YKL-40 and Lysosome-Associated Membrane Proteins as Potential Discriminative Biomarkers in Central Nervous System Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients’ Selection
- age > 18 years
- individuals with clinical presentation of acute CNS infection—fever, headache, vomiting, seizures, disturbances of consciousness, signs of meningeal irritation (neck stiffness, Kernig’s and Brudzinski’s signs).
- patients with abnormal CSF findings consistent with acute CNS infections including the presence of CSF pleocytosis, increased protein levels, changes in CSF glucose levels or in the cerebrospinal fluid/serum glucose ratio.
2.2. Control Group
2.3. Laboratory Methods
2.3.1. Microbiological Evaluation
2.3.2. Gene Expression of YKL-40, LAMP-1, LAMP-2
- Isolation of RNA from blood cells—RNA was isolated from WBC using TRIzol (Thermo Fisher Scientific, Waltham, MA, USA, Lot. No. 1559602,) following the manufacturer’s instructions. After total RNA extraction, samples were treated with the TURBO DNA-free kit (Thermo Fisher Scientific, Waltham, MA, USA, Lot. No. AM1907) to remove residual DNA. Extracted RNAs were quantified at 260/280 nm absorbance by NanoDrop Nucleic Acid Quantification (Thermo Fisher Scientific, Waltham, MA, USA).
- Reverse transcription and qPCR—2 µg of total RNA was reverse transcribed by RevertAid H minus First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA, Lot. No. 01189716) according to the manufacturer’s instructions. The resulting cDNA was used to quantify YKL-40, LAMP-1, LAMP-2 expression. The cDNA was then used as a template for amplification in a quantitative PCR reaction by Genaxon GreenMasterMix (2×) (Genaxxon bioscience GmbH, Ulm, Germany, Cat. No. M3023.0500) following the manufacturer’s recommendations. The following specific primers were used for RNA transcripts of: YKL-40 (Fw 5′-CTGCTCCAGTGCTGCTCT-3′, Rev 5′-TACAGAGGAAGCGGTCCAAGG-3′), LAMP-1 (Fw 5′-CTCTAATGTCTGCAGCTCAAGG-3′, Rev 5′-TGTACACAGCGCAGAACAGG-3′), LAMP-2 (Fw5′-ACAACAGTGGATCAGACAGTACG-3′, Rev5′-AGCAGCAAGCATCAGTTCTTC-3′).
2.3.3. Detection of YKL-40, LAMP-1 and LAMP-2 in Plasma and CSF by ELISA
2.4. Statistical Analysis
3. Results
- 1.
- Microbiological results and groups of observation
- 2.
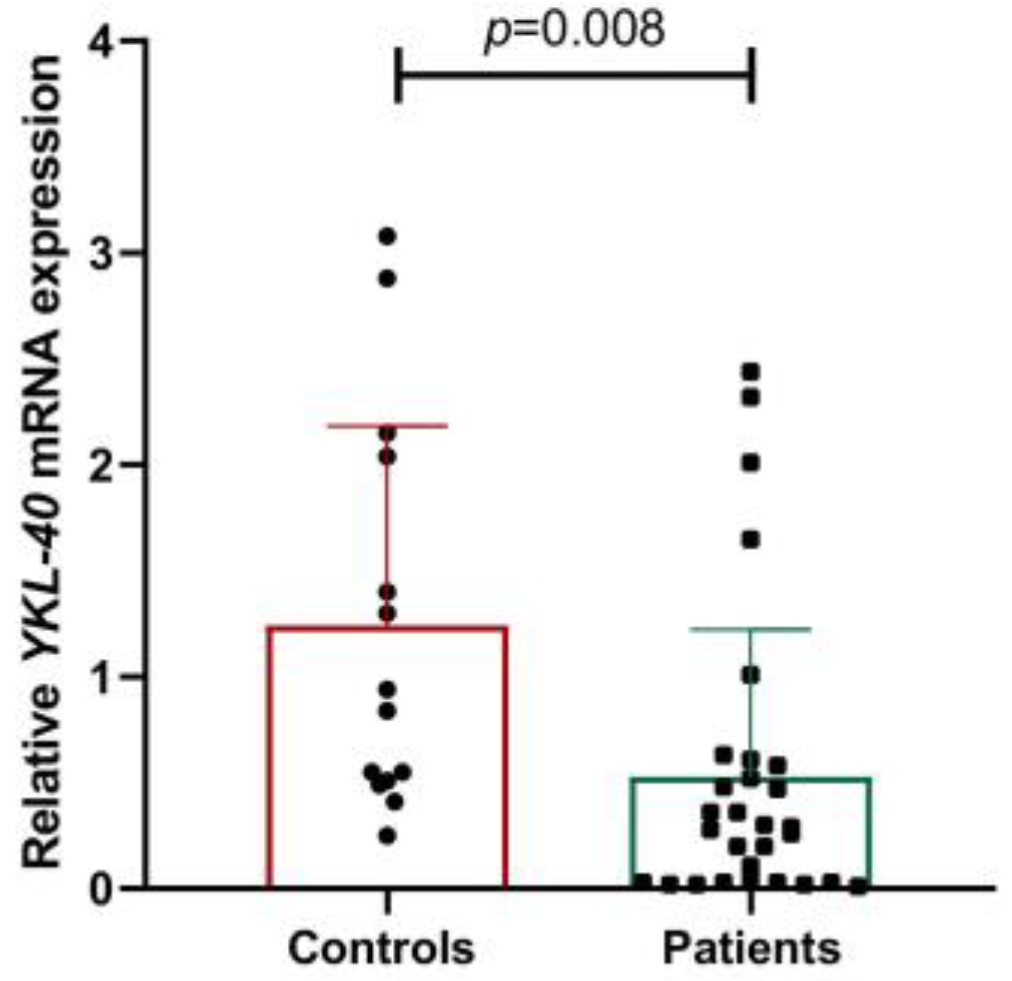
- Gene and protein expression of YKL-40
- 3.
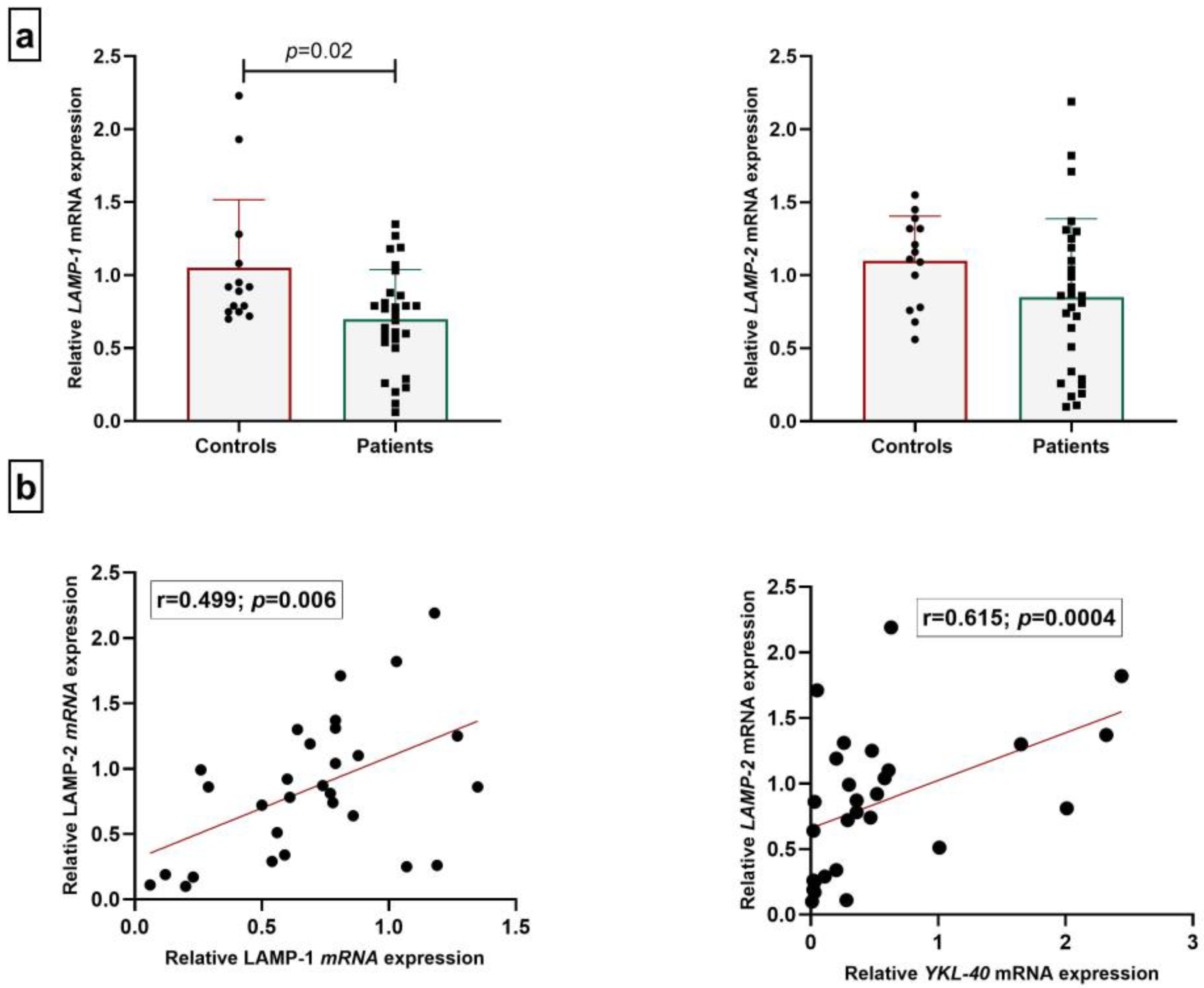
- Gene and protein expression of LAMPs
- 4.
- Discriminative power of YKL-40 and LAMPs
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| WBCs | White blood cells |
| LAMPs | Lysosome-associated membrane proteins |
| ESR | Erythrocyte sedimentation rate |
| CRP | C-reactive protein |
| LP | Lumbar puncture |
| VN | Viral neuroinfections |
| BN | Bacterial neuroinfections |
| LOQ | Limit of quantification |
| HSV-1 | Herpes simplex virus type 1 |
| eIF2D | Translation initiation factor |
| LGI1 | Anti-leucine-rich glioma inactivated 1 |
References
- Melhuish, F. Viral meningitis and encephalitis. Medicine 2021, 49, 675–680. [Google Scholar] [CrossRef]
- Hasbun, R. Progress and Challenges in Bacterial Meningitis: A Review. JAMA 2022, 328, 2147–2154, Erratum in JAMA 2023, 329, 515. [Google Scholar] [CrossRef] [PubMed]
- Scarborough, M.; Thwaites, G.E. The diagnosis and management of acute bacterial meningitis in resource-poor settings. Lancet Neurol. 2008, 7, 637–648. [Google Scholar] [CrossRef]
- Yekani, M.; Memar, M.Y. Immunologic biomarkers for bacterial meningitis. Clin. Chim. Acta 2023, 548, 117470. [Google Scholar] [CrossRef]
- Pedemonte, G.; Mancardi, D.; Giunti, A.; Corcione, F.; Benvenuto, V.; Pistoia, A. Mechanisms of the adaptive immune response inside the central nervous system during inflammatory and autoimmune diseases. Pharmacol. Ther. 2006, 111, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Kalita, J.; Haldar, R.; Misra, U.K. Blood-CSF-barrier permeability in tuberculous meningitis and its association with clinical, MRI and inflammatory cytokines. J. Neuroimmunol. 2022, 372, 577954. [Google Scholar] [CrossRef]
- Bailey, S.L.; Carpentier, P.A.; McMahon, E.J.; Begolka, W.S.; Miller, S.D. Innate and adaptive immune responses of the central nervous system. Crit. Rev. Immunol. 2006, 26, 149–188. [Google Scholar] [CrossRef]
- Thorsdottir, S.; Henriques-Normark, B.; Iovino, F. The Role of Microglia in Bacterial Meningitis: Inflammatory Response, Experimental Models and New Neuroprotective Therapeutic Strategies. Front. Microbiol. 2019, 10, 576. [Google Scholar] [CrossRef]
- Dorsett, M.; Liang, S.Y. Diagnosis and Treatment of Central Nervous System Infections in the Emergency Department. Emerg. Med. Clin. N. Am. 2016, 34, 917–942. [Google Scholar] [CrossRef]
- Kazakova, M.; Sarafian, V. YKL-40 in health and disease: A challenge for joint inflammation. Biomed. Rev. 2013, 24, 49–56. [Google Scholar] [CrossRef]
- Väänänen, T.; Koskinen, A.; Paukkeri, E.L.; Hämäläinen, M.; Moilanen, T.; Moilanen, E.; Vuolteenaho, K. YKL-40 as a novel factor associated with inflammation and catabolic mechanisms in osteoarthritic joints. Mediat. Inflamm. 2014, 1, 215140. [Google Scholar] [CrossRef]
- Çiledağ, A.; Akın, A.P.; Çelik, G.; Demir, N.; Yüksel, C.; Köycü, G.; Gökmen, Ö.D.; Rad, A.Y.; Kaya, A.; Kutlay, H.; et al. High serum YKL-40 level is associated with poor prognosis in patients with lung cancer. Tuberk Toraks 2018, 66, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Shao, R. YKL-40 acts as an angiogenic factor to promote tumor angiogenesis. Front. Physiol. 2013, 4, 122. [Google Scholar] [CrossRef]
- Bonneh-Barkay, D.; Wang, G.; Starkey, A.; Hamilton, R.L.; Wiley, C.A. In vivo CHI3L1 (YKL-40) expression in astrocytes in acute and chronic neurological diseases. J. Neuroinflamm. 2010, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Shahim, P.; Tegner, Y.; Marklund, N.; Höglund, K.; Portelius, E.; Brody, D.L.; Blennow, K.; Zetterberg, H. Astroglial activation and altered amyloid metabolism in human repetitive concussion. Neurology 2017, 88, 1400–1407. [Google Scholar] [CrossRef]
- Kazakova, M.; Pavlov, G.; Dikov, D.; Simitchiev, K.; Dichev, V.; Stefanov, C.; Sarafian, V. Protein YKL-40 in cerebrospinal fluid in traumatic brain injury. Acta Morphol. Anthropol. 2018, 25, 61–66. [Google Scholar]
- Eskelinen, E.L.; Illert, A.L.; Tanaka, Y.; Schwarzmann, G.; Blanz, J.; Von Figura, K.; Saftig, P. Role of LAMP-2 in lysosome biogenesis and autophagy. Mol. Biol. Cell 2002, 13, 2977–3368. [Google Scholar] [CrossRef]
- Eskelinen, E.L. Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol. Asp. Med. 2006, 27, 495–502. [Google Scholar] [CrossRef]
- Jordi, V.; Jordi, B.; Carmen, M. Molecular diagnosis of the central nervous system (CNS) infections. In Enfermedades Infecciosas y Microbiología Clínica; David, N.O., Jose, P.M., Eds.; Elsevier: Madrid, Spain, 2021; pp. 403–410. [Google Scholar]
- Clemens, D.L.; Horwitz, M.A. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J. Exp. Med. 1995, 181, 257–270. [Google Scholar] [CrossRef]
- Kiel, J.A. Autophagy in unicellular eukaryotes. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 819–830. [Google Scholar] [CrossRef]
- Pang, Y.; Wu, L.; Tang, C.; Wang, H.; Wei, Y. Autophagy-Inflammation Interplay During Infection: Balancing Pathogen Clearance and Host Inflammation. Front. Pharmacol. 2022, 13, 832750. [Google Scholar] [CrossRef] [PubMed]
- Orvedahl, A.; Alexander, D.; Tallóczy, Z.; Sun, Q.; Wei, Y.; Zhang, W.; Burns, D.; Leib, D.A.; Levine, B. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe 2007, 1, 23–35. [Google Scholar] [CrossRef]
- Sarafian, V.; Jadot, M.; Foidart, J.M.; Letesson, J.J.; Van den Brûle, F.; Castronovo, V.; Wattiaux, R.; Coninck, S.W. Expression of Lamp-1 and Lamp-2 and their interactions with galectin-3 in human tumor cells. Int. J. Cancer 1998, 75, 105–111. [Google Scholar] [CrossRef]
- Sarafian, V.S.; Koev, I.; Mehterov, N.; Kazakova, M.; Dangalov, K. LAMP-1 gene is overexpressed in high grade glioma. Apmis 2018, 126, 657–662. [Google Scholar] [CrossRef]
- Kristen, H.; Sastre, I.; Aljama, S.; Fuentes, M.; Recuero, M.; Frank-García, A.; Martin, A.; Sanchez-Juan, P.; Lage, C.; Bullido, M.J.; et al. LAMP2 deficiency attenuates the neurodegeneration markers induced by HSV-1 infection. Neurochem. Int. 2021, 146, 105032. [Google Scholar] [CrossRef]
- Deretic, V. Autophagy in inflammation, infection, and immunometabolism. Immunity 2021, 54, 437–453. [Google Scholar] [CrossRef] [PubMed]
- Rubinsztein, D.C.; Mariño, G.; Kroemer, G. Autophagy and aging. Cell 2011, 146, 682–695. [Google Scholar] [CrossRef]
- Kirkpatrick, R.B.; Emery, J.G.; Connor, J.R.; Dodds, R.; Lysko, P.G.; Rosenberg, M. Induction and expression of human cartilage glycoprotein 39 in rheumatoid inflammatory and peripheral blood monocyte-derived macrophages. Exp. Cell Res. 1997, 237, 46–54. [Google Scholar] [CrossRef]
- Muszyński, P.; Groblewska, M.; Kulczyńska-Przybik, A.; Kułakowska, A.; Mroczko, B. YKL-40 as a Potential Biomarker and a Possible Target in Therapeutic Strategies of Alzheimer’s Disease. Curr. Neuropharmacol. 2017, 15, 906–917. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Wang, Y.; Zhao, X.; Whang, S.; Li, L. CHI3L1 in the CSF is a potential biomarker for anti-leucine-rich glioma inactivated 1 encephalitis. Front. Immunol. 2022, 13, 1071219. [Google Scholar] [CrossRef]
- Hermansson, L.; Yilmaz, A.; Axelsson, M.; Blennow, K.; Fuchs, D.; Hagberg, L.; Lycke, J.; Zetterberg, H.; Gisslén, M. Cerebrospinal fluid levels of glial marker YKL-40 strongly associated with axonal injury in HIV infection. J. Neuroinflamm. 2019, 16, 1–9. [Google Scholar] [CrossRef]
- Létuvé, S.; Kozhich, A.; Arouche, N.; Grandsaigne, M.; Reed, J.; Dombret, M.C.; Kiener, P.A.; Aubier, M.; Coyle, A.J.; Pretolani, M. YKL-40 is elevated in patients with chronic obstructive pulmonary disease and activates alveolar macrophages. J. Immunol. 2008, 181, 5167–5173. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Palmero, V.A.; Rubio-Fernández, M.; Antequera, D.; Villarejo-Galende, A.; Molina, J.A.; Ferrer, I.; Bartolome, F.; Carro, E. Increased YKL-40 but Not C-Reactive Protein Levels in Patients with Alzheimer’s Disease. Biomedicines 2021, 9, 1094. [Google Scholar] [CrossRef]
- Gispert, J.D.; Monté, G.C.; Falcon, C.; Tucholka, A.; Rojas, S.; Sánchez-Valle, R.; Antonell, A.; Lladó, A.; Rami, L.; Molinuevo, J.L. CSF YKL-40 and pTau181 are related to different cerebral morphometric patterns in early AD. Neurobiol. Aging 2016, 38, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ng, K.P.; Therriault, J.; Kang, M.S.; Pascoal, T.A.; Rosa-Neto, P.; Gauthier, S. Alzheimer’s Disease Neuroimaging Initiative. Cerebrospinal fluid phosphorylated tau, visinin-like protein-1, and chitinase-3-like protein 1 in mild cognitive impairment and Alzheimer’s disease. Transl. Neurodegener. 2018, 7, 23. [Google Scholar] [CrossRef]
- Dichev, V.; Mehterov, N.; Kazakova, M.; Karalilova, R.; Batalov, A.; Sarafian, V. The lncRNAs/miR-30e/CHI3L1 Axis Is Dysregulated in Systemic Sclerosis. Biomedicines 2022, 10, 496. [Google Scholar] [CrossRef]


| No | Age | M/F | Microbiological Result | CSF WBC [×106/L] | CSF Protein [g/L] | CSF Glucose [mmol/L] | Serum/ CSF Glucose Ratio | CSF Appear-ance | Clinical Course | Study Group Definition |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 55 | M | no etiology | 20.00 | 0.77 | 3.70 | 0.58 | clear | mild | suspected viral |
| 2 | 94 | F | no etiology | 528.00 | 0.66 | 3.20 | 0.60 | clear | mild | suspected viral |
| 3 | 17 | M | no etiology | 2.00 | 1.79 | 7.80 | 0.62 | clear | mild | suspected viral |
| 4 | 68 | M | no etiology | 11.00 | 0.84 | 4.70 | 0.70 | clear | mild | suspected viral |
| 5 | 32 | M | no etiology | 3.00 | 1.40 | 2.70 | 0.56 | clear | mild | suspected viral |
| 6 | 49 | F | no etiology | 176.00 | 1.10 | 2.20 | 0.32 | clear | mild | suspected viral |
| 7 | 32 | M | no etiology | 1.00 | 0.64 | 2.00 | 0.51 | clear | mild | suspected viral |
| 8 | 48 | M | no etiology | 126.00 | 1.30 | 4.00 | 0.72 | clear | mild | suspected viral |
| 9 | 67 | M | no etiology | 2.00 | 1.10 | 4.00 | 0.77 | clear | mild | suspected viral |
| 10 | 63 | M | no etiology | 7.00 | 0.68 | 5.50 | 0.57 | clear | mild | suspected viral |
| 11 | 69 | F | no etiology | 3.00 | 0.32 | 9.40 | 1.20 | clear | mild | suspected viral |
| 12 | 70 | F | no etiology | 6.00 | 0.82 | 6.10 | 0.91 | clear | mild | suspected viral |
| 13 | 79 | M | no etiology | 18.00 | 0.58 | 4.20 | 0.79 | clear | mild | suspected viral |
| 14 | 49 | F | no etiology | 6.00 | 0.45 | 4.00 | 0.65 | clear | mild | suspected viral |
| 15 | 69 | M | VZV | 32.00 | 0.87 | 3.40 | 0.63 | clear | moderate | viral |
| 16 | 62 | M | HSV-1 | 288.00 | 1.77 | 2.90 | 0.51 | clear | moderate | viral |
| 17 | 47 | M | no etiology | 1.00 | 0.83 | 5.20 | 0.85 | clear | mild | suspected viral |
| 18 | 49 | M | no etiology | 21 | 0.3 | 6 | 0.77 | clear | mild | suspected viral |
| 19 | 8 | M | no etiology | 2 | 0.21 | 3.7 | 0.79 | clear | mild | suspected viral |
| 20 | 42 | F | no etiology | 12 | 0.37 | 3 | 0.67 | clear | mild | suspected viral |
| 21 | 80 | F | no etiology | 9 | 0.46 | 6.9 | 0.68 | clear | mild | suspected viral |
| 22 | 49 | F | no etiology | 0 | 0.26 | 4.1 | 0.61 | clear | mild | suspected viral |
| 23 | 35 | M | S. pneumoniae | 1190.00 | 6.53 | 0.00 | 0 | turbid | severe | bacterial |
| 24 | 25 | M | no etiology | 129.00 | 2.40 | 0.60 | 0.09 | turbid | severe | suspected bacterial |
| 25 * | 43 | M | no etiology | 1.00 | 0.37 | 3.00 | 0.42 | turbid | moderate | suspected bacterial |
| 26 | 22 | M | N. meningitidis C | 9386.00 | 7.07 | 0.00 | 0 | turbid | severe | bacterial |
| 27 | 82 | F | L. monocytogenes | 64 | 5.43 | 2 | 0.29 | turbid | severe | bacterial |
| 28 | 34 | F | H. influenzae b | 480 | 1.81 | 2.3 | 0.30 | turbid | severe | bacterial |
| 29 | 70 | M | S. pneumoniae | 2218 | 2.2 | 0.2 | 0.03 | turbid | severe | bacterial |
| Characteristics | Verified Viral or Suspected Viral Neuroinfection (VN) (n = 22) | Verified Bacterial or Suspected Bacterial Neuroinfection (BN) (n = 7) | ||||
|---|---|---|---|---|---|---|
| 25th Percentile | Median | 75th Percentile | 25th Percentile | Median | 75th Percentile | |
| Age at sampling (years) | 46 | 52 | 69 | 25 | 35 | 70 |
| CSF protein [g/L] | 0.4 | 0.7 | 1.1 | 2.1 | 3.9 | 6.7 |
| CSF glucose [mmol/L] | 3.15 | 4.00 | 5.63 | 0.00 | 0.60 | 2.30 |
| CSF WBC [×106/L] | 2.0 | 8.0 | 23.8 | 112.8 | 835.0 | 4010.0 |
| SER WBC [×109/L] | 5.3 | 9.3 | 13.0 | 20.0 | 24.9 | 27.2 |
| SER NEU [×109/L] | 5.8 | 7.8 | 13.2 | 15.9 | 19.6 | 25.0 |
| LY SER [×109/L] | 0.5 | 0.8 | 1.4 | 0.5 | 0.9 | 1.6 |
| SER FIB [g/L] | 3.4 | 4.8 | 6.0 | 4.5 | 6.7 | 7.5 |
| SER CRP [mg/L] | 10.0 | 40.0 | 91.8 | 99.8 | 133.0 | 327.8 |
| ESR [mm/h] | 8.0 | 20.0 | 33.0 | 16.8 | 45.0 | 74.0 |
| YKL-40 SER [ng/mL] | 43 | 119 | 332 | 53 | 110 | 306 |
| YKL-40 CSF [ng/mL] | 110 | 269 | 377 | 76 | 376 | 386 |
| Viral or Potential Viral Neuroinfection | |||||
|---|---|---|---|---|---|
| Patient Numbers (n) | Plasma LAMP-1 | CSF LAMP-1 | Plasma LAMP-2 | CSF LAMP-2 | |
| Concentrations | |||||
| <LOQ ng/mL | 3 | 14 | 4 | 14 | |
| (LOQ–4.99) ng/mL | 15 | 0 | 4 | 3 | |
| (5.00–9.99) ng/mL | 2 | 2 | 7 | 0 | |
| (10.00–19.99) ng/mL | 1 | 0 | 6 | 0 | |
| >20.00 ng/mL | 0 | 2 | 0 | 0 | |
| Bacterial or Potential Bacterial Neuroinfection | |||||
| Patient Numbers (n) | Plasma LAMP-1 | CSF LAMP-1 | Plasma LAMP-2 | CSF LAMP-2 | |
| Concentrations | |||||
| <LOQ ng/mL | 0 | 3 | 1 | 3 | |
| (LOQ–4.99) ng/mL | 5 | 3 | 2 | 3 | |
| (5.00–9.99) ng/mL | 2 | 0 | 2 | 0 | |
| (10.00–19.99) ng/mL | 0 | 0 | 1 | 1 | |
| >20.00 ng/mL | 0 | 1 | 1 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazakova, M.; Kalchev, Y.; Dichev, V.; Argirova, P.; Simitchiev, K.; Murdjeva, M.; Sarafian, V. YKL-40 and Lysosome-Associated Membrane Proteins as Potential Discriminative Biomarkers in Central Nervous System Infections. Microbiol. Res. 2025, 16, 84. https://doi.org/10.3390/microbiolres16040084
Kazakova M, Kalchev Y, Dichev V, Argirova P, Simitchiev K, Murdjeva M, Sarafian V. YKL-40 and Lysosome-Associated Membrane Proteins as Potential Discriminative Biomarkers in Central Nervous System Infections. Microbiology Research. 2025; 16(4):84. https://doi.org/10.3390/microbiolres16040084
Chicago/Turabian StyleKazakova, Maria, Yordan Kalchev, Valentin Dichev, Petya Argirova, Kiril Simitchiev, Mariana Murdjeva, and Victoria Sarafian. 2025. "YKL-40 and Lysosome-Associated Membrane Proteins as Potential Discriminative Biomarkers in Central Nervous System Infections" Microbiology Research 16, no. 4: 84. https://doi.org/10.3390/microbiolres16040084
APA StyleKazakova, M., Kalchev, Y., Dichev, V., Argirova, P., Simitchiev, K., Murdjeva, M., & Sarafian, V. (2025). YKL-40 and Lysosome-Associated Membrane Proteins as Potential Discriminative Biomarkers in Central Nervous System Infections. Microbiology Research, 16(4), 84. https://doi.org/10.3390/microbiolres16040084





