Carriage of Rifampicin- and Multidrug-Resistant Pseudomonas aeruginosa in Apparently Healthy Camels: A View Through a Zoonosis Lens
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Isolation and Identification of Pseudomonas aeruginosa
2.3. Antimicrobial Susceptibility Testing
2.4. Phenotypic Identification of ESBL-Producing P. aeruginosa Isolates
2.5. DNA Extraction
2.6. Genotypic Detection of Beta-Lactamase-Encoding Genes in ESBL-Producing P. aeruginosa Isolates
2.7. Molecular Detection of the rpoB Gene in P. aeruginosa Isolates
2.8. Partial DNA Sequencing of the P. aeroginosa rpoB Gene and Phylogenetic Analysis
3. Results
3.1. Occurrence of P. aeruginosa in Apparently Healthy Slaughtered Camels
3.2. Antimicrobial Susceptibility Pattern of P. aeruginosa Isolates
3.3. Phenotypic and Genotypic Detection of ESBL-Producing P. aeruginosa Isolates
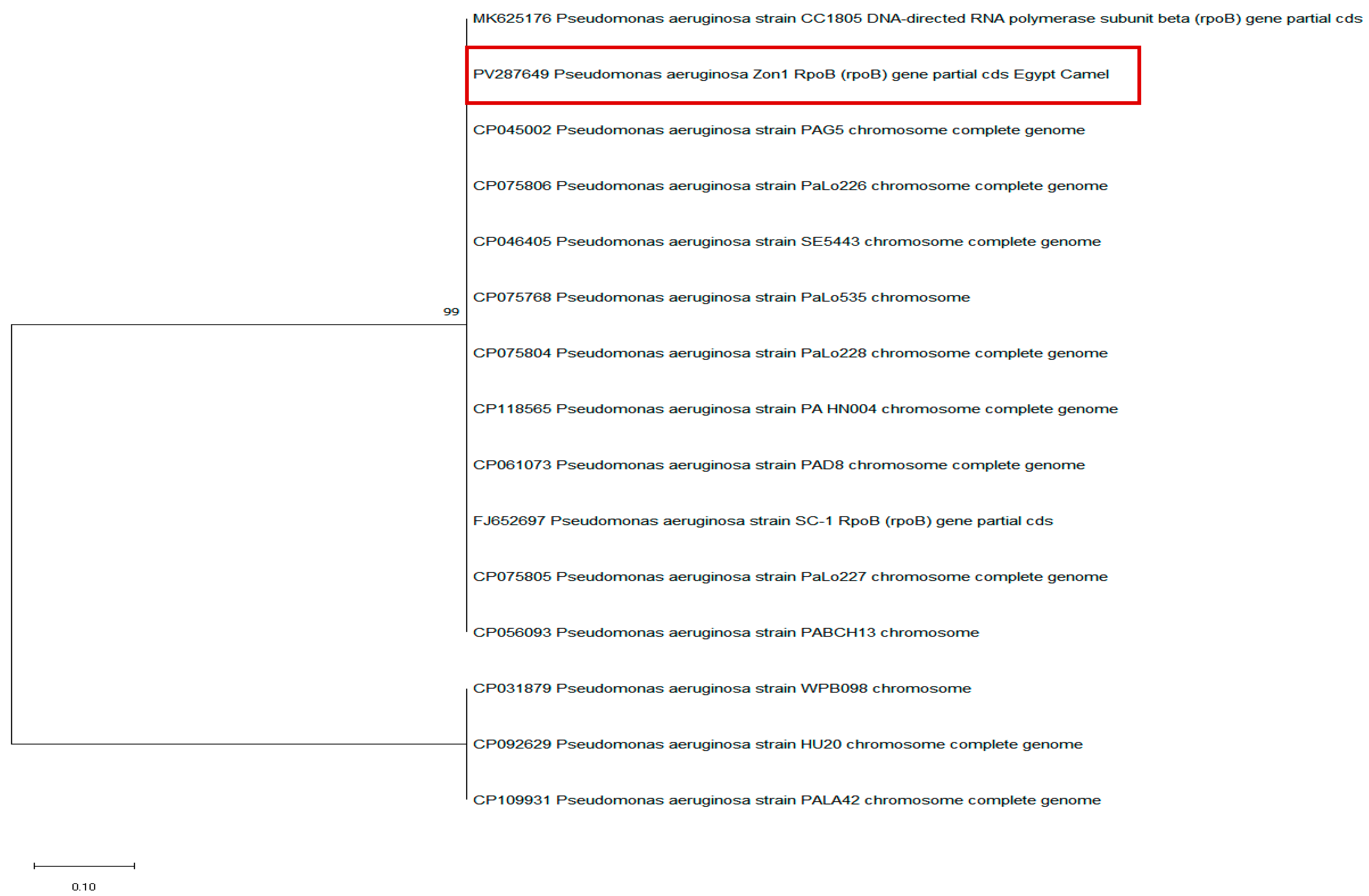
3.4. Occurrence of the rpoB Gene in P. aeruginosa Isolates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elfadadny, A.; Ragab, R.F.; AlHarbi, M.; Badshah, F.; Ibáñez-Arancibia, E.; Farag, A.; Hendawy, A.O.; De Los Ríos-Escalante, P.R.; Aboubakr, M.; Zakai, S.A.; et al. Antimicrobial resistance of Pseudomonas aeruginosa: Navigating clinical impacts, current resistance trends, and innovations in breaking therapies. Front. Microbiol. 2024, 15, 1374466. [Google Scholar] [CrossRef] [PubMed]
- Secker, B.; Shaw, S.; Atterbury, R.J. Pseudomonas spp. in Canine Otitis Externa. Microorganisms 2023, 11, 2650. [Google Scholar] [CrossRef] [PubMed]
- Schauer, B.; Wald, R.; Urbantke, V.; Loncaric, I.; Baumgartner, M. Tracing Mastitis Pathogens-Epidemiological Investigations of a Pseudomonas aeruginosa Mastitis Outbreak in an Austrian Dairy Herd. Animals 2021, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.F.; Fayez, M.; Swelum, A.A.; Alswat, A.S.; Alkafafy, M.; Alzahrani, O.M.; Alsunaini, S.J.; Almuslem, A.; Al Amer, A.S.; Yusuf, S. Genetic Diversity, Biofilm Formation, and Antibiotic Resistance of Pseudomonas aeruginosa Isolated from Cow, Camel, and Mare with Clinical Endometritis. Vet. Sci. 2022, 9, 239. [Google Scholar] [CrossRef]
- Cabassi, C.S.; Sala, A.; Santospirito, D.; Alborali, G.L.; Carretto, E.; Ghibaudo, G.; Taddei, S. Activity of AMP2041 against human and animal multidrug resistant Pseudomonas aeruginosa clinical isolates. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 17. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance, 2024. Available online: https://www.who.int/publications/i/item/9789240093461 (accessed on 21 May 2024).
- Elmansoury, E.A.; Zaki, M.E.S.; Salem, M.M.M.; Montasser, K.A.; Hamam, S.S.M. Genetic study of extended spectrum beta-lactamase genes; bla-TEM, blaOXA-10, blaSHV and per-1 in Pseudomonas aeruginosa from hospital-acquired infections. Antonie Van Leeuwenhoek 2025, 118, 59. [Google Scholar] [CrossRef]
- Shalmashi, H.; Farajnia, S.; Sadeghi, M.; Tanoumand, A.; Veissi, K.; Hamishekar, H.; Gotaslou, R. Detection of ESBLs types blaCTX-M, blaSHV and blaTEM resistance genes among clinical isolates of Pseudomonas aeruginosa. Gene Rep. 2022, 28, 101637. [Google Scholar] [CrossRef]
- Horcajada, J.P.; Montero, M.; Oliver, A.; Sorlí, L.; Luque, S.; Gómez-Zorrilla, S.; Benito, N.; Grau, S. Epidemiology and Treatment of Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa Infections. Clin. Microbiol. Rev. 2019, 32, e00031-19. [Google Scholar] [CrossRef]
- Abdel-Aziem, S.H.; Mabrouk, D.M.; Abd El-Kader, H.A.; Alam, S.S.; Othman, O.E. Genetic similarity and diversity among three camel populations reared in Egypt. J. Genet. Eng. Biotechnol. 2022, 20, 154. [Google Scholar] [CrossRef]
- Zhu, S.; Zimmerman, D.; Deem, S.L. A Review of Zoonotic Pathogens of Dromedary Camels. EcoHealth 2019, 16, 356–377. [Google Scholar] [CrossRef]
- Abdelrahman, A.M.; Mohamed, S.R.; Soliman, S.M.; Marouf, S. Pseudomonas species isolated from camels: Phenotypic, genotypic and antimicrobial profile. Adv. Anim. Vet. Sci. 2022, 10, 219–225. [Google Scholar] [CrossRef]
- Almansour, A.M.; Alhadlaq, M.A.; Alzahrani, K.O.; Mukhtar, L.E.; Alharbi, A.L.; Alajel, S.M. The Silent Threat: Antimicrobial-Resistant Pathogens in Food-Producing Animals and Their Impact on Public Health. Microorganisms 2023, 11, 2127. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Fang, R.; Wang, C.; Tian, X.; Lin, J.; Zeng, W.; Zhou, T.; Xu, C. Resistance Profiles and Biological Characteristics of Rifampicin-Resistant Staphylococcus aureus Small-Colony Variants. Infect. Drug Resist 2021, 14, 1527–1536. [Google Scholar] [CrossRef]
- Li, M.C.; Lu, J.; Lu, Y.; Xiao, T.Y.; Liu, H.C.; Lin, S.Q.; Xu, D.; Li, G.L.; Zhao, X.Q.; Liu, Z.G.; et al. rpoB Mutations and Effects on Rifampin Resistance in Mycobacterium tuberculosis. Infect. Drug Resist 2021, 14, 4119–4128. [Google Scholar] [CrossRef]
- Mandell, G.L.; Vest, T.K. Killing of intraleukocytic Staphylococcus aureus by rifampin: In-vitro and in-vivo studies. J. Infect. Dis. 1972, 125, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.J.; Gross, C.A. Mapping and sequencing of mutations in the Escherichia coli rpoB gene that lead to rifampicin resistance. J. Mol. Biol. 1988, 202, 45–58. [Google Scholar] [CrossRef]
- Giannouli, M.; Di Popolo, A.; Durante-Mangoni, E.; Bernardo, M.; Cuccurullo, S.; Amato, G.; Tripodi, M.F.; Triassi, M.; Utili, R.; Zarrilli, R. Molecular epidemiology and mechanisms of rifampicin resistance in Acinetobacter baumannii isolates from Italy. Int. J. Antimicrob. Agents 2012, 39, 58–63. [Google Scholar] [CrossRef]
- Shi, S.; Xu, M.; Zhao, Y.; Feng, L.; Liu, Q.; Yao, Z.; Sun, Y.; Zhou, T.; Ye, J. Tigecycline-Rifampicin Restrains Resistance Development in Carbapenem-Resistant Klebsiella pneumoniae. ACS Infect. Dis. 2023, 9, 1858–1866. [Google Scholar] [CrossRef]
- Amin, W.; Gadallah, M.; Salah, A.; Rady, M. Prevalence of Rifampicin resistance tuberculosis among presumptive tuberculosis patients in Egypt-2021: A national health facility-based survey. BMC Infect. Dis. 2024, 24, 210. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, S.S.; Choi, S.M.; Bae, C.J.; Oh, T.H.; Kim, S.E.; Kim, U.J.; Kang, S.J.; Jung, S.I.; Park, K.H. Rifamycin resistance, rpoB gene mutation and clinical outcomes of Staphylococcus species isolates from prosthetic joint infections in Republic of Korea. J. Glob. Antimicrob. Resist 2022, 28, 43–48. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, F.; Zhang, J.; Dai, J.; Rong, D.; Zhao, M.; Wang, J.; Ding, Y.; Chen, M.; Xue, L.; et al. Molecular Characterization of Rifampicin-Resistant Staphylococcus aureus Isolates from Retail Foods in China. Antibiotics 2021, 10, 1487. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Toll-Riera, M.; Heilbron, K.; Preston, G.M.; MacLean, R.C. The genomic basis of adaptation to the fitness cost of rifampicin resistance in Pseudomonas aeruginosa. Proc. Biol. Sci. 2016, 283, 20152452. [Google Scholar]
- Yee, Y.C.; Kisslinger, B.; Yu, V.L.; Jin, D.J. A mechanism of rifamycin inhibition and resistance in Pseudomonas aeruginosa. J. Antimicrob. Chemother. 1996, 38, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Ward, H.; Perron, G.G.; Maclean, R.C. The cost of multiple drug resistance in Pseudomonas aeruginosa. J. Evol. Biol. 2009, 22, 997–1003. [Google Scholar] [CrossRef]
- Quinn, P.J.; Markey, B.K.; Donnelly, W.J.; Leonard, F.C.; Fanning, S.; Maguire, D. Veterinary Microbiology and Microbial Disease; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). M100-S25 Performance Standards for Antimicrobial Susceptibility Testing: 25th Informational Supplement; CLSI: Wayne, PA, USA, 2015. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Motoshima, M.; Yanagihara, K.; Fukushima, K.; Matsuda, J.; Sugahara, K.; Hirakata, Y.; Yamada, Y.; Kohno, S.; Kamihira, S. Rapid and accurate detection of Pseudomonas aeruginosa by real-time polymerase chain reaction with melting curve analysis targeting gyrB gene. Diagn. Microbiol. Infect. Dis. 2007, 58, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Neyestanaki, D.K.; Mirsalehian, A.; Rezagholizadeh, F.; Jabalameli, F.; Taherikalani, M.; Emaneini, M. Determination of extended spectrum beta-lactamases, metallo-beta-lactamases and AmpC-beta-lactamases among carbapenem resistant Pseudomonas aeruginosa isolated from burn patients. Burns 2014, 40, 1556–1561. [Google Scholar] [CrossRef]
- M’Zali, F.H.; Gascoyne-Binzi, D.M.; Heritage, J.; Hawkey, P.M. Detection of mutations conferring extended-spectrum activity on SHV beta-lactamases using polymerase chain reaction single strand conformational polymorphism (PCR-SSCP). J. Antimicrob. Chemother. 1996, 37, 797–802. [Google Scholar] [CrossRef]
- Celenza, G.; Pellegrini, C.; Caccamo, M.; Segatore, B.; Amicosante, G.; Perilli, M. Spread of bla(CTX-M-type) and bla(PER-2) beta-lactamase genes in clinical isolates from Bolivian hospitals. J. Antimicrob. Chemother. 2006, 57, 975–978. [Google Scholar] [CrossRef]
- Ait Tayeb, L.; Ageron, E.; Grimont, F.; Grimont, P.A. Molecular phylogeny of the genus Pseudomonas based on rpoB sequences and application for the identification of isolates. Microbiol. Res. 2005, 156, 763–773. [Google Scholar] [CrossRef]
- Al Tarazi, Y.H. Bacteriological and Pathological Study on Pneumonia in the One-Humped Camel (Camelus dromedarius) in Jordan. Revue d’Elevage et de Médecine Vétérinaire des Pays Tropicaux 2001, 54, 93–97. [Google Scholar]
- Nahed, S.S.; Tarek, R.A.E.; Amani, A.H.; Iman, A.E.E.; Asmaa, A.D. Clinicopathological and Bacteriological Studies on Pneumonia in Camel (Camelus dromedarius). J. Vet. Adv. 2016, 6, 1228–1236. [Google Scholar]
- Gebru, M.; Tefera, G.; Dawo, F.; Tessema, T.S. Aerobic bacteriological studies on the respiratory tracts of apparently healthy and pneumonic camels (Camelus dromedaries) in selected districts of Afar Region, Ethiopia. Trop. Anim. Health Prod. 2018, 50, 603–611. [Google Scholar] [CrossRef]
- Elhariri, M.; Hamza, D.; Elhelw, R.; Dorgham, S.M. Extended-spectrum beta-lactamase-producing Pseudomonas aeruginosa in camel in Egypt: Potential human hazard. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 21. [Google Scholar] [CrossRef]
- Vukić Lušić, D.; Maestro, N.; Cenov, A.; Lušić, D.; Smolčić, K.; Tolić, S.; Maestro, D.; Kapetanović, D.; Marinac-Pupavac, S.; Tomić Linšak, D.; et al. Occurrence of P. aeruginosa in Water Intended for Human Consumption and in Swimming Pool Water. Environments 2021, 8, 132. [Google Scholar] [CrossRef]
- Morales-Espinosa, R.; Delgado, G.; Espinosa-Camacho, F.; Flores-Alanis, A.; Rodriguez, C.; Mendez, J.L.; Gonzalez-Pedraza, A.; Cravioto, A. Pseudomonas aeruginosa strains isolated from animal with high virulence genes content and highly sensitive to antimicrobials. J. Glob. Antimicrob. Resist 2024, 37, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wu, Q.; Zhang, J.; Guo, W.; Gu, Q.; Wu, H.; Wang, J.; Lei, T.; Xue, L.; Zhang, Y.; et al. Prevalence, Virulence, Antimicrobial Resistance, and Molecular Characterization of Pseudomonas aeruginosa Isolates From Drinking Water in China. Front. Microbiol. 2020, 11, 544653. [Google Scholar] [CrossRef]
- De Jonghe, V.; Coorevits, A.; Van Hoorde, K.; Messens, W.; Van Landschoot, A.; De Vos, P.; Heyndrickx, M. Influence of storage conditions on the growth of Pseudomonas species in refrigerated raw milk. Appl. Environ. Microbiol. 2011, 77, 460–470. [Google Scholar] [CrossRef]
- Kishilova, S.A.; Rozhkova, I.V.; Fomenko, O.Y. Public health and sanitation issues related to the bacterium Pseudomonas aeruginosa. Food Syst. 2025, 8, 49–57. [Google Scholar] [CrossRef]
- Al-Kadmy, I.M.S.; Abid, S.A.; Aziz, S.N.; Al-Kadmy, Z.; Suhail, A.; Al-Jubori, S.S.; Naji, E.N.; Alhomaidi, E.; Yahia, R.; Algammal, A.M.; et al. The secrets of environmental Pseudomonas aeruginosa in slaughterhouses: Antibiogram profile, virulence, and antibiotic resistance genes. Folia Microbiol. 2024, 69, 805–822. [Google Scholar] [CrossRef]
- Tuon, F.F.; Dantas, L.R.; Suss, P.H.; Tasca Ribeiro, V.S. Pathogenesis of the Pseudomonas aeruginosa Biofilm: A Review. Pathogens 2022, 11, 300. [Google Scholar] [CrossRef]
- Li, X.; Gu, N.; Huang, T.Y.; Zhong, F.; Peng, G. Pseudomonas aeruginosa: A typical biofilm forming pathogen and an emerging but underestimated pathogen in food processing. Front. Microbiol. 2023, 13, 1114199. [Google Scholar] [CrossRef]
- Gupta, N.; Chauhan, K.; Singh, G.; Chaudhary, S.; Rathore, J.S. Decoding antibiotic resistance in Pseudomonas aeruginosa: Embracing innovative therapies beyond conventional antibiotics. Microbe 2025, 6, 100233. [Google Scholar] [CrossRef]
- Edward, E.A.; El Shehawy, M.R.; Abouelfetouh, A.; Aboulmagd, E. Phenotypic and molecular characterization of extended spectrum- and metallo- beta lactamase producing Pseudomonas aeruginosa clinical isolates from Egypt. Infection 2024, 52, 2399–2414. [Google Scholar] [CrossRef]
- Umadevi, S.; Joseph, N.M.; Kumari, K.; Easow, J.M.; Kumar, S.; Stephen, S.; Srirangaraj, S.; Raj, S. Detection of extended spectrum beta lactamases, ampc beta lactamases and metallobetalactamases in clinical isolates of ceftazidime resistant Pseudomonas aeruginosa. Braz. J. Microbiol. 2011, 42, 1284–1288. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, S.A.; Cruz-Córdova, A.; Rodea, G.E.; Cázares-Domínguez, V.; Escalona, G.; Arellano-Galindo, J.; Hernández-Castro, R.; Reyes-López, A.; Xicohtencatl-Cortes, J. Phenotypic characterization of multidrug-resistant Pseudomonas aeruginosa strains isolated from pediatric patients associated to biofilm formation. Microbiol. Res. 2015, 172, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Farhan, S.M.; Ibrahim, R.A.; Mahran, K.M.; Hetta, H.F.; Abd El-Baky, R.M. Antimicrobial resistance pattern and molecular genetic distribution of metallo-β-lactamases producing Pseudomonas aeruginosa isolated from hospitals in Minia, Egypt. Infect. Drug Resist 2019, 12, 2125–2133. [Google Scholar] [CrossRef]
- Castanheira, M.; Doyle, T.B.; Smith, C.J.; Mendes, R.E.; Sader, H.S. Combination of MexAB-OprM overexpression and mutations in efflux regulators, PBPs and chaperone proteins is responsible for ceftazidime/avibactam resistance in Pseudomonas aeruginosa clinical isolates from US hospitals. J. Antimicrob. Chemother. 2019, 74, 2588–2595. [Google Scholar] [CrossRef]
- Castanheira, M.; Mills, J.C.; Farrell, D.J.; Jones, R.N. Mutation-driven β-lactam resistance mechanisms among contemporary ceftazidime-nonsusceptible Pseudomonas aeruginosa isolates from U.S. hospitals. Antimicrob. Agents Chemother. 2014, 58, 6844–6850. [Google Scholar] [CrossRef]
- Ramsay, K.A.; Rehman, A.; Wardell, S.T.; Martin, L.W.; Bell, S.C.; Patrick, W.M.; Winstanley, C.; Lamont, I.L. Ceftazidime resistance in Pseudomonas aeruginosa is multigenic and complex. PLoS ONE 2023, 18, e0285856. [Google Scholar] [CrossRef]
- El-Ghareeb, W.R.; Mulla, Z.S.; Meligy, A.M.A.; Darwish, W.S.; Edris, A.M. Antibiotic Residue Levels In Camel, Cattle and Sheep Tissues Using LC-MS/MS Method. J. Anim. Plant Sci. 2019, 29, 2019. [Google Scholar]
- Mohmed, S.A.; Barre, A.; Mohamud, A.H.; Gaciye, M.M.; Hirsi, F.I. Antibiotics use and Resistance Knowledge, Attitude, and Practice Towards Dairy Camel Farmers in Banadir Region, Somalia. J. Vet. Res. Clin. Care 2024, 9, 20–27. [Google Scholar] [CrossRef]
- Brown, K.; Mugoh, M.; Call, D.R.; Omulo, S. Antibiotic residues and antibiotic-resistant bacteria detected in milk marketed for human consumption in Kibera, Nairobi. PLoS ONE 2020, 15, e0233413. [Google Scholar] [CrossRef]
- Yang, A.F.; Huang, V.; Samaroo-Campbell, J.; Augenbraun, M. Multi-drug resistant Pseudomonas aeruginosa: A 2019-2020 single center retrospective case control study. Infect. Prev. Prac. 2023, 5, 100296. [Google Scholar] [CrossRef] [PubMed]
- Al-Orphaly, M.; Hadi, H.A.; Eltayeb, F.K.; Al-Hail, H.; Samuel, B.G.; Sultan, A.A.; Skariah, S. Epidemiology of Multidrug-Resistant Pseudomonas aeruginosa in the Middle East and North Africa Region. mSphere 2021, 6, e00202-21. [Google Scholar] [CrossRef]
- Aloush, V.; Navon-Venezia, S.; Seigman-Igra, Y.; Cabili, S.; Carmeli, Y. Multidrug-resistant Pseudomonas aeruginosa: Risk factors and clinical impact. Antimicrob. Agents Chemother. 2006, 50, 43–48. [Google Scholar] [CrossRef]
- Płókarz, D.; Rypuła, K. A One Health Perspective on the Human-Pets Pseudomonas aeruginosa Transmission. Appl. Microbiol. Open Access 2022, 8, 227. [Google Scholar]
- Haenni, M.; Hocquet, D.; Ponsin, C.; Cholley, P.; Guyeux, C.; Madec, J.Y.; Bertrand, X. Population structure and antimicrobial susceptibility of Pseudomonas aeruginosa from animal infections in France. BMC Vet. Res. 2015, 11, 9. [Google Scholar] [CrossRef]
- Samir, A.; Abdel-Moein, K.A.; Zaher, H.M. Molecular Detection of Toxigenic Clostridioides difficile among Diarrheic Dogs and Cats: A Mounting Public Health Concern. Vet. Sci. 2021, 8, 88. [Google Scholar] [CrossRef]
- Shaker, A.A.; Samir, A.; Zaher, H.M.; Abdel-Moein, K.A. Emergence of Virulent Extensively Drug-Resistant Vancomycin-Resistant Enterococci Among Diarrheic Pet Animals: A Possible Public Health Threat on the Move. Vector Borne Zoonotic Dis. 2024, 24, 600–606. [Google Scholar] [CrossRef]
- Abdelaziz, A.A.; Kamer, A.M.A.; Al-Monofy, K.B.; Al-Madboly, L.A. Pseudomonas aeruginosa’s greenish-blue pigment pyocyanin: Its production and biological activities. Microb. Cell Fact. 2023, 22, 110. [Google Scholar] [CrossRef] [PubMed]
- Jayaseelan, S.; Ramaswamy, D.; Dharmaraj, S. Pyocyanin: Production, applications, challenges and new insights. World J. Microbiol. Biotechnol. 2014, 30, 1159–1168. [Google Scholar] [CrossRef]
- Khalefa, H.S.; Arafa, A.A.; Hamza, D.; Abd El-Razik, K.A.; Ahmed, Z. Emerging biofilm formation and disinfectant susceptibility of ESBL-producing Klebsiella pneumoniae. Sci. Rep. 2025, 15, 1599. [Google Scholar] [CrossRef] [PubMed]
- Samir, A.; Mosallam, T.; Aboul-Ella, H.; Ali, A.; Samir, O.; Hegab, M.; Erian, M.; Youssef, F.; Zaher, H. Zoonotic relevance of multidrug-resistant bacteria in parrots with respiratory illness. Vet. Res. Commun. 2025, 49, 194. [Google Scholar] [CrossRef]
- Falodun, O.I.; Ikusika, E.O.; Musa, I.B.; Oyelade, A.A. Extended-spectrum beta-lactamase genes distribution in Pseudomonas species from livestock samples in Ibadan, Nigeria. Gene Rep. 2020, 21, 100950. [Google Scholar] [CrossRef]
- Nordmann, P.; Ronco, E.; Naas, T.; Duport, C.; Michel-Briand, Y.; Labia, R. Characterization of a novel extended-spectrum beta-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1993, 37, 962–969. [Google Scholar] [CrossRef]
- Polse, R.F.; Khalid, H.M.; Mero, W.M.S. Distribution of blaOXA-10, blaPER-1, and blaSHV genes in ESBL-producing Pseudomonas aeruginosa strains isolated from burn patients. Sci. Rep. 2023, 13, 18402. [Google Scholar] [CrossRef]
- Azzam, A.; Khaled, H.; Samer, D.; Nageeb, W.M. Prevalence and molecular characterization of ESBL-producing Enterobacteriaceae in Egypt: A systematic review and meta-analysis of hospital and community-acquired infections. Antimicrob. Resist. Infect. Control 2024, 13, 145. [Google Scholar] [CrossRef]
- Walther-Rasmussen, J.; Høiby, N. Cefotaximases (CTX-M-ases), an expanding family of extended-spectrum beta-lactamases. Can. J. Microbiol. 2004, 50, 137–165. [Google Scholar] [CrossRef]
- Franco, M.M.J.; Ribeiro, M.G.; Pavan, F.R.; Miyata, M.; Heinemann, M.B.; de Souza Filho, A.F.; Cardoso, R.F.; de Almeida, A.L.; Sakate, R.I.; Paes, A.C. Genotyping and rifampicin and isoniazid resistance in Mycobacterium bovis strains isolated from the lymph nodes of slaughtered cattle. Tuberculosis 2017, 104, 30–37. [Google Scholar] [CrossRef]
- Khan, A.U.; Shell, W.S.; Melzer, F.; Sayour, A.E.; Ramadan, E.S.; Elschner, M.C.; Moawad, A.A.; Roesler, U.; Neubauer, H.; El-Adawy, H. Identification, Genotyping and Antimicrobial Susceptibility Testing of Brucella spp. Isolated from Livestock in Egypt. Microorganisms 2019, 7, 603. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Feßler, A.T.; Jiang, N.; Fan, R.; Wang, Y.; Wu, C.; Shen, J.; Schwarz, S. Molecular basis of rifampicin resistance in multiresistant porcine livestock-associated MRSA. J. Antimicrob. Chemother. 2016, 71, 3313–3315. [Google Scholar] [CrossRef]
- Stevenson, S.M.; McAllister, T.A.; Selinger, L.B.; Yanke, L.J.; Olson, M.E.; Morck, D.W.; Read, R.R. Transfer of a rifampicin-resistant Escherichia coli strain among feedlot cattle. J. Appl. Microbiol. 2003, 95, 398–410. [Google Scholar] [CrossRef][Green Version]
- Jatsenko, T.; Tover, A.; Tegova, R.; Kivisaar, M. Molecular characterization of Rif(r) mutations in Pseudomonas aeruginosa and Pseudomonas putida. Mutat. Res. 2010, 683, 106–114. [Google Scholar] [CrossRef]
- Armengol, E.; Kragh, K.N.; Tolker-Nielsen, T.; Sierra, J.M.; Higazy, D.; Ciofu, O.; Viñas, M.; Høiby, N. Colistin Enhances Rifampicin’s Antimicrobial Action in Colistin-Resistant Pseudomonas aeruginosa Biofilms. Antimicrob. Agents Chemother. 2023, 67, e0164122. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, J.S.; Laulund Siebert, A.S.; Ciofu, O.; Høiby, N.; Moser, C.; Franzyk, H. Synergistic combinations of novel polymyxins and rifampicin with improved eradication of colistin-resistant Pseudomonas aeruginosa biofilms. Biofilm 2024, 8, 100224. [Google Scholar] [CrossRef]
- Mogashoa, T.; Loubser, J.; Choga, O.T.; Ngom, J.T.; Choga, W.T.; Mbulawa, M.B.; Molefi, T.; Stephen, O.; Makhondo, T.; Seru, K.; et al. Whole genomic analysis uncovers high genetic diversity of rifampicin-resistant Mycobacterium tuberculosis strains in Botswana. Front. Microbiol. 2025, 16, 1535160. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.A.; Korzheva, N.; Mustaev, A.; Murakami, K.; Nair, S.; Goldfarb, A.; Darst, S.A. Structural mechanism for rifampicin inhibition of bacterial rna polymerase. Cell 2001, 104, 901–912. [Google Scholar] [CrossRef]
- Severinov, K.; Soushko, M.; Goldfarb, A.; Nikiforov, V. RifR mutations in the beginning of the Escherichia coli rpoB gene. Mol. Gen. Genet. 1994, 244, 120–126. [Google Scholar] [CrossRef]
- Vogler, A.J.; Busch, J.D.; Percy-Fine, S.; Tipton-Hunton, C.; Smith, K.L.; Keim, P. Molecular analysis of rifampin resistance in Bacillus anthracis and Bacillus cereus. Antimicrob. Agents Chemother. 2002, 46, 511–513. [Google Scholar] [CrossRef]
- Aubry-Damon, H.; Galimand, M.; Gerbaud, G.; Courvalin, P. rpoB mutation conferring rifampin resistance in Streptococcus pyogenes. Antimicrob. Agents Chemother. 2002, 46, 1571–1573. [Google Scholar] [CrossRef] [PubMed]
- Heep, M.; Odenbreit, S.; Beck, D.; Decker, J.; Prohaska, E.; Rieger, U.; Lehn, N. Mutations at four distinct regions of the rpoB gene can reduce the susceptibility of Helicobacter pylori to rifamycins. Antimicrob. Agents Chemother. 2000, 44, 1713–1715. [Google Scholar] [CrossRef] [PubMed]
- Hellmark, B.; Söderquist, B.; Unemo, M. Simultaneous species identification and detection of rifampicin resistance in staphylococci by sequencing of the rpoB gene. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 183–190. [Google Scholar] [CrossRef] [PubMed]
| No. of Examined Camels | No. of Positive Camels | Total | |
|---|---|---|---|
| 10 camels | 6 camels | 16 camels (16%) | |
| 100 | 10 nasal swabs (10 isolates) | 6 nasal swabs (6 isolates) | 16 isolates |
| 6 tissue samples (6 isolates) | 6 isolates |
| Sample Type | Isolate No. | CAZ | CPM | CTR | CTX | AT | AK | GEN | RIF | CIP | SXT | MRP | IPM | MDR Pattern |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nasal swab | 1 | R | R | R | R | R | S | S | R | S | R | R | R | MDR |
| Nasal swab | 2 | R | R | R | R | R | S | S | R | S | R | S | S | MDR |
| Nasal swab | 3 | R | R | R | R | R | S | S | R | S | R | S | S | MDR |
| Nasal swab | 4 | R | R | R | R | S | S | S | R | S | R | S | S | MDR |
| Nasal swab | 5 | R | R | R | R | S | S | S | R | S | R | S | S | MDR |
| Nasal swab | 6 | R | R | R | R | S | S | S | R | S | S | S | S | - |
| Nasal swab | 7 | R | R | R | R | S | S | S | S | S | S | S | S | - |
| Nasal swab | 8 | R | R | R | R | S | S | S | S | S | S | S | S | - |
| Nasal swab | 9 | R | R | R | R | R | S | R | R | S | R | R | S | MDR |
| Nasal swab | 10 | R | R | R | R | R | S | S | R | S | R | S | S | MDR |
| Nasal swab | 11 | R | R | R | R | R | S | S | R | S | R | S | S | MDR |
| Nasal swab | 12 | R | R | R | R | R | S | S | R | S | R | S | S | MDR |
| Nasal swab | 13 | R | R | R | R | S | S | S | R | S | R | S | S | MDR |
| Nasal swab | 14 | R | R | R | R | S | S | S | S | S | S | S | S | - |
| Nasal swab | 15 | R | R | R | R | S | S | S | S | S | S | S | S | - |
| Nasal swab | 16 | R | R | R | R | S | S | S | S | R | S | S | S | - |
| Tissue sample | 17 | R | R | R | R | S | S | S | R | S | S | S | S | - |
| Tissue sample | 18 | R | S | R | R | R | S | S | R | S | S | S | S | MDR |
| Tissue sample | 19 | R | R | R | R | S | S | S | S | S | S | S | S | - |
| Tissue sample | 20 | R | R | R | R | S | S | S | R | S | R | S | S | MDR |
| Tissue sample | 21 | R | R | R | R | S | S | S | R | S | S | S | S | - |
| Tissue sample | 22 | R | R | R | R | S | S | S | R | S | S | S | S | - |
| Total (22) | 22/22 (100%) | 21/22 (95.5%) | 22/22 (100%) | 22/22 (100%) | 8/22 (36.4%) | 0/22 (0%) | 1/22 (4.5%) | 16/22 (72.7%) | 1/22 (4.5%) | 11/22 (50%) | 2/22 (9.1%) | 1/22 (4.5%) | 12/22 (54.5%) |
| Isolate No. | Beta-Lactamase-Encoding Genes | rpoB Gene | |||
|---|---|---|---|---|---|
| blaPER | blaCTX-M | blaTEM | blaSHV | ||
| 1 | + | + | + | + | |
| 2 | + | + | + | ||
| 3 | + | + | + | ||
| 4 | + | + | + | + | |
| 5 | + | + | + | ||
| 6 | |||||
| 7 | + | ||||
| 8 | |||||
| 9 | + | + | + | ||
| 10 | + | + | |||
| 11 | + | + | + | ||
| 12 | + | + | + | ||
| 13 | + | ||||
| 14 | + | ||||
| 15 | |||||
| 16 | + | ||||
| 17 | + | + | |||
| 18 | + | + | + | ||
| 19 | + | ||||
| 20 | + | + | |||
| 21 | |||||
| 22 | + | ||||
| Total | 9/22 (40.9%) | 8/22 (36.4%) | 7/22 (31.8%) | 6/22 (27.3%) | 11/22 (50%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamza, D.; Zaher, H.M. Carriage of Rifampicin- and Multidrug-Resistant Pseudomonas aeruginosa in Apparently Healthy Camels: A View Through a Zoonosis Lens. Microbiol. Res. 2025, 16, 107. https://doi.org/10.3390/microbiolres16060107
Hamza D, Zaher HM. Carriage of Rifampicin- and Multidrug-Resistant Pseudomonas aeruginosa in Apparently Healthy Camels: A View Through a Zoonosis Lens. Microbiology Research. 2025; 16(6):107. https://doi.org/10.3390/microbiolres16060107
Chicago/Turabian StyleHamza, Dalia, and Hala M. Zaher. 2025. "Carriage of Rifampicin- and Multidrug-Resistant Pseudomonas aeruginosa in Apparently Healthy Camels: A View Through a Zoonosis Lens" Microbiology Research 16, no. 6: 107. https://doi.org/10.3390/microbiolres16060107
APA StyleHamza, D., & Zaher, H. M. (2025). Carriage of Rifampicin- and Multidrug-Resistant Pseudomonas aeruginosa in Apparently Healthy Camels: A View Through a Zoonosis Lens. Microbiology Research, 16(6), 107. https://doi.org/10.3390/microbiolres16060107






