Microb. Drug Resist. 2001, 7, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.A.; Austin, B.; Meaden, P.G.; McIntosh, D. Molecular characterization of plasmid-mediated oxytetracycline resistance in Aeromonas salmonicida. Appl. Environ. Microbiol. 1998, 64, 4194–4201. [Google Scholar] [CrossRef]
- Trudel, M.V.; Vincent, A.T.; Attéré, S.A.; Labbé, M.; Derome, N.; Culley, A.I.; Charette, S.J. Diversity of antibiotic-resistance genes in Canadian isolates of Aeromonas salmonicida subsp. salmonicida: Dominance of pSN254b and discovery of pAsa8. Sci. Rep. 2016, 6, 35617. [Google Scholar] [CrossRef]
- Attéré, S.A.; Vincent, A.T.; Paccaud, M.; Frenette, M.; Charette, S.J. The Role for the Small Cryptic Plasmids As Moldable Vectors for Genetic Innovation in Aeromonas salmonicida subsp. salmonicida. Front. Genet. 2017, 8, 211. [Google Scholar] [CrossRef]
- Vincent, A.T.; Trudel, M.V.; Paquet, V.E.; Boyle, B.; Tanaka, K.H.; Dallaire-Dufresne, S.; Daher, R.K.; Frenette, M.; Derome, N.; Charette, S.J. Detection of variants of the pRAS3, pAB5S9, and pSN254 plasmids in Aeromonas salmonicida subsp. salmonicida: Multidrug resistance, interspecies exchanges, and plasmid reshaping. Antimicrob. Agents Chemother. 2014, 58, 7367–7374. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vincent, A.T.; Emond-Rheault, J.G.; Barbeau, X.; Attéré, S.A.; Frenette, M.; Lagüe, P.; Charette, S.J. Antibiotic resistance due to an unusual ColE1-type replicon plasmid in Aeromonas salmonicida. Microbiology 2016, 162, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Attéré, S.A.; Vincent, A.T.; Trudel, M.V.; Chanut, R.; Charette, S.J. Diversity and Homogeneity among Small Plasmids of Aeromonas salmonicida subsp. salmonicida Linked with Geographical Origin. Front. Microbiol. 2015, 6, 1274. [Google Scholar] [CrossRef]
- Dacanay, A.; Knickle, L.; Solanky, K.S.; Boyd, J.M.; Walter, J.A.; Brown, L.L.; Johnson, S.C.; Reith, M. Contribution of the type III secretion system (TTSS) to virulence of Aeromonas salmonicida subsp. salmonicida. Microbiology 2006, 152 Pt 6, 1847–1856. [Google Scholar] [CrossRef]
- Stuber, K.; Burr, S.E.; Braun, M.; Wahli, T.; Frey, J. Type III secretion genes in Aeromonas salmonicida subsp salmonicida are located on a large thermolabile virulence plasmid. J. Clin. Microbiol. 2003, 41, 3854–3856. [Google Scholar] [CrossRef]
- Tanaka, K.H.; Vincent, A.T.; Emond-Rheault, J.G.; Adamczuk, M.; Frenette, M.; Charette, S.J. Plasmid composition in Aeromonas salmonicida subsp. salmonicida 01-B526 unravels unsuspected type three secretion system loss patterns. BMC Genom. 2017, 18, 528. [Google Scholar] [CrossRef]
- Belland, R.J.; Trust, T.J. Aeromonas salmonicida Plasmids: Plasmid-directed Synthesis of Proteins in vitro and in Escherichia coli Minicells. Microbiology 1989, 135, 513–524. [Google Scholar] [CrossRef]
- Brown, R.L.; Sanderson, K.; Kirov, S.M. Plasmids and Aeromonas virulence. FEMS Immunol. Med. Microbiol. 1997, 17, 217–223. [Google Scholar]
- Boyd, J.; Williams, J.; Curtis, B.; Kozera, C.; Singh, R.; Reith, M. Three small, cryptic plasmids from Aeromonas salmonicida subsp. salmonicida A449. Plasmid 2003, 50, 131–144. [Google Scholar] [CrossRef]
- Fehr, D.; Casanova, C.; Liverman, A.; Blazkova, H.; Orth, K.; Dobbelaere, D.; Frey, J.; Burr, S.E. AopP, a type III effector protein of Aeromonas salmonicida, inhibits the NF-kappaB signalling pathway. Microbiology 2006, 152 Pt 9, 2809–2818. [Google Scholar] [CrossRef]
- Emond-Rheault, J.G.; Vincent, A.T.; Trudel, M.V.; Frey, J.; Frenette, M.; Charette, S.J. AsaGEI2b: A new variant of a genomic island identified in the Aeromonas salmonicida subsp. salmonicida JF3224 strain isolated from a wild fish in Switzerland. FEMS Microbiol. Lett. 2015, 362, fnv093. [Google Scholar] [CrossRef][Green Version]
- Juhas, M.; van der Meer, J.R.; Gaillard, M.; Gaillard, M.; Harding, R.M.; Hood, D.W.; Crook, D.W. Genomic islands: Tools of bacterial horizontal gene transfer and evolution. FEMS Microbiol. Rev. 2009, 33, 376–393. [Google Scholar] [CrossRef] [PubMed]
- Bellanger, X.; Payot, S.; Leblond-Bourget, N.; Guédon, G. Conjugative and mobilizable genomic islands in bacteria: Evolution and diversity. FEMS Microbiol. Rev. 2014, 38, 720–760. [Google Scholar] [CrossRef]
- Darmon, E.; Leach, D.R. Bacterial genome instability. Microbiol. Mol. Biol. Rev. 2014, 78, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ni, X.D.; Liu, Y.J.; Lu, C.P. Detection of three virulence genes alt, ahp and aerA in Aeromonas hydrophila and their relationship with actual virulence to zebrafish. J. Appl. Microbiol. 2011, 110, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, Y.H.; Huang, J.; Dasheng, H.U.; Liang, J.; Peng, Y.; Long, S.; Niu, Z.; Han, S.; Zhi, Q. Studies on the correlation with pathogenicity and virulence genes of Aeromonas hydrophila by Truogx sinensis. J. Fish. Sci. China 2015, 22, 698–706. [Google Scholar]
- Zhou, Q.L.; Wang, Y.J.; Xie, J.; Ge, X.P.; Xi, B.W.; Liu, B. Distribution and virulence gene comparison of Aeromonas strains isolated from diseased fish and water environment. Pol. J. Microbiol. 2013, 62, 299–302. [Google Scholar] [CrossRef]
- Liu, Z.P. Screening of Aeromonas salmonicida Strainand Identificaiton of Its Cytotoxin. Master’s Thesis, Hunan Normal University, Changsha, China, 2020. [Google Scholar]
- Yang, X.; Pan, J.M.; Zhang, P.; Hu, A.D.; Zhang, Y.Z.; Cheng, Z.T.; Jiang, H.B.; Wen, M. Molecular typing and virulence gene detection of achromogenes subspecies of Aeromonas salmonicida. J. Yangzhou Univ. (Agric. Life Sci. Ed.) 2021, 42, 24–29. [Google Scholar]
- Diao, J.; Li, L.; Wang, X.L.; Fan, Y.; Yu, X.Q.; La, X.U.; Gai, C.L.; Wang, Y.Q.; Hai-Bin, Y.E. Isolation, identification and virulence factor detection of pathogenic Aeromonas salmonicida from rainbow trout Oncorhynchus mykiss. J. Dalian Ocean Univ. 2018, 33, 435–443. [Google Scholar]
- Zhu, Y.X. The Study on the Virulence and Resistance of Aeromonas in the Yellow River Drainage Basin of Henan. Master’s Thesis, Henan Normal University, Xinxiang, China, 2018. [Google Scholar]
- Liu, M.Z.; Ye, X.; Tian, Y.Y.; Ma, D.M.; Zhang, L.L.; Chi, Y.Y.; Deng, G.C. Expression and immunogenicity analysis of the outer membrane protein W gene of Aeromonas hydrophila. Microbiology/Weishengwuxue Tongbao 2011, 38, 437–445. [Google Scholar]
- Nong, X.W.; Huang, Y.H.; Long, S.; Liang, J.Z.; Niu, Z.W.; Liu, J.; Mi, Q.; Huang, J. Isolation, identification and virulence gene detection of pathogenic Aeromonas sobria from Truogx sinensis. J. South. Agric. 2015, 46, 1322–1328. [Google Scholar]
- Wong, C.Y.F.; Heuzenroeder, M.W.; Flower, R.L.P. Inactivation of two haemolytic toxin genes in Aeromonas hydrophila attenuates virulence in a suckling mouse model. Microbiology 1998, 144 Pt 2, 291–298. [Google Scholar] [CrossRef]
- Song, M.F.; Zhang, D.X.; Zhang, H.P.; Chen, L.; Kang, Y.H.; Zhang, L.; Shan, X.F.; Qian, A.D. Research advances of virulence factors in Aeromonas veronii. Chin. Vet. Sci./Zhongguo Shouyi Kexue 2018, 48, 1038–1042. [Google Scholar]
- Chakraborty, T.; Huhle, B.; Hof, H.; Bergbauer, H.; Goebel, W. Marker exchange mutagenesis of the aerolysin determinant in Aeromonas hydrophila demonstrates the role of aerolysin in A. hydrophila-associated systemic infections. Infect. Immun. 1987, 55, 2274–2280. [Google Scholar] [CrossRef] [PubMed]
- Hirono, I.; Aoki, T. Cloning and characterization of three hemolysin genes from Aeromonas salmonicida. Microb. Pathog. 1993, 15, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.M.; Lin, T.L.; Gong, H.; Yang, J.X.; Liu, X.D. Preparing the ISCOMs of Aeromonas hydrophila β-hemolysin expressed in E. coli. Fujian J. Agric. Sci. 2004, 19, 238–242. [Google Scholar]
- Li, L.; Diao, J.; Yu, X.Q.; Wang, X.L.; Wang, Y.H.; Ye, H.B.; Wu, H.Y. Study on the Immune Protective Effects of Recombinant Hemolysin and Pilin against Aeromonas salmonicida. J. Guangxi Acad. Sci. 2021, 37, 101–108. [Google Scholar]
- Chen, Y.; Wang, J.; Cai, H.; Lin, M.; Zhang, Y.; Huang, L. Enhanced Hemolytic Activity of Mesophilic Aeromonas salmonicida SRW-OG1 Is Brought about by Elevated Temperatures. Microorganisms 2022, 10, 2033. [Google Scholar] [CrossRef]
- Kroniger, T.; Mehanny, M.; Schlüter, R.; Trautwein-Schult, A.; Köllner, B.; Becher, D. Effect of Iron Limitation, Elevated Temperature, and Florfenicol on the Proteome and Vesiculation of the Fish Pathogen Aeromonas salmonicida. Microorganisms 2022, 10, 1735. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.H.; Huang, H.; Liu, X.D.; Zhang, L.W.; Yang, T.; Hu, L.B.; Li, Z.J. Isolation, identification and virulence gene of atypical Aeromonas salmonicida from cultured turbot Scophthalmus maximus. J. Dalian Fish. Univ. 2022, 37, 558–567. [Google Scholar]
- Eggset, G.; Bjørnsdottir, R.; Leifson, R.M.; Arnesen, J.A.; Coucheron, D.H.; JØRGENSEN, T.Ø. Extracellular glycerophospholipid: Cholesterol acyltransferase from Aeromonas salmonicida: Activation by serine protease. J. Fish Dis. 2010, 17, 17–29. [Google Scholar] [CrossRef]
- Vipond, R.; Bricknell, I.R.; Durant, E.; Bowden, T.J.; Ellis, A.E.; Smith, M.; MacIntyre, S. Defined deletion mutants demonstrate that themajor secreted toxins are not essential for the virulence of Aeromonas salmonicida. Infect. Immun. 1998, 66, 1990. [Google Scholar] [CrossRef] [PubMed]
- Bricknell, I.R.; Bowden, T.J.; Lomax, J.; Ellis, A.E. Antibody response and protection of Atlantic salmon (Salmo salar) immunised with an extracellular polysaccharide of Aeromonas salmonicida. Fish Shellfish Immunol. 1997, 7, 1–16. [Google Scholar] [CrossRef]
- Ausio, J.; van der Goot, F.G.; Buckley, J.T. Physical and chemical characterization of the oligomerization state of the Aeromonas hydrophila lipase/acyltransferase. FEBS Lett. 1993, 333, 296–300. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ebanks, R.O.; Goguen, M.; McKinnon, S.; Pinto, D.M.; Ross, N.W. Identification of the major outer membrane proteins of Aeromonas salmonicida. Dis. Aquat. Organ. 2005, 68, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Arnesen, K.R.; Mikkelsen, H.; Schrøder, M.B.; Lund, V. Impact of reattaching various Aeromonas salmonicida A-layer proteins on vaccine efficacy in Atlantic cod (Gadus morhua). Vaccine 2010, 28, 4703–4708. [Google Scholar] [CrossRef]
- Lund, V.; Mikkelsen, H.; Schrøder, M.B. Atypical furunculosis vaccines for Atlantic cod Gadhus morhua: Impact of reattached Aeromonas salmonicida A-layer protein on vaccine efficacy. Dis. Aquat. Organ. 2009, 85, 115–122. [Google Scholar] [CrossRef][Green Version]
- Leduc, G.R.; Paquet, V.E.; Vincent, A.T.; Charette, S.J. Characterization of bacteriophage T7-Ah reveals its lytic activity against a subset of both mesophilic and psychrophilic Aeromonas salmonicida strains. Arch. Virol. 2021, 166, 521–533. [Google Scholar] [CrossRef]
- Paquet, V.E.; Vincent, A.T.; Moineau, S.; Charette, S.J. Beyond the A-layer: Adsorption of lipopolysaccharides and characterization of bacteriophage-insensitive mutants of Aeromonas salmonicida subsp. salmonicida. Mol. Microbiol. 2019, 112, 667–677. [Google Scholar] [CrossRef]
- Leduc, G.R.; Paquet, V.E.; Piché, L.C.; Vincent, A.T.; Charette, S.J. Isolation of vB_AsaM_LPM4 reveals the dynamics of Prophage 3 in Aeromonas salmonicida subsp. salmonicida. Arch. Virol. 2023, 168, 72. [Google Scholar] [CrossRef]
- Yu, H.B.; Zhang, Y.L.; Lau, Y.L.; Yao, F.; Vilches, S.; Merino, S.; Tomas, J.M.; Howard, S.P.; Leung, K.Y. Identification and characterization of putative virulence genes and gene clusters in Aeromonas hydrophila PPD134/91. Appl. Environ. Microbiol. 2005, 71, 4469–4477. [Google Scholar] [CrossRef]
- Lee, H.J.; Storesund, J.E.; Lunestad, B.T.; Hoel, S.; Lerfall, J.; Jakobsen, A.N. Whole genome sequence analysis of Aeromonas spp. isolated from ready-to-eat seafood: Antimicrobial resistance and virulence factors. Front. Microbiol. 2023, 14, 1175304. [Google Scholar] [CrossRef] [PubMed]
- Vanden Bergh, P.; Frey, J. Aeromonas salmonicida subsp. salmonicida in the light of its type-three secretion system. Microb. Biotechnol. 2014, 7, 381–400. [Google Scholar] [CrossRef]
- Vanden Bergh, P.; Heller, M.; Braga-Lagache, S.; Frey, J. The Aeromonas salmonicida subsp. salmonicida exoproteome: Global analysis, moonlighting proteins and putative antigens for vaccination against furunculosis. Proteome Sci. 2013, 11, 44. [Google Scholar] [CrossRef]
- Burr, S.E.; Stuber, K.; Frey, J. The ADP-ribosylating toxin, AexT, from Aeromonas salmonicida subsp. salmonicida is translocated via a type III secretion pathway. J. Bacteriol. 2003, 185, 6583–6591. [Google Scholar] [CrossRef]
- Braun, M.; Stuber, K.; Schlatter, Y.; Wahli, T.; Kuhnert, P.; Frey, J. Characterization of an ADP-Ribosyltransferase Toxin (AexT) from Aeromonas salmonicida subsp. salmonicida. J. Bacteriol. 2002, 184, 1851. [Google Scholar] [CrossRef] [PubMed]
- Bergh, P.V.; Burr, S.E.; Benedicenti, O.; von Siebenthal, B.; Frey, J.; Wahli, T. Antigens of the type-three secretion system of Aeromonas salmonicida subsp. salmonicida prevent protective immunity in rainbow trout. Vaccine 2013, 31, 5256–5261. [Google Scholar] [CrossRef]
- Rangrez, A.Y.; Dayananda, K.M.; Atanur, S.; Joshi, R.; Patole, M.S.; Shouche, Y.S. Detection of conjugation related type four secretion machinery in Aeromonas culicicola. PLoS ONE 2006, 1, e115. [Google Scholar] [CrossRef] [PubMed]
- Bröms, J.E.; Meyer, L.; Lavander, M.; Larsson, P.; Sjöstedt, A. Core components of type VI secretion systems are essential for Francisella LVS pathogenicity. PLoS ONE 2012, 7, e34639. [Google Scholar] [CrossRef]
- Carniel, E.; Guilvout, I.; Prentice, M. Characterization of a large chromosomal “high-pathogenicity island” in biotype 1B Yersinia enterocolitica. J. Bacteriol. 1996, 178, 6743–6751. [Google Scholar] [CrossRef]
- Cai, H.; Yu, J.; Qiao, Y.; Ma, Y.; Zheng, J.; Lin, M.; Yan, Q.; Huang, L. Effect of the Type VI Secretion System Secreted Protein Hcp on the Virulence of Aeromonas salmonicida. Microorganisms 2022, 10, 2307. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, R.; Joseph, S.W.; Chopra, A.K.; Sha, J.; Shaw, J.; Graf, J.; Haft, D.; Wu, M.; Ren, Q.; Rosovitz, M.J.; et al. Genome sequence of Aeromonas hydrophila ATCC 7966T: Jack of all trades. J. Bacteriol. 2006, 188, 8272–8282. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Zou, Z.; Huang, H.; Ren, Y.; Zhang, Y.; Li, G.; Zhou, Z.; Wang, L. Complete genome sequence of Aeromonas veronii strain B565. J. Bacteriol. 2011, 193, 3389–3390. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Miller, A.; Bull, H.; Howard, S.P. Assembly of the type II secretion system: Identification of ExeA residues critical for peptidoglycan binding and secretin multimerization. J. Bacteriol. 2011, 193, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Suarez, G.; Sierra, J.C.; Sha, J.; Wang, S.; Erova, T.E.; Fadl, A.A.; Foltz, S.M.; Horneman, A.J.; Chopra, A.K. Molecular characterization of a functional type VI secretion system from a clinical isolate of Aeromonas hydrophila. Microb. Pathog. 2008, 44, 344–361. [Google Scholar] [CrossRef]
- Suarez, G.; Sierra, J.C.; Erova, T.E.; Sha, J.; Horneman, A.J.; Chopra, A.K. A type VI secretion system effector protein, VgrG1, from Aeromonas hydrophila that induces host cell toxicity by ADP ribosylation of actin. J. Bacteriol. 2010, 192, 155–168. [Google Scholar] [CrossRef]
- Ratledge, C.; Dover, L.G. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 2000, 54, 881–941. [Google Scholar] [CrossRef]
- Wandersman, C.; Delepelaire, P. Bacterial iron sources: From siderophores to hemophores. Annu. Rev. Microbiol. 2004, 58, 611–647. [Google Scholar] [CrossRef]
- Balado, M.; Souto, A.; Vences, A.; Careaga, V.P.; Valderrama, K.; Segade, Y.; Rodríguez, J.; Osorio, C.R.; Jiménez, C.; Lemos, M.L. Two Catechol Siderophores, Acinetobactin and Amonabactin, Are Simultaneously Produced by Aeromonas salmonicida subsp. salmonicida Sharing Part of the Biosynthetic Pathway. ACS Chem. Biol. 2015, 10, 2850–2860. [Google Scholar] [CrossRef]
- Najimi, M.; Lemos, M.L.; Osorio, C.R. Identification of siderophore biosynthesis genes essential for growth of Aeromonas salmonicida under iron limitation conditions. Appl. Environ. Microbiol. 2008, 74, 2341–2348. [Google Scholar] [CrossRef]
- Ebanks, R.O.; Dacanay, A.; Goguen, M.; Pinto, D.M.; Ross, N.W. Differential proteomic analysis of Aeromonas salmonicida outer membrane proteins in response to low iron and in vivo growth conditions. Proteomics 2004, 4, 1074–1085. [Google Scholar] [CrossRef] [PubMed]
- Hirst, I.D.; Ellis, A.E. Iron-regulated outer membrane proteins of Aeromonas salmonicida are important protective antigens in Atlantic salmon against furunculosis. Fish Shellfish Immunol. 1994, 1, 29–45. [Google Scholar] [CrossRef]
- Huang, W.; Wilks, A. Extracellular Heme Uptake and the Challenge of Bacterial Cell Membranes. Annu. Rev. Biochem. 2017, 86, 799–823. [Google Scholar] [CrossRef] [PubMed]
- Richard, K.L.; Kelley, B.R.; Johnson, J.G. Heme Uptake and Utilization by Gram-Negative Bacterial Pathogens. Front. Cell Infect. Microbiol. 2019, 9, 81. [Google Scholar] [CrossRef]
- Najimi, M.; Lemos, M.L.; Osorio, C.R. Identification of heme uptake genes in the fish pathogen Aeromonas salmonicida subsp. salmonicida. Arch. Microbiol. 2008, 190, 439–449. [Google Scholar] [CrossRef]
- Lemos, M.L.; Balado, M. Iron uptake mechanisms as key virulence factors in bacterial fish pathogens. J. Appl. Microbiol. 2020, 129, 104–115. [Google Scholar] [CrossRef]
- Padra, J.T.; Loibman, S.O.; Thorell, K.; Sundh, H.; Sundell, K.; Lindén, S.K. Atlantic Salmon Mucins Inhibit LuxS-Dependent, A. Salmonicida AI-2 Quorum Sensing in an N-Acetylneuraminic Acid-Dependent Manner. Int. J. Mol. Sci. 2022, 23, 4326. [Google Scholar] [CrossRef]
- Ojaimi Loibman, S.; Quintana-Hayashi, M.P.; Santos, L.; Lindén, S.K. Aeromonas salmonicida AI-1 and AI-2 quorum sensing pathways are differentially regulated by rainbow trout mucins and during in vivo colonization. Fish Shellfish Immunol. 2024, 153, 109862. [Google Scholar] [CrossRef]
- Majik, M.S.; Gawas, U.B.; Mandrekar, V.K. Next generation quorum sensing inhibitors: Accounts on structure activity relationship studies and biological activities. Bioorg. Med. Chem. 2020, 28, 115728. [Google Scholar] [CrossRef]
- Gonzales, M.; Kergaravat, B.; Jacquet, P.; Billot, R.; Grizard, D.; Chabrière, É.; Plener, L.; Daudé, D. Disrupting quorum sensing as a strategy to inhibit bacterial virulence in human, animal, and plant pathogens. Pathog. Dis. 2024, 82, ftae009. [Google Scholar] [CrossRef]
- Cao, C.Y.; Wang, K.Y.; Wang, L.; Huang, X.L. Isolation and Identification of Pathogenic Bacteria Causing Ulcer Disease of Atlantic salmon. Freshw. Fish. 2009, 39, 54–57. [Google Scholar]
- Jin, H.Y.; Liu, Y.K.; Gao, Y.; Xia, S.D.; Chen, S.Q.; Mo, Z.L.; Bian, L.; Li, J. Isolation and Identification of Aeromonas salmonicida from Thamnaconus septentrionalis and Sebastes schlegeli. Prog. Fish. Sci. 2023, 44, 191–200. [Google Scholar]
- Yu, X.H.; Wang, Y.H.; Tang, C.; Yue, H. Progress in studies on Aeromonas hydrophila. J. Southwest Univ. Natl. (Nat. Sci. Ed.) 2007, 33, 508–514. [Google Scholar]
- Huang, X.; Xiong, G.; Feng, Y.; Wang, K.; Liu, Y.; Zhong, L.; Liu, S.; Geng, Y.; Ouyang, P.; Chen, D. Ulcerative disease emergence in grass carp (Ctenopharyngodon idellus) aquaculture in China: Possible impact of temperature abnormality. Aquaculture 2020, 517, 734811. [Google Scholar] [CrossRef]
- Hussain Bhat, R.A.; Thakuria, D.; Dubey, M.K.; Tandel, R.S.; Sharma, P.; Khangembam, V.C.; Dash, P.; Tripathi, G.; Sarma, D. Lethal dose and histopathological alterations induced by Aeromonas salmonicida in experimentally challenged common carp, Cyprinus carpio. Microb. Pathog. 2021, 158, 105110. [Google Scholar] [CrossRef] [PubMed]
- Tewari, R.; Dudeja, M.; Nandy, S.; Das, A.K. Isolation of Aeromonas salmonicida from Human Blood Sample: A Case Report. J. Clin. Diagn. Res. 2014, 8, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Kamble, R. Aeromonas salmonicida furunculosis in an adult male. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 59–63. [Google Scholar]
- Varshney, A.; Das, M.; Chaudhary, P.; Kumari, R.; Yadav, K. Aeromonas Salmonicida as a Causative Agent for Postoperative Endophthalmitis. Middle East Afr. J. Ophthalmol. 2017, 24, 213–215. [Google Scholar] [CrossRef]
- Vincent, A.T.; Fernández-Bravo, A.; Sanchis, M.; Mayayo, E.; Figueras, M.J.; Charette, S.J. Investigation of the virulence and genomics of Aeromonas salmonicida strains isolated from human patients. Infect. Genet. Evol. 2019, 68, 1–9. [Google Scholar] [CrossRef]
- Vincent, A.T.; Bernatchez, A.; Frey, J.; Charette, S.J. A Mesophilic Aeromonas salmonicida Strain Isolated from an Unsuspected Host, the Migratory Bird Pied Avocet. Microorganisms 2019, 7, 592. [Google Scholar] [CrossRef]
- Deng, T.W.; Jiang, Z.H.; Huo, Y.N.; Song, H.; He, Q.G. Characterization of antibiotic resistance and detection of virulence genes in Aeromonas salmonicida isolated from pig. Chin. J. Vet. Med. 2019, 55, 74–76+79+125. [Google Scholar]
- Wang, B.; Yan, Z.Y.; Kong, L.C.; Jia, B.Y.; Wei, X.; Liu, S.M.; Ma, H.X. Isolation, Identification and Antimicrobial Susceptibility Test of Aeromonas salmonicida from Goat. Chin. J. Vet. Drug 2018, 52, 1–6. [Google Scholar]
- Bakiyev, S.; Smekenov, I.; Zharkova, I.; Kobegenova, S.; Sergaliyev, N.; Absatirov, G.; Bissenbaev, A. Characterization of atypical pathogenic Aeromonas salmonicida isolated from a diseased Siberian sturgeon (Acipenser baerii). Heliyon 2023, 9, e17775. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Devi, R.; Khan, M.I.R.; Kamilya, D.; Gon Choudhury, T.; Parhi, J. Isolation of Aeromonas salmonicida subspecies salmonicida from aquaculture environment in India: Polyphasic identification, virulence characterization, and antibiotic susceptibility. Microb. Pathog. 2023, 179, 106100. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.J.; Kim, S.S.; Kim, A.; Cho, M.Y.; Do, J.W. Isolation, Identification, and Characteristics of Aeromonas salmonicida subsp. masoucida from Diseased Starry Flounder (Platichthys stellatus). Pathogens 2025, 14, 257. [Google Scholar]
- Sun, X.N.; Wang, Q.; Wang, Y.F.; Tao, Y.; Zheng, C.L.; Wang, M.H.; Che, M.Y.; Cui, Z.H.; Li, X.L.; Zhang, Q.; et al. Isolation and identification of vapA-absent Aeromonas salmonicida in diseased snakehead Channa argus in China. Int. Microbiol. 2024, 27, 1137–1150. [Google Scholar] [CrossRef]
- Nikapitiya, C.; Dananjaya, S.H.S.; Chandrarathna, H.P.S.U.; Senevirathne, A.; De Zoysa, M.; Lee, J. Isolation and Characterization of Multidrug Resistance Aeromonas salmonicida subsp. salmonicida and Its Infecting Novel Phage ASP-1 from Goldfish (Carassius auratus). Indian J. Microbiol. 2019, 59, 161–170. [Google Scholar] [PubMed]
- Kala, K.; Mallik, S.K.; Shahi, N.; Pathak, R.; Sharma, P.; Chandra, S.; Patiyal, R.S.; Pande, V.; Pandey, N.; Pande, A.; et al. Emergence of Aeromonas salmonicida subsp. masoucida MHJM250: Unveiling pathological characteristics and antimicrobial susceptibility in golden mahseer, Tor putitora (Hamilton, 1822) in India. Vet. Res. Commun. 2024, 48, 3751–3772. [Google Scholar]
- Rostang, A.; Bachelet, F.; Fournel, C.; Carabin, T.; Navarro-Gonzalez, N.; Calvez, S. Susceptibility of Aeromonas salmonicida subsp. salmonicida bacteria from French farmed trout to antibiotics commonly used in fish farming, and attempt to set epidemiological cut-off values. Front. Microbiol. 2025, 16, 1532748. [Google Scholar]
- Snieszko, S.F.; Friddle, S.B. Prophylaxis of furunculosis in brook trout (Salvelinus fontinalis) by oral immunization and sulfamerazine. Progress. Fish-Cult. 1949, 11, 161–168. [Google Scholar] [CrossRef]
- Rodgers, C.J.; Furones, M.D. Antimicrobial agents in aquaculture: Practice, needs and issues. Options Méditerranéennes 2009, 86, 41–59. [Google Scholar]
- Gu, Q.H. Establishment of a Detection Method for Aeromonas salmonicida and Screening of Antibacterial Drugs. Master’s Thesis, Jiangsu Ocean University, Lianyungang, China, 2022. [Google Scholar]
- Li, J. Pathogenicity of Aeromonas salmonicida, Host Immune Response in Odontobutis potamophila and Screening of Antibacterial Chinese Herbal Medicines. Master’s Thesis, Yangzhou University, Yangzhou, China, 2024. [Google Scholar]
- Park, S.Y.; Han, J.E.; Kwon, H.; Park, S.C.; Kim, J.H. Recent Insights into Aeromonas salmonicida and Its Bacteriophages in Aquaculture: A Comprehensive Review. J. Microbiol. Biotechnol. 2020, 30, 1443–1457. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Jin, P.; Zhou, X.; Zhang, Y.; Wang, Q.; Liu, X.; Shao, S.; Liu, Q. Isolation of a Virulent Aeromonas salmonicida subsp. masoucida Bacteriophage and Its Application in Phage Therapy in Turbot (Scophthalmus maximus). Appl. Environ. Microbiol. 2021, 87, e0146821. [Google Scholar]
- Hosseini, N.; Paquet, V.E.; Chehreghani, M.; Moineau, S.; Charette, S.J. Phage Cocktail Development against Aeromonas salmonicida subsp. salmonicida Strains Is Compromised by a Prophage. Viruses 2021, 13, 2241. [Google Scholar]
- Zhou, Y.; Yuan, S.J.; Yan, T.W.; Ma, Y.F. Isolation and Characterization of a Novel Lytic T4-Like Bacteriophage Asfd-1 Infecting Aeromonas salmonicide. J. Integr. Technol. 2019, 8, 10–18. [Google Scholar]
- Kim, J.H.; Choresca, C.H.; Shin, S.P.; Han, J.E.; Jun, J.W.; Park, S.C. Biological control of Aeromonas salmonicida subsp. salmonicida infection in rainbow trout (Oncorhynchus mykiss) using Aeromonas phage PAS-1. Transbound. Emerg. Dis. 2015, 62, 81–86. [Google Scholar]
- Cui, Z.L.; Guo, X.K.; Li, L.; Feng, T.T. Establishment of safety assessment system of phage therapy. Acta Microbiol. Sin. 2018, 58, 2033–2039. [Google Scholar]

| Antibiotics | Classification | Sensitivity | References |
|---|---|---|---|
| Oxytetracycline | Tetracyclines | I | [116] |
| Tetracycline | S | [117] | |
| Vancomycin | Glycopeptides | R | [118] |
| Florfenicol | Amides | S | [119] |
| Kanamycin | Aminoglycosides | S | [118] |
| Gentamicin | S | [120] | |
| Cefoxitin | Cephalosporins | S | [121] |
| Ceftriaxone | S | ||
| Cefepime | S | ||
| Cefpodoxime | S | ||
| Cephalothin | S | [120] | |
| Rifamycin | R | [117] | |
| Erythromycin | Macrolides | I | [116] |
| Trimethoprim | Sulfonamides | R | [122] |
| Sulfadiazine | R | ||
| Enrofloxacin | Quinolones | S | [118] |
| Norfloxacin | S | [116] | |
| Ciprofloxacin | S | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, X.; Li, Z.; Guo, J.; Bai, F.; Ling, X. Beyond Fish Pathogens: A Comprehensive Overview of Aeromonas salmonicida. Microbiol. Res. 2025, 16, 157. https://doi.org/10.3390/microbiolres16070157
Qin X, Li Z, Guo J, Bai F, Ling X. Beyond Fish Pathogens: A Comprehensive Overview of Aeromonas salmonicida. Microbiology Research. 2025; 16(7):157. https://doi.org/10.3390/microbiolres16070157
Chicago/Turabian StyleQin, Xiaotong, Zhongduo Li, Jinglan Guo, Feng Bai, and Xiaodong Ling. 2025. "Beyond Fish Pathogens: A Comprehensive Overview of Aeromonas salmonicida" Microbiology Research 16, no. 7: 157. https://doi.org/10.3390/microbiolres16070157
APA StyleQin, X., Li, Z., Guo, J., Bai, F., & Ling, X. (2025). Beyond Fish Pathogens: A Comprehensive Overview of Aeromonas salmonicida. Microbiology Research, 16(7), 157. https://doi.org/10.3390/microbiolres16070157