Dual Detection of Pathogenic tdh and trh Genes of Vibrio parahaemolyticus in Oysters Using Multienzyme Isothermal Rapid Amplification (MIRA) Combined with Lateral-Flow Dipstick (LFD) Assay
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and DNA Extraction
2.2. Primers and Probes
2.3. Multienzyme Isothermal Rapid Amplification (MIRA) and Lateral-Flow Dipstick (LFD)
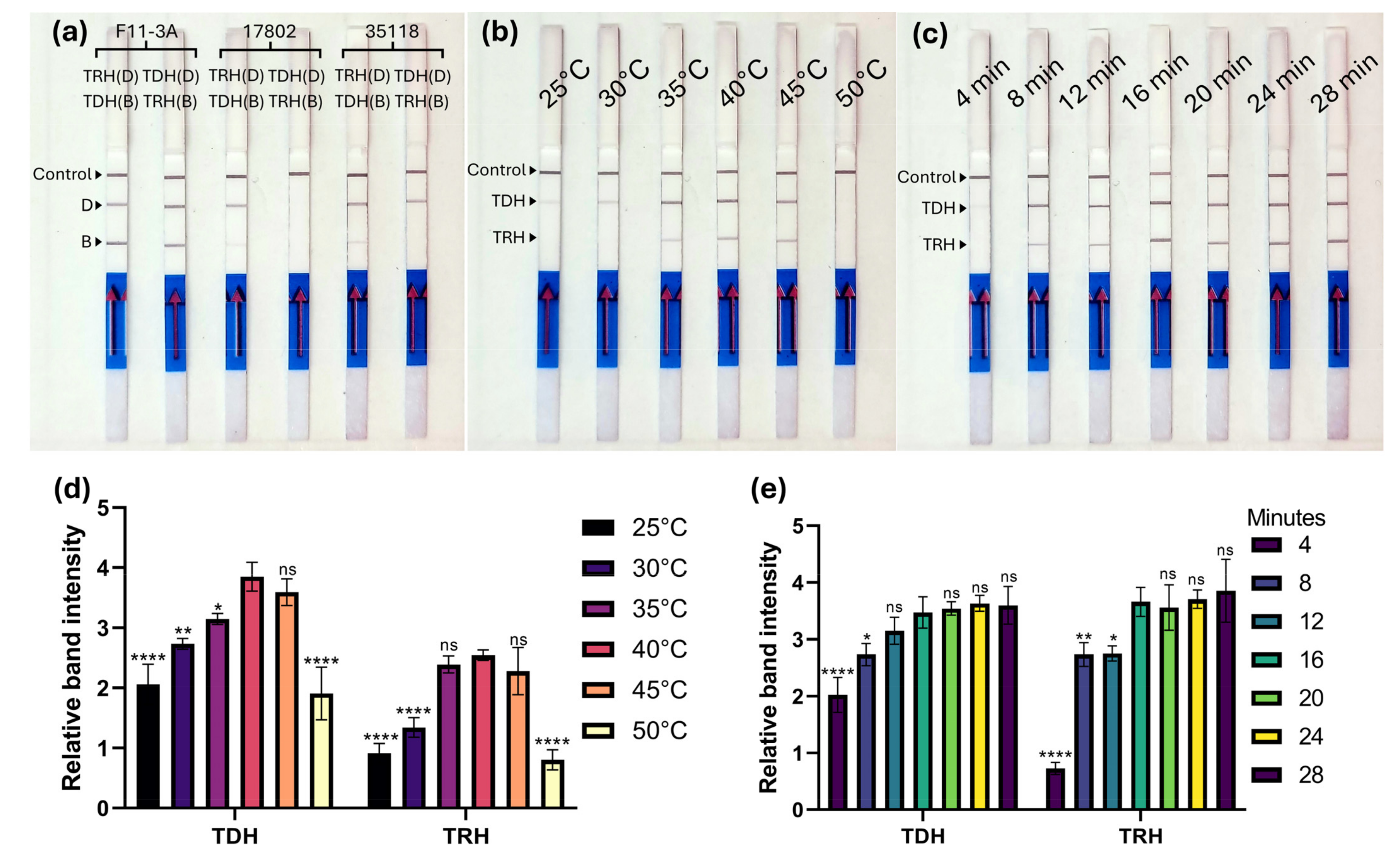
2.4. Optimization of MIRA-LFD Assay
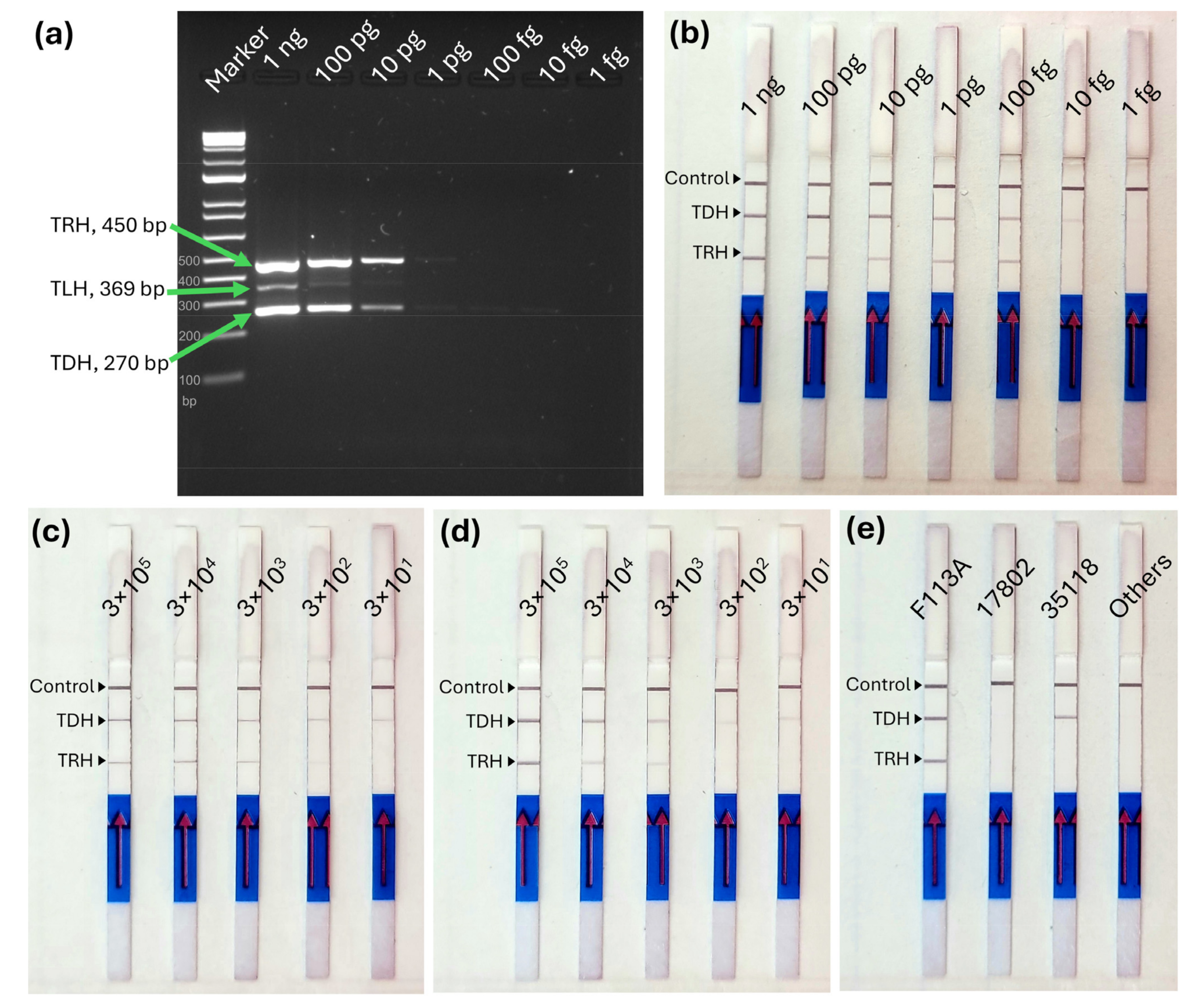
2.5. Sensitivity and Specificity of MIRA-LFD Assay
2.6. Multiplex PCR
2.7. Statistical Analysis
3. Results and Discussion
3.1. Validation of Primers and Probes
3.2. Optimization of MIRA-LFD
3.3. Sensitivity and Specificity of MIRA-LFD Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, R.; Zhong, Y.; Gu, X.; Yuan, J.; Saeed, A.F.; Wang, S. The pathogenesis, detection, and prevention of Vibrio parahaemolyticus. Front. Microbiol. 2015, 6, 144. [Google Scholar] [CrossRef] [PubMed]
- Baker-Austin, C.; Trinanes, J.; Gonzalez-Escalona, N.; Martinez-Urtaza, J. Non-cholera vibrios: The microbial barometer of climate change. Trends Microbiol. 2017, 25, 76–84. [Google Scholar] [CrossRef] [PubMed]
- DePaola, A.; Nordstrom, J.L.; Bowers, J.C.; Wells, J.G.; Cook, D.W. Seasonal abundance of total and pathogenic Vibrio parahaemolyticus in Alabama oysters. Appl. Environ. Microbiol. 2003, 69, 1521–1526. [Google Scholar] [CrossRef] [PubMed]
- Froelich, B.A.; Noble, R.T. Vibrio bacteria in raw oysters: Managing risks to human health. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150209. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Urtaza, J.; Baker-Austin, C.; Jones, J.L.; Newton, A.E.; Gonzalez-Aviles, G.D.; DePaola, A. Spread of Pacific northwest Vibrio parahaemolyticus strain. N. Engl. J. Med. 2013, 369, 1573–1574. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.E.; Garrett, N.; Stroika, S.G.; Halpin, J.L.; Turnsek, M.; Mody, R.K. Notes from the field: Increase in Vibrio parahaemolyticus infections associated with consumption of atlantic coast shellfish-2013. MMWR Morb. Mortal Wkly. Rep. 2014, 63, 335–336. [Google Scholar]
- DePaola, A. Managing Vibrio Risk in Oysters. Food Prot. Trends 2019, 39, 338–347. [Google Scholar]
- Gutierrez West, C.K.; Klein, S.L.; Lovell, C.R. High frequency of virulence factor genes tdh, trh, and tlh in Vibrio parahaemolyticus strains isolated from a pristine estuary. Appl. Environ. Microbiol. 2013, 79, 2247–2252. [Google Scholar] [CrossRef]
- Li, L.; Meng, H.; Gu, D.; Li, Y.; Jia, M. Molecular mechanisms of Vibrio parahaemolyticus pathogenesis. Microbiol. Res. 2019, 222, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tong, J.; Wu, Q.; Liu, J.; Yuan, M.; Tian, C.; Xu, H.; Malakar, P.K.; Pan, Y.; Zhao, Y. Natural inhibitors targeting the localization of lipoprotein system in Vibrio parahaemolyticus. Int. J. Mol. Sci. 2022, 23, 14352. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Ono, T.; Rokuda, M.; Jang, M.H.; Okada, K.; Iida, T.; Honda, T. Functional characterization of two type III secretion systems of Vibrio parahaemolyticus. Infect. Immun. 2004, 72, 6659–6665. [Google Scholar] [CrossRef] [PubMed]
- Shirai, H.; Ito, H.; Hirayama, T.; Nakamoto, Y.; Nakabayashi, N.; Kumagai, K.; Takeda, Y.; Nishibuchi, M. Molecular epidemiologic evidence for association of thermostable direct hemolysin (TDH) and TDH-related hemolysin of Vibrio parahaemolyticus with gastroenteritis. Infect. Immun. 1990, 58, 3568–3573. [Google Scholar] [CrossRef]
- Broberg, C.A.; Calder, T.J.; Orth, K. Vibrio parahaemolyticus cell biology and pathogenicity determinants. Microbes Infect. 2011, 13, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Iida, T. The pathogenicity of Vibrio parahaemolyticus and the role of the thermostable direct haemolysin and related haemolysins. Rev. Res. Med. Microbiol. 1993, 4, 106–113. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Jiang, T.; Bao, Y.; Zhou, X. Insufficiency of the Kanagawa hemolytic test for detecting pathogenic Vibrio parahaemolyticus in Shanghai, China. Diagn. Microbiol. Infect. Dis. 2011, 69, 7–11. [Google Scholar]
- Vieira, R.H.; Costa, R.A.; Menezes, F.G.; Silva, G.C.; Theophilo, G.N.; Rodrigues, D.P.; Maggioni, R. Kanagawa-negative, tdh-and trh-positive Vibrio parahaemolyticus isolated from fresh oysters marketed in Fortaleza, Brazil. Curr. Microbiol. 2011, 63, 126–130. [Google Scholar] [CrossRef]
- Thongjun, J.; Mittraparp-Arthorn, P.; Yingkajorn, M.; Kongreung, J.; Nishibuchi, M.; Vuddhakul, V. The trend of Vibrio parahaemolyticus infections in Southern Thailand from 2006 to 2010. Trop. Med. Health 2013, 41, 151–156. [Google Scholar] [CrossRef]
- Ohnishi, K.; Nakahira, K.; Unzai, S.; Mayanagi, K.; Hashimoto, H.; Shiraki, K.; Honda, T.; Yanagihara, I. Relationship between heat-induced fibrillogenicity and hemolytic activity of thermostable direct hemolysin and a related hemolysin of Vibrio parahaemolyticus. FEMS Microbiol. Lett. 2011, 318, 10–17. [Google Scholar] [CrossRef]
- Bej, A.K.; Patterson, D.P.; Brasher, C.W.; Vickery, M.C.; Jones, D.D.; Kaysner, C.A. Detection of total and hemolysin-producing Vibrio parahaemolyticus in shellfish using multiplex PCR amplification of tl, tdh and trh. J. Microbiol. Methods 1999, 36, 215–225. [Google Scholar] [CrossRef]
- Kaysner, C.A.; DePaola, A.; Jones, J. Bacteriological Analytical Manual Chapter 9: Vibrio; Food and Drug Administration: Silver Spring, MD, USA, 2004. Available online: https://www.fda.gov/food/laboratory-methods-food/bam-chapter-9-vibrio (accessed on 15 May 2024).
- Nordstrom, J.L.; Vickery, M.C.; Blackstone, G.M.; Murray, S.L.; DePaola, A. Development of a multiplex real-time PCR assay with an internal amplification control for the detection of total and pathogenic Vibrio parahaemolyticus bacteria in oysters. Appl. Environ. Microbiol. 2007, 73, 5840–5847. [Google Scholar] [CrossRef]
- Park, S.B.; Zhang, Y. Development of multienzyme isothermal rapid amplification (MIRA) combined with lateral-flow dipstick (LFD) assay to detect species-specific tlh and pathogenic trh and tdh genes of Vibrio parahaemolyticus. Pathogens 2024, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, L.; Yang, Y.; Li, J.; Luan, X.; Gong, S.; Ma, Y.; Gu, W.; Du, J.; Meng, Q. Development and application of the MIRA and MIRA-LFD detection methods of Spiroplasma eriocheiris. J. Invertebr. Pathol. 2023, 201, 108017. [Google Scholar] [CrossRef]
- Ji, C.; Feng, Y.; Sun, R.; Gu, Q.; Zhang, Y.; Ma, J.; Pan, Z.; Yao, H. Development of a multienzyme isothermal rapid amplification and lateral flow dipstick combination assay for bovine coronavirus detection. Front. Vet. Sci. 2022, 9, 1059934. [Google Scholar] [CrossRef] [PubMed]
- Glover, W.A. Laboratory Method for Vibrio parahaemolyticus (V.p.) Enumeration and detection through MPN and real-time PCR. In Proceedings of the Interstate Shellfish Sanitation Conference (ISSC), Columbia, SC, USA, 11 January 2016. [Google Scholar]
- Rizvi, A.V.; Bej, A.K. Multiplexed real-time PCR amplification of tlh, tdh and trh genes in Vibrio parahaemolyticus and its rapid detection in shellfish and Gulf of Mexico water. Antonie Van Leeuwenhoek 2010, 98, 279–290. [Google Scholar] [CrossRef]
- Park, S.B.; Zhang, Y. Innovative multiplex PCR assay for detection of tlh, trh, and tdh genes in Vibrio parahaemolyticus with reference to the US FDA’s Bacteriological Analytical Manual (BAM). Pathogens 2024, 13, 774. [Google Scholar] [CrossRef]
- Nordstrom, J.; Kaysner, C.; Blackstone, G.; Vickery, M.; Bowers, J.; DePaola, A. Effect of intertidal exposure on Vibrio parahaemolyticus levels in Pacific Northwest oysters. J. Food Prot. 2004, 67, 2178–2182. [Google Scholar] [CrossRef] [PubMed]
- National Shellfish Sanitation Program (NSSP)—Guide for the Control of Molluscan Shellfish 2023 Revision. Available online: https://www.fda.gov/food/federal-state-local-tribal-and-territorial-cooperative-human-food-programs/national-shellfish-sanitation-program-nssp (accessed on 19 May 2024).
- Daher, R.K.; Stewart, G.; Boissinot, M.; Bergeron, M.G. Recombinase polymerase amplification for diagnostic applications. Clin. Chem. 2016, 62, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Crannell, Z.A.; Rohrman, B.; Richards-Kortum, R. Equipment-free incubation of recombinase polymerase amplification reactions using body heat. PLoS ONE 2014, 9, e112146. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, J.L. Development of a multiplex real-time recombinase polymerase amplification (RPA) assay for rapid quantitative detection of Campylobacter coli and jejuni from eggs and chicken products. Food Control 2017, 73, 1247–1255. [Google Scholar] [CrossRef]
- Larrea-Sarmiento, A.; Stack, J.P.; Alvarez, A.M.; Arif, M. Multiplex recombinase polymerase amplification assay developed using unique genomic regions for rapid on-site detection of genus Clavibacter and C. nebraskensis. Sci. Rep. 2021, 11, 12017. [Google Scholar] [CrossRef]
- Liu, H.B.; Du, X.J.; Zang, Y.X.; Li, P.; Wang, S. SERS-based lateral flow strip biosensor for simultaneous detection of Listeria monocytogenes and Salmonella enterica serotype enteritidis. J. Agric. Food Chem. 2017, 65, 10290–10299. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Li, J.; Chen, K.; Yu, X.; Sun, C.; Zhang, M. Multiplex recombinase polymerase amplification assay for the simultaneous detection of three foodborne pathogens in seafood. Foods 2020, 9, 278. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Liao, C.; Liang, L.; Yi, X.; Zhou, Z.; Wei, G. Recent advances in recombinase polymerase amplification: Principle, advantages, disadvantages and applications. Front. Cell. Infect. Microbiol. 2022, 12, 1744. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H. Monoplex and multiplex immunoassays: Approval, advancements, and alternatives. Comp. Clin. Pathol. 2022, 31, 333–345. [Google Scholar] [CrossRef]
- Ali, M.E.; Razzak, M.A.; Hamid, S.B.A. Multiplex PCR in species authentication: Probability and prospects—A review. Food Anal. Methods 2014, 7, 1933–1949. [Google Scholar] [CrossRef]
- Becherer, L.; Borst, N.; Bakheit, M.; Frischmann, S.; Zengerle, R.; von Stetten, F. Loop-mediated isothermal amplification (LAMP)–review and classification of methods for sequence-specific detection. Anal. Methods 2020, 12, 717–746. [Google Scholar] [CrossRef]
- Kreitmann, L.; Miglietta, L.; Xu, K.; Malpartida-Cardenas, K.; D’Souza, G.; Kaforou, M.; Brengel-Pesce, K.; Drazek, L.; Holmes, A.; Rodriguez-Manzano, J. Next-generation molecular diagnostics: Leveraging digital technologies to enhance multiplexing in real-time PCR. TrAC Trends Anal. Chem. 2023, 160, 116963. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.W.; He, J.W.; Guo, S.L.; Li, J. Development and evaluation of a rapid and sensitive multienzyme isothermal rapid amplification with a lateral flow dipstick assay for detection of Acinetobacter baumannii in spiked blood specimens. Front. Cell. Infect. Microbiol. 2022, 12, 1010201. [Google Scholar] [CrossRef]
- Drake, S.L.; DePaola, A.; Jaykus, L.A. An overview of Vibrio vulnificus and Vibrio parahaemolyticus. Compr. Rev. Food Sci. Food Saf. 2007, 6, 120–144. [Google Scholar] [CrossRef]
- Saetang, J.; Sukkapat, P.; Palamae, S.; Singh, P.; Senathipathi, D.N.; Buatong, J.; Benjakul, S. Multiplex PCR-Lateral Flow Dipstick Method for Detection of Thermostable Direct Hemolysin (TDH) Producing V. parahaemolyticus. Biosensors 2023, 13, 698. [Google Scholar] [CrossRef]
- Heng, P.; Liu, J.; Song, Z.; Wu, C.; Yu, X.; He, Y. Rapid detection of Staphylococcus aureus using a novel multienzyme isothermal rapid amplification technique. Front. Microbiol. 2022, 13, 1027785. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Yao, J.; Yuan, S.; Liu, H.; Wei, N.; Zhang, J.; Shan, W. Development of a lateral flow recombinase polymerase amplification assay for rapid and visual detection of Cryptococcus neoformans/C. gattii in cerebral spinal fluid. BMC Infect. Dis. 2019, 19, 108. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Niu, J.; Sun, M.; Li, Z.; Wang, X.; He, Y.; Qi, J. Rapid and sensitive detection of Streptococcus iniae in Trachinotus ovatus based on multienzyme isothermal rapid amplification. Int. J. Mol. Sci. 2023, 24, 7733. [Google Scholar] [CrossRef]
- Zhu, L.; Gong, F.; Liu, X.; Sun, X.; Yu, Y.; Shu, J.; Pan, Z. Integrating filter paper extraction, isothermal amplification, and lateral flow dipstick methods to detect Streptococcus agalactiae in milk within 15 min. Front. Vet. Sci. 2023, 10, 1100246. [Google Scholar] [CrossRef]
- Zhan, Z.; He, S.; Cui, Y.; Yang, J.; Shi, X. Development of a multiplex recombinase polymerase amplification coupled with lateral flow dipsticks for the simultaneous rapid detection of Salmonella spp., Salmonella typhimurium and Salmonella enteritidis. Food Qual. Saf. 2024, 8, fyad059. [Google Scholar] [CrossRef]
- Moon, Y.J.; Lee, S.Y.; Oh, S.W. A review of isothermal amplification methods and food-origin inhibitors against detecting food-borne pathogens. Foods 2022, 11, 322. [Google Scholar] [CrossRef]
- Hossain, M.T.; Kim, Y.O.; Kong, I.S. Multiplex PCR for the detection and differentiation of Vibrio parahaemolyticus strains using the groEL, tdh and trh genes. Mol. Cell. Probes 2013, 27, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Izumiya, H.; Matsumoto, K.; Yahiro, S.; Lee, J.; Morita, M.; Yamamoto, S.; Arakawa, E.; Ohnishi, M. Multiplex PCR assay for identification of three major pathogenic Vibrio spp., Vibrio cholerae, Vibrio parahaemolyticus, and Vibrio vulnificus. Mol. Cell. Probes 2011, 25, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Nishibuchi, M.; Janda, J.M.; Ezaki, T. The thermostable direct hemolysin gene (tdh) of Vibrio hollisae is dissimilar in prevalence to and phylogenetically distant from the tdh genes of other vibrios implications in the horizontal transfer of the tdh gene. Microbiol. Immunol. 1996, 40, 59–65. [Google Scholar] [CrossRef]
- Wong, H.C.; You, W.Y.; Chen, S.Y. Detection of toxigenic Vibrio cholerae, V. parahaemolyticus and V. vulnificus in oyster by multiplex-PCR with internal amplification control. J. Food Drug Anal. 2012, 20, 11. [Google Scholar] [CrossRef]

| Gene | Name | Sequence (5′-3′) | Amplicon Size (bp) |
|---|---|---|---|
| TDH | VP_TDH_F1 | CTGTGAACATTAATGATAAAGACTATACAA | 145 |
| VP_TDH_Probe | /56-FAM/AGCTTCAACATTCCTATGATTCTGTAGCTA/idSp/CTTTGTTGGTGAAGA/3SpC3/ | ||
| VP_TDH_R1_Dig | Digoxigenin-ATTACCAATATATTACCACTACCACTCTCATA | ||
| VP_TDH_R1_Bio | Biotin-ATTACCAATATATTACCACTACCACTCTCATA | ||
| TRH | VP_TRH_F1 | ACTCTACTTTGCCTTCAGTTTGCTATTGGCTTC | 141 |
| VP_TRH_Probe | /56-FAM/TGAGCTACTATTTGTCGTTAGAAATACAAC/idSp/ATAAAAACTGAATCA/3SpC3/ | ||
| VP_TRH_R1_Bio | Biotin-GAAGTCGTGAAAATAGATTGACCGTGAACGCT | ||
| VP_TRH_R1_Dig | Digoxigenin-GAAGTCGTGAAAATAGATTGACCGTGAACGCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.B.; Chang, S.K.C.; Bi, L.; Cha, Y.; Zhang, Y. Dual Detection of Pathogenic tdh and trh Genes of Vibrio parahaemolyticus in Oysters Using Multienzyme Isothermal Rapid Amplification (MIRA) Combined with Lateral-Flow Dipstick (LFD) Assay. Microbiol. Res. 2025, 16, 87. https://doi.org/10.3390/microbiolres16050087
Park SB, Chang SKC, Bi L, Cha Y, Zhang Y. Dual Detection of Pathogenic tdh and trh Genes of Vibrio parahaemolyticus in Oysters Using Multienzyme Isothermal Rapid Amplification (MIRA) Combined with Lateral-Flow Dipstick (LFD) Assay. Microbiology Research. 2025; 16(5):87. https://doi.org/10.3390/microbiolres16050087
Chicago/Turabian StylePark, Seong Bin, Sam K. C. Chang, Lin Bi, Yunim Cha, and Yan Zhang. 2025. "Dual Detection of Pathogenic tdh and trh Genes of Vibrio parahaemolyticus in Oysters Using Multienzyme Isothermal Rapid Amplification (MIRA) Combined with Lateral-Flow Dipstick (LFD) Assay" Microbiology Research 16, no. 5: 87. https://doi.org/10.3390/microbiolres16050087
APA StylePark, S. B., Chang, S. K. C., Bi, L., Cha, Y., & Zhang, Y. (2025). Dual Detection of Pathogenic tdh and trh Genes of Vibrio parahaemolyticus in Oysters Using Multienzyme Isothermal Rapid Amplification (MIRA) Combined with Lateral-Flow Dipstick (LFD) Assay. Microbiology Research, 16(5), 87. https://doi.org/10.3390/microbiolres16050087







