The Potential Role of rpoS and ompR in the Acid Resistance and Desiccation Tolerance of Cronobacter malonaticus Strains
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Genetic Analysis
2.3. Acid Resistance
2.4. Generation of Sub-Lethally Injured C. malonaticus Following Desiccation
2.5. Statistical Analysis
3. Results
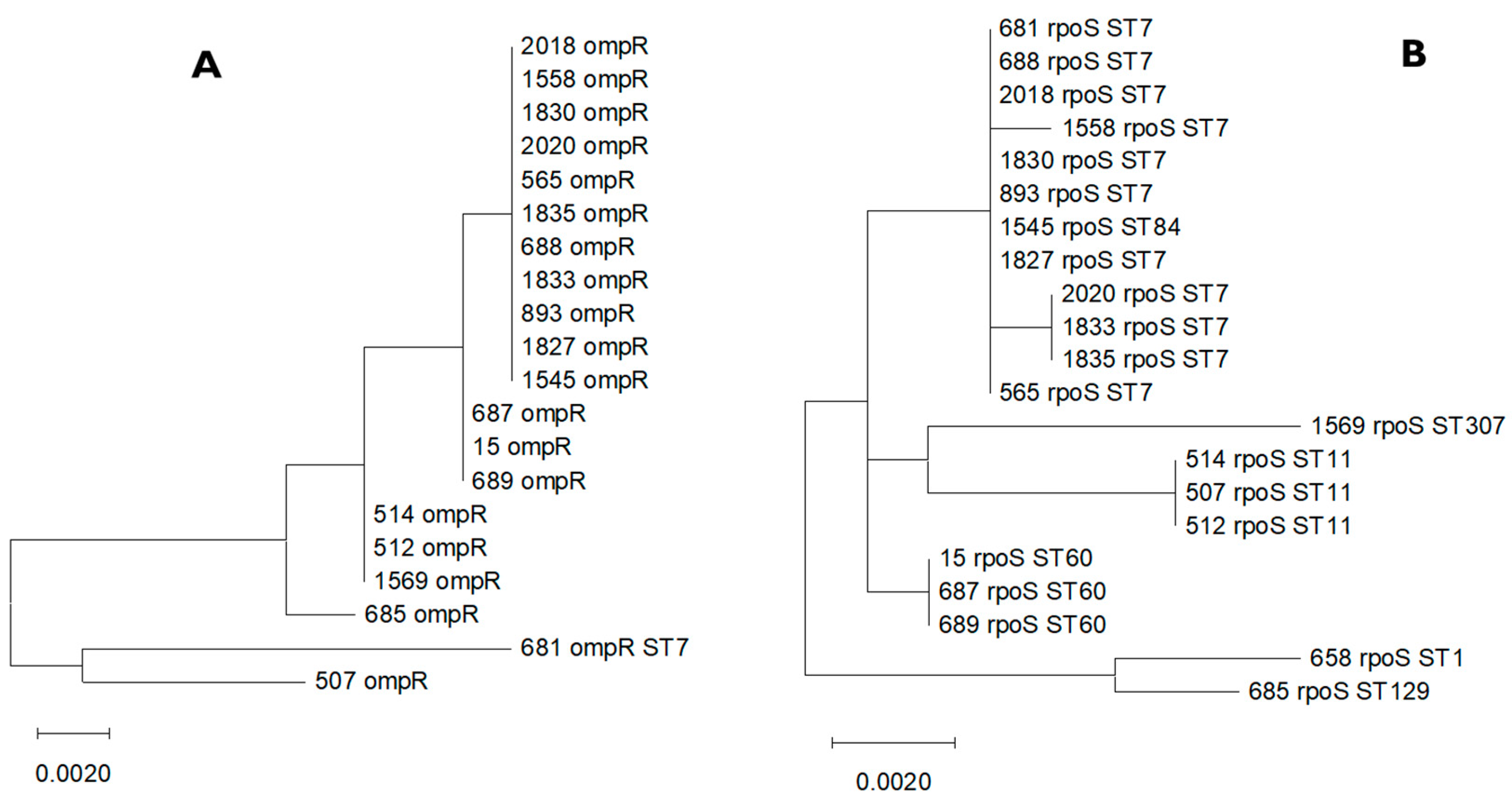
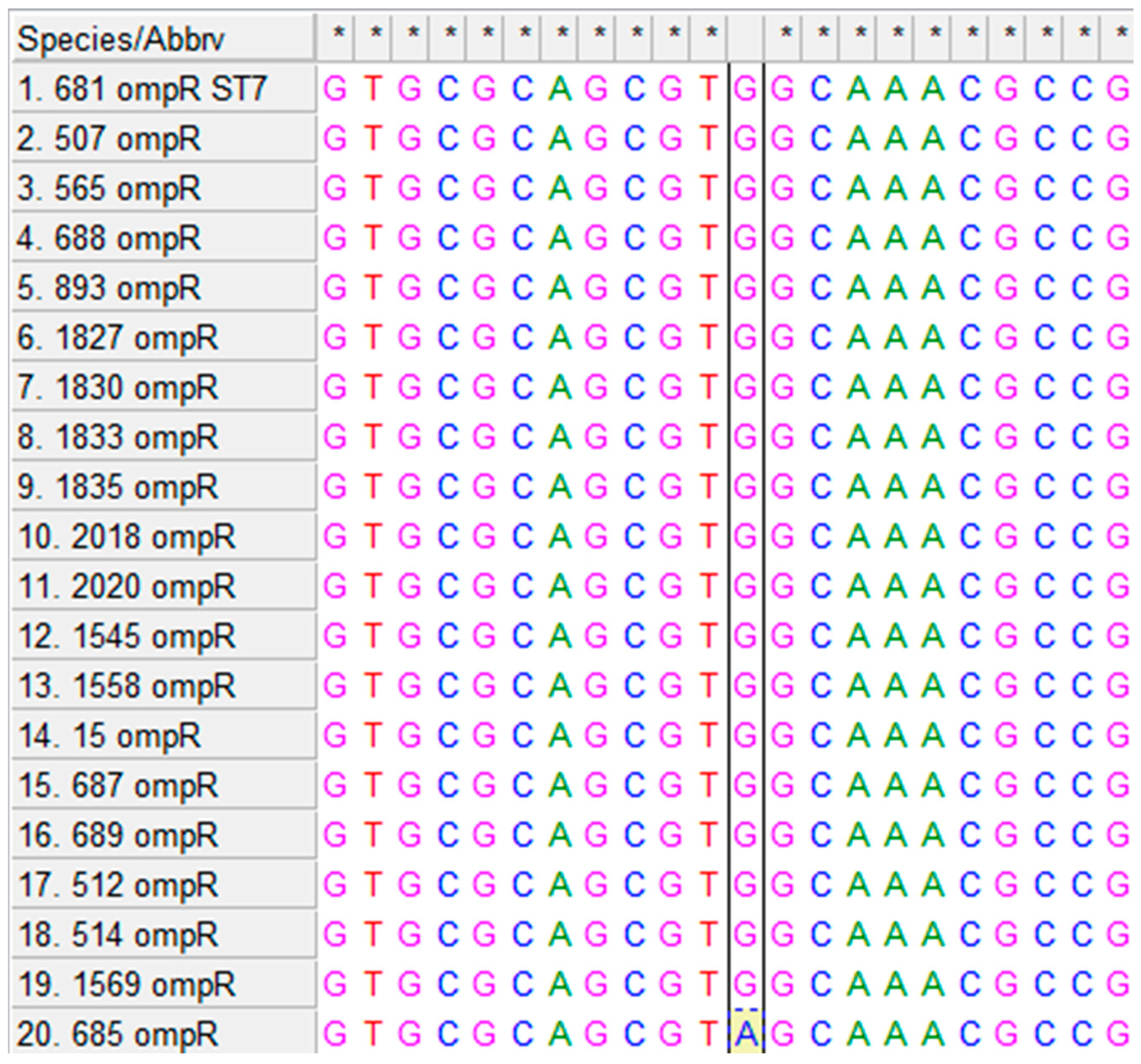
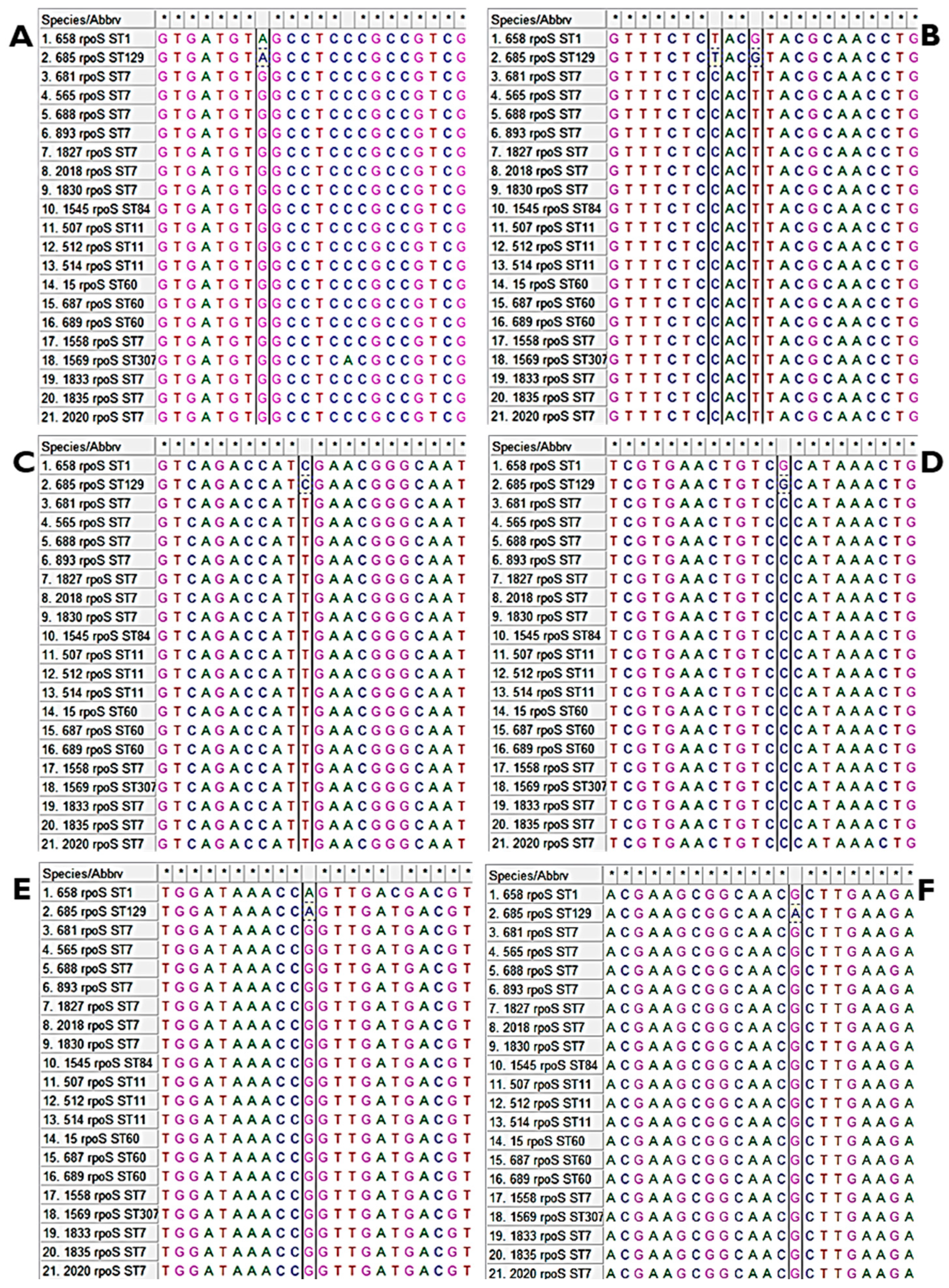
3.1. Multiple Alignments of ompR and rpoS Genes and Their Homologues
3.2. Acid Stress Response in C. malonaticus
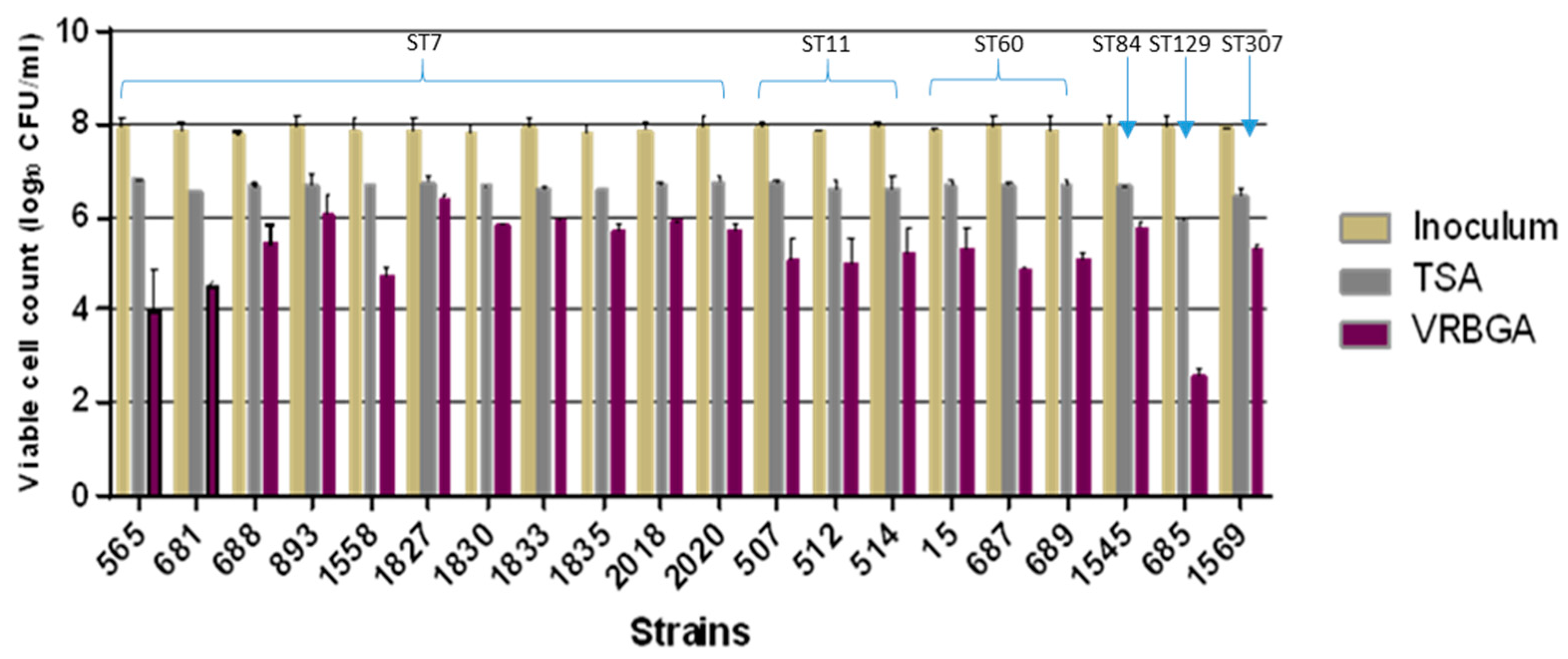
3.3. Sub-Lethal Injury and Desiccation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hariri, S.; Joseph, S.; Forsythe, S. Cronobacter sakazakii ST4 strains and neonatal meningitis, United States. Emerg. Infect. Dis. 2013, 19, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Alsonosi, A.; Hariri, S.; Kajsík, M.; Oriešková, M.; Hanulík, V.; Röderová, M.; Petrželová, J.; Kollárová, H.; Drahovská, H.; Forsythe, S.; et al. The speciation and genotyping of Cronobacter isolates from hospitalized patients. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1979–1988. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, S.J.; Dickins, B.; Jolley, K.A. Cronobacter, the Emergent bacterial pathogen Enterobacter sakazakii comes of age; MLST and whole genome sequence analysis. BMC Genom. 2014, 15, 1121. [Google Scholar] [CrossRef]
- Asato, V.C.; Vilches, V.; Pineda, M.G.; Casanueva, E.; Cané, A.; Moroni, M.; Brengi, S.P.; Pichel, M. First Clinical Isolates of Cronobacter spp. (Enterobacter sakazakii) in Argentina: Characterization and subtyping by pulsed-field gel electrophoresis. Rev. Argent. Microbiol. 2013, 45, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Brandão, M.L.L.; Umeda, N.S.; Carvalho, K.R.; De Filippis, I. Investigação de um Surto Causado por Cronobacter malonaticus em um Hospital Maternidade em Teresina, Piauí: Caracterização e Tipificação por Eletroforese em Gel de Campo Pulsado. Vigil. Sanit. Em Debate 2015, 3, 290. [Google Scholar] [CrossRef]
- Aldubyan, M.A.; Almami, I.S.; Benslimane, F.; Alsonosi, A.; Forsythe, S. Comparative outer membrane protein analysis of high and low-invasive strains of Cronobacter malonaticus. Front. Microbiol. 2017, 8, 2268. [Google Scholar] [CrossRef]
- Alsonosi, A.M.; Holý, O.; Forsythe, S.J. Characterization of the pathogenicity of clinical Cronobacter malonaticus strains based on tissue culture investigations. Antonie Van Leeuwenhoek 2019, 112, 435–450. [Google Scholar] [CrossRef]
- Song, X.; Teng, H.; Chen, L.; Kim, M. Cronobacter species in powdered infant formula and their detection methods. Korean J. Food Sci. Anim. Resour. 2018, 38, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; He, J.; Ma, Y.; Dong, G.; Chang, Y.; Meng, Z.; Jiang, S.; Xie, Q.; Li, S.; Chen, X.; et al. Cronobacter spp. in commercial powdered infant formula collected from nine provinces in China: Prevalence, genotype, biofilm formation, and antibiotic Susceptibility. Front. Microbiol. 2022, 13, 900690. [Google Scholar] [CrossRef]
- Caubilla-Barron, J.; Forsythe, S. Dry stress and survival time of Enterobacter sakazakii and other Enterobacteriaceae. J. Food Prot. 2007, 70, 2111–2117. [Google Scholar] [CrossRef]
- Ibrahim, K.M.; Alsonosi, A.M.; Agena, M.B.; Elgamoudi, B.A.; Forsythe, S.J. Multiplex determination of K-Antigen and Colanic acid capsule variants of Cronobacter sakazakii. Genes 2024, 15, 1282. [Google Scholar] [CrossRef] [PubMed]
- Breeuwer, P.; Lardeau, A.; Peterz, M.; Joosten, H. Desiccation and heat tolerance of Enterobacter sakazakii. J. Appl. Microbiol. 2003, 95, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Wesche, A.M.; Gurtler, J.B.; Marks, B.P.; Ryser, E.T. Stress, Sublethal injury, resuscitation, and virulence of bacterial foodborne pathogens. J. Food Prot. 2009, 72, 1121–1138. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, A.; Petruzzi, L.; Speranza, B.; Campaniello, D.; Ciuffreda, E.; Altieri, C.; Sinigaglia, M.; Corbo, M.R. Viability, sublethal injury, and release of cellular components from Alicyclobacillus acidoterrestris spores and cells after the application of physical treatments, natural extracts, or their components. Front. Nutr. 2021, 8, 700500. [Google Scholar] [CrossRef] [PubMed]
- Feeney, A.; Sleator, R.D. An In Silico Analysis of Osmotolerance in the emerging gastrointestinal pathogen Cronobacter sakazakii. Bioeng. Bugs 2011, 2, 260–270. [Google Scholar] [CrossRef]
- Stasic, A.J.; Wong, A.C.L.; Kaspar, C.W. Osmotic and desiccation tolerance in Escherichia coli O157 requires rpoS (σ38). Curr. Microbiol. 2012, 65, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Ordóñez, A.; Begley, M.; Hill, C. Polymorphisms in rpoS and stress tolerance heterogeneity in natural isolates of Cronobacter sakazakii. Appl. Environ. Microbiol. 2012, 78, 3975–3984. [Google Scholar] [CrossRef] [PubMed]
- Schellhorn, H.E. Function, Evolution, and composition of the RpoS regulon in Escherichia coli. Front. Microbiol. 2020, 11, 560099. [Google Scholar] [CrossRef] [PubMed]
- Handler, S.; Kirkpatrick, C.L. New Layers of regulation of the general stress response sigma factor RpoS. Front. Microbiol. 2024, 15, 1363955. [Google Scholar] [CrossRef]
- Zhan, J.; Qiao, J.; Wang, X. Role of sigma factor RpoS in Cronobacter sakazakii environmental stress tolerance. Bioengineered 2021, 12, 2791–2809. [Google Scholar] [CrossRef]
- Xue, J.; Lv, J.; Liu, L.; Duan, F.; Shi, A.; Ji, X.; Ding, L. Maltodextrin-binding protein as a key factor in Cronobacter sakazakii survival under desiccation stress. Food Res. Int. 2024, 177, 113871. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Ordóñez, A.; Begley, M.; Clifford, T.; Deasy, T.; Collins, B.; Hill, C. Transposon mutagenesis reveals genes involved in osmotic stress and drying in Cronobacter sakazakii. Food Res. Int. 2014, 55, 45–54. [Google Scholar] [CrossRef]
- Johler, S.; Stephan, R.; Hartmann, I.; Kuehner, K.; Lehner, A. Genes involved in yellow pigmentation of Cronobacter sakazakii ES5 and influence of pigmentation on persistence and growth under environmental stress. Appl. Environ. Microbiol. 2009, 76, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Grim, C.; Kotewicz, M.; Power, K.; Gopinath, G.; Franco, A.; Jarvis, K.; Yan, Q.; Jackson, S.; Sathyamoorthy, V.; Hu, L.; et al. Pan-Genome analysis of the emerging foodborne pathogen Cronobacter spp. suggests a species-level bidirectional divergence driven by niche adaptation. BMC Genom. 2013, 14, 366. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Power, K.A.; Cooney, S.; Fox, E.M.; Gopinath, G.; Grim, C.J.; Tall, B.D.; McCusker, M.P.; Fanning, S. Complete genome sequence and phenotype microarray analysis of Cronobacter sakazakii SP291: A Persistent isolate cultured from a powdered infant formula production facility. Front. Microbiol. 2013, 4, 256. [Google Scholar] [CrossRef] [PubMed]
- Carver, T.; Rutherford, K.; Berriman, M.; Rajandream, M.; Barrell, B.; Parkhill, J. ACT: The artemis comparison tool. Bioinformatics 2005, 21, 3422–3423. [Google Scholar] [CrossRef]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C. Open-Access bacterial population genomics: BIGSdb software, the pubmlst.org Website, and their applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef] [PubMed]
- Edelson-Mammel, S.; Porteous, M.; Buchanan, R. Acid resistance of twelve strains of Enterobacter sakazakii, and the impact of habituating the cells to an acidic environment. J. Food Sci. 2006, 71, M201–M207. [Google Scholar] [CrossRef]
- Gardiner, G.; O’Sullivan, E.; Kelly, J.; Auty, M.A.; Fitzgerald, G.F.; Collins, J.; Ross, R.P.; Stanton, C. Comparative survival rates of human-derived probiotic Lactobacillus paracasei and L. salivarius strains during heat treatment and spray drying. Appl. Environ. Microbiol. 2000, 66, 2605–2612. [Google Scholar] [CrossRef]
- Ramos, J.L.; Gallegos, M.; Marqués, S.; Ramos-González, M.I.; Espinosa-Urgel, M.; Segura, A. Responses of gram-negative bacteria to certain environmental stressors. Curr. Opin. Microbiol. 2001, 4, 166–171. [Google Scholar] [CrossRef]
- Lian, W.; Hsiao, H.; Chou, C. Survival of bifidobacteria after spray-drying. Int. J. Food Microbiol. 2002, 74, 79–86. [Google Scholar] [CrossRef]
- Osaili, T.M.; Forsythe, S. Desiccation resistance and persistence of Cronobacter species in infant formula. Int. J. Food Microbiol. 2009, 136, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, E.; Kucerova, E.; Loughlin, M.; Caubilla-Barron, J.; Hilton, A.; Armstrong, R.; Smith, C.; Grant, J.; Shoo, S.; Forsythe, S. Neonatal enteral feeding tubes as loci for colonization by members of the Enterobacteriaceae. BMC Infect. Dis. 2009, 9, 146. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Kenney, L.J. A New role of OmpR in acid and osmotic stress in Salmonella and E. coli. Front. Microbiol. 2018, 9, 2656. [Google Scholar] [CrossRef]
- Hengge-Aronis, R. Recent insights into the general stress response regulatory network in Escherichia coli. PubMed 2002, 4, 341–346. [Google Scholar]
- Meléndez, K.F.; Crenshaw, K.; Barrila, J.; Yang, J.; Gangaraju, S.; Davis, R.R.; Forsyth, R.J.; Ott, C.M.; Kader, R.; Curtiss, R.; et al. Role of RpoS in Regulating Stationary Phase Salmonella Typhimurium Pathogenesis-Related Stress Responses under Physiological Low Fluid Shear Force Conditions. mSphere 2022, 7, e00210-22. [Google Scholar] [CrossRef]
- Pérez-Palacios, P.; Rodríguez-Ochoa, J.L.; Velázquez-Escudero, A.; Rodríguez-Baño, J.; Rodríguez-Martínez, J.M.; Pascual, Á.; Docobo-Pérez, F. Implications of two-component systems EnvZ/OmpR and BaeS/BaeR in in vitro temocillin resistance in Escherichia coli. J. Antimicrob. Chemother. 2024, 79, 641–647. [Google Scholar] [CrossRef] [PubMed]

| Strain No. | ST | Source | Country | Year of Isolation |
|---|---|---|---|---|
| 565 | 7 | Faecal isolate | USA | 1973 |
| 681 | 7 | Breast abscess isolate | USA | 1977 |
| 688 | 7 | Sputum | Czech Republic | 2004 |
| 983 | 7 | Infant formula | Brazil | 2007 |
| 1558 | 7 | Faecal isolate | Czech Republic | Unknown |
| 1827 | 7 | Cannula (blood) | Czech Republic | 2007 |
| 1830 | 7 | Throat swab | Czech Republic | 2007 |
| 1833 | 7 | Faecal isolate | Czech Republic | 2010 |
| 1835 | 7 | Throat swab | Czech Republic | 2012 |
| 2018 | 7 | Sputum | Czech Republic | 2013 |
| 2020 | 7 | Faecal isolate | Czech Republic | 2013 |
| 507 | 11 | Faecal isolate | Czech Republic | 1984 |
| 512 | 11 | Clinical | Czech Republic | 1983 |
| 514 | 11 | Clinical | Czech Republic | 1983 |
| 15 | 60 | Faecal isolate | Czech Republic | 2003 |
| 687 | 60 | Sputum | Czech Republic | 2004 |
| 689 | 60 | Faecal isolate | Czech Republic | 2005 |
| 1545 | 84 | Faecal isolate | Czech Republic | Unknown |
| 685 | 129 | Blood isolate | USA | 1977 |
| 1569 | 307 | Blood isolate | USA | 2011 |
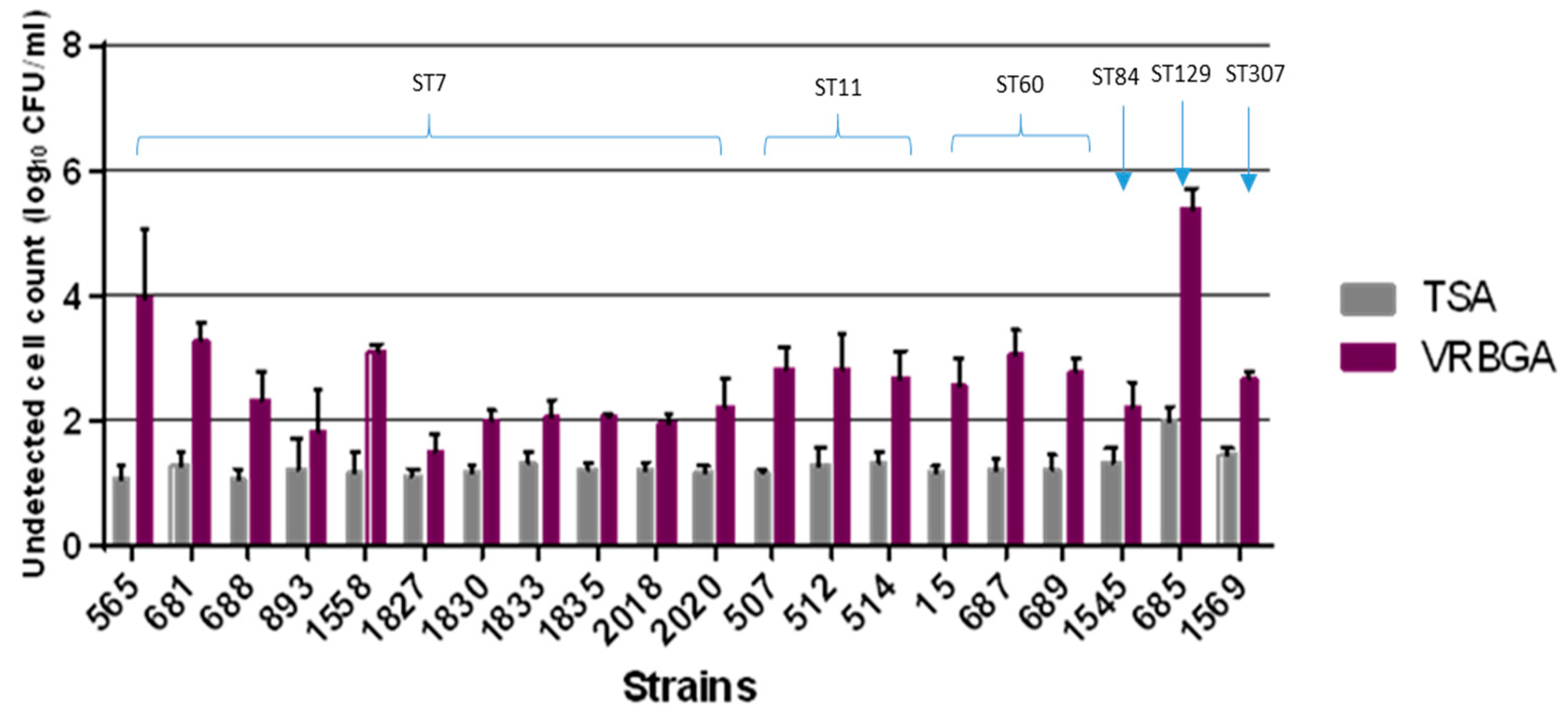
| Isolate ID | ST | Non-Detected Cells on VRBGA in log CFU/mL | Non-Detected Cells on TSA in log CFU/mL | Sub-Lethally Injured Cells in log CFU/mL |
|---|---|---|---|---|
| 565 | 7 | 3.93 | 1.08 | 2.85 |
| 681 | 7 | 3.30 | 1.27 | 2.02 |
| 688 | 7 | 2.32 | 1.06 | 1.26 |
| 893 | 7 | 1.82 | 1.21 | 0.62 |
| 1558 | 7 | 3.10 | 1.18 | 1.92 |
| 1827 | 7 | 1.48 | 1.12 | 0.36 |
| 1830 | 7 | 1.97 | 1.18 | 0.79 |
| 1833 | 7 | 2.03 | 1.30 | 0.73 |
| 1835 | 7 | 2.07 | 1.20 | 0.87 |
| 2018 | 7 | 1.94 | 1.20 | 0.74 |
| 2020 | 7 | 2.22 | 1.19 | 1.02 |
| 507 | 11 | 2.84 | 1.19 | 1.65 |
| 512 | 11 | 2.85 | 1.28 | 1.57 |
| 514 | 11 | 2.69 | 1.32 | 1.37 |
| 1545 | 84 | 2.20 | 1.32 | 0.88 |
| 15 | 60 | 2.55 | 1.17 | 1.38 |
| 687 | 60 | 3.06 | 1.21 | 1.86 |
| 689 | 60 | 2.79 | 1.20 | 1.59 |
| 685 | 129 | 5.38 | 2.01 | 3.37 |
| 1569 | 307 | 2.65 | 1.45 | 1.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsonosi, A.M.; Ibrahim, K.M.; Elgamoudi, B.A.; Agena, M.B.; Forsythe, S.J. The Potential Role of rpoS and ompR in the Acid Resistance and Desiccation Tolerance of Cronobacter malonaticus Strains. Microbiol. Res. 2025, 16, 53. https://doi.org/10.3390/microbiolres16030053
Alsonosi AM, Ibrahim KM, Elgamoudi BA, Agena MB, Forsythe SJ. The Potential Role of rpoS and ompR in the Acid Resistance and Desiccation Tolerance of Cronobacter malonaticus Strains. Microbiology Research. 2025; 16(3):53. https://doi.org/10.3390/microbiolres16030053
Chicago/Turabian StyleAlsonosi, Abdlrhman M., Khaled M. Ibrahim, Bassam A. Elgamoudi, Mahmoud B. Agena, and Stephen J. Forsythe. 2025. "The Potential Role of rpoS and ompR in the Acid Resistance and Desiccation Tolerance of Cronobacter malonaticus Strains" Microbiology Research 16, no. 3: 53. https://doi.org/10.3390/microbiolres16030053
APA StyleAlsonosi, A. M., Ibrahim, K. M., Elgamoudi, B. A., Agena, M. B., & Forsythe, S. J. (2025). The Potential Role of rpoS and ompR in the Acid Resistance and Desiccation Tolerance of Cronobacter malonaticus Strains. Microbiology Research, 16(3), 53. https://doi.org/10.3390/microbiolres16030053






