Hydroethanolic Extract of Punica granatum Inhibits Cryptococcus by Depolarising Mitochondrial Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Hydroalcoholic Extract from the Leaves of P. granatum L.
2.2. Strains
2.3. Inoculum Preparation
2.4. Determination of Minimum Inhibitory Concentration (MIC)
2.5. Determination of Fractional Inhibitory Concentration (FIC)
2.6. Evaluation of Mitochondrial Membrane Potential (ΔΨm) Using Flow Cytometry
2.7. Statistical Analysis
3. Results
3.1. Phytochemical Analysis of P. Granatum Leaf (PgL)
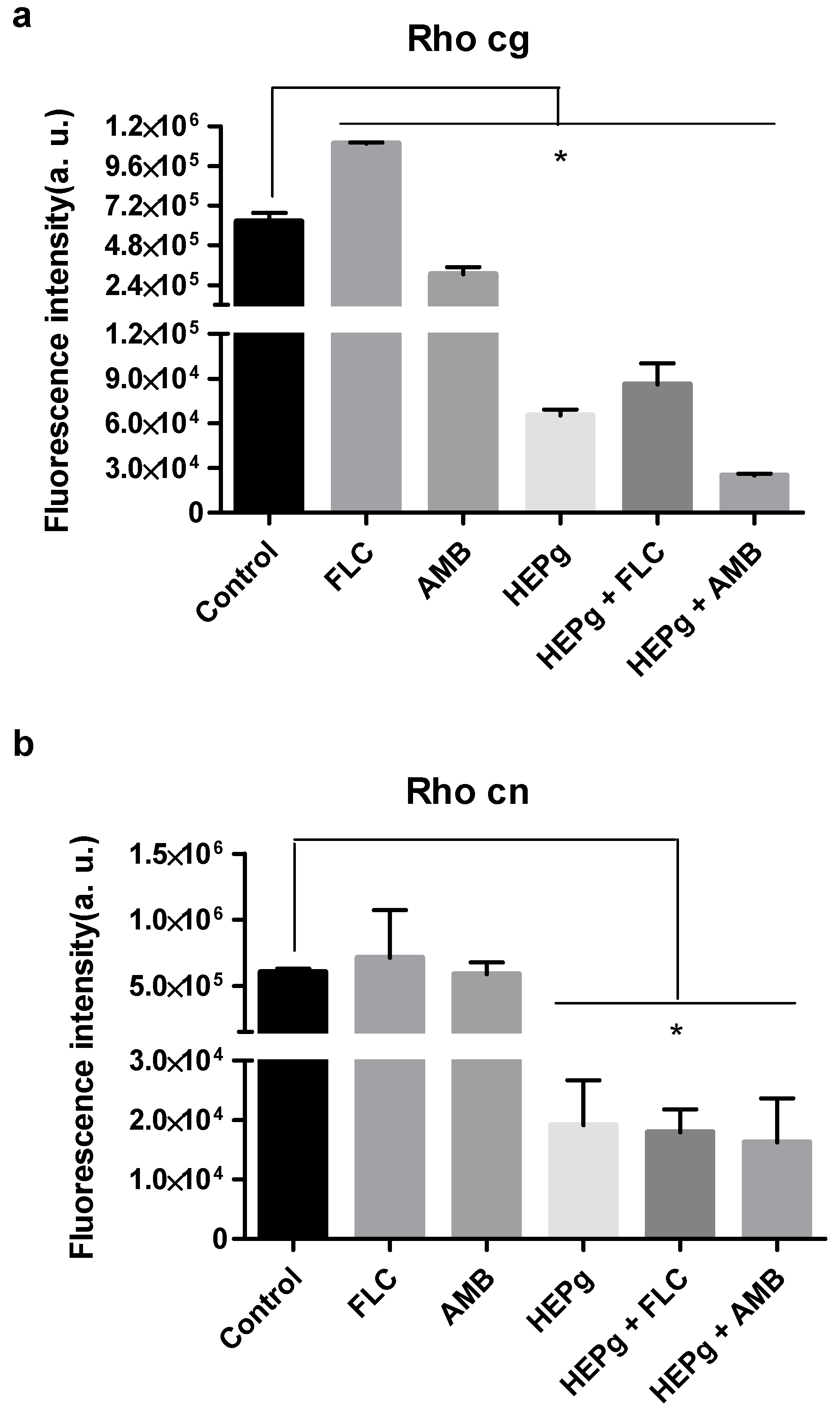
3.2. HEPg Alone and in Combination with Antifungals Showed Activity Against Cryptococcus
3.3. HEPg in Combination with Fluconazole Showed Synergism Against C. gattii
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gushiken, A.C.; Saharia, K.K.; Baddley, J.W. Cryptococcosis. Infect. Dis. Clin. N. Am. 2021, 35, 493–514. [Google Scholar] [CrossRef] [PubMed]
- Armstrong-James, D.; Bicanic, T.; Brown, G.D.; Hoving, J.C.; Meintjes, G.; Nielsen, K.; Working Group from the EMBO Workshop on AIDS-Related Mycoses. AIDS-Related Mycoses: Current Progress in the Field and Future Priorities. Trends Microbiol. 2017, 25, 428–430. [Google Scholar] [CrossRef]
- Iyer, K.R.; Revie, N.M.; Fu, C.; Robbins, N.; Cowen, L.E. Treatment strategies for cryptococcal infection: Challenges, advances and future outlook. Nat. Rev. Microbiol. 2021, 19, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.L.; Arnoldi, A.; Kelly, D.E. Molecular genetic analysis of azole antifungal mode of action. Biochem. Soc. Trans. 1993, 21, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Gray, K.C.; Palacios, D.S.; Dailey, I.; Endo, M.M.; Uno, B.E.; Wilcock, B.C.; Burke, M.D. Amphotericin primarily kills yeast by simply binding ergosterol. Proc. Natl. Acad. Sci. USA 2012, 109, 2234–2239. [Google Scholar] [CrossRef] [PubMed]
- Loyse, A.; Dromer, F.; Day, J.; Lortholary, O.; Harrison, T.S. Flucytosine and cryptococcosis: Time to urgently address the worldwide accessibility of a 50-year-old antifungal. J. Antimicrob. Chemother. 2013, 68, 2435–2444. [Google Scholar] [CrossRef] [PubMed]
- Melhem, M.S.; Leite Júnior, D.P.; Takahashi, J.P.; Macioni, M.B.; Oliveira, L.D.; de Araújo, L.S.; Fava, W.S.; Bonfietti, L.X.; Paniago, A.M.; Venturini, J.; et al. Antifungal Resistance in Cryptococcal Infections. Pathogens 2024, 13, 128. [Google Scholar] [CrossRef] [PubMed]
- Szerencsés, B.; Vörös, M.; Bagi, K.; Háznagy, M.B.; Hunyadi, A.; Vágvölgyi, C.; Pfeiffer, I.; Vágvölgyi, M. Semi-Synthetic Ecdysteroid 6-Oxime Derivatives of 20-Hydroxyecdysone Possess Anti-Cryptococcal Activity. Microbiol. Res. 2022, 13, 985–994. [Google Scholar] [CrossRef]
- Sousa, M.N.; Macedo, A.T.; Ferreira, G.F.; Furtado, H.L.A.; Pinheiro, A.J.M.C.R.; Lima-Neto, L.G.; Fontes, V.C.; Ferreira, R.L.P.S.; Monteiro, C.A.; Falcai, A.; et al. Hydroalcoholic Leaf Extract of Punica granatum, alone and in Combination with Calcium Hydroxide, Is Effective against Mono- and Polymicrobial Biofilms of Enterococcus faecalis and Candida albicans. Antibiotics 2022, 11, 584. [Google Scholar] [CrossRef] [PubMed]
- Villis, P.C.M.; de Macedo, A.T.; Furtado, H.L.A.; Fontenelle, P.H.C.; Gonçalves, I.S.; Mendes, T.L.; Motta, B.L.A.; Marinho, P.L.L.; Pinheiro, A.J.M.C.R.; Lima-Neto, L.G.; et al. A Study of the Disruptive Effect of the Acetate Fraction of Punica granatum Extract on Cryptococcus Biofilms. Front. Microbiol. 2021, 11, 568258. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Mohanram, K.; Kannan, I. Antifungal activity, gas chromatographic-mass spectrometric analysis and in silico study of Punica Granatum peel extracts against fluconazole resistant strains of Candida species. Curr. Pharm. Biotechnol. 2018, 19, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Gouvinhas, I.; Chen, J.; Zhu, Y.; Deng, J.; Xiang, Z.; Oliveira, P.; Xia, C.; Barros, A. Unlocking the therapeutic treasure of pomegranate leaf: A comprehensive review on phytochemical compounds, health benefits, and future prospects. Food Chem. X 2024, 23, 101587. [Google Scholar] [CrossRef] [PubMed]
- Marques, L.C.F.; Pinheiro, A.J.M.C.R.; Araujo, J.G.G.; Oliveira, R.A.G.; Silva, S.N.; Abreu, I.C.; Sousa, E.M.; Fernandes, E.S.; Luchessi, A.D.; Silbiger, V.N.; et al. Anti-Inflammatory Effects of a Pomegranate Leaf Extract in LPS-Induced Peritonitis. Planta Med. 2016, 82, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.J.M.C.R.; Gonçalves, J.S.; Dourado, Á.W.A.; de Sousa, E.M.; Brito, N.M.; Silva, L.K.; Batista, M.C.A.; de Sá, J.C.; Monteiro, C.R.A.V.; Fernandes, E.S.; et al. Punica granatum L. Leaf Extract Attenuates Lung Inflammation in Mice with Acute Lung Injury. J. Immunol. Res. 2018, 2018, 6879183. [Google Scholar] [PubMed]
- Approved Standard M27-A3, 3rd ed.; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008.
- Bidaud, A.L.; Schwarz, P.; Herbreteau, G.; Dannaoui, E. Techniques for the Assessment of In Vitro and In Vivo Antifungal Combinations. J. Fungi 2021, 7, 113. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Brazil, Ministério da Saúde. Portaria MS nº 21, de 28 de Maio de 2021. Torna Pública a Decisão de Incorporar, no Âmbito do Sistema Único de Saúde—SUS, a Flucitosina Para o Tratamento de Pacientes com Meningite Criptocócica e Demais Formas de Neurocriptococose. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/sctie/2021/prt0021_02_06_2021.html (accessed on 17 July 2024).
- Bongomin, F.; Oladele, R.O.; Gago, S.; Moore, C.B.; Richardson, M.D. A systematic review of fluconazole resistance in clinical isolates of Cryptococcus species. Mycoses 2018, 61, 290–297. [Google Scholar] [CrossRef]
- Bertout, S.; Laroche, L.; Roger, F.; Krasteva, D.; Drakulovski, P.; Bellet, V. Fluconazole Resistance and Virulence in In Vitro Induced-Fluconazole Resistant Strains and in Clinical Fluconazole Resistant Strain of Cryptococcus deuterogattii. Pathogens 2023, 12, 758. [Google Scholar] [CrossRef] [PubMed]
- Kommalapati, V.; Rajkumar, N.G.; Karri, R.L.; Ashok, S.; Kumar, A.S.; Srilakshmi, D. Evaluation of antifungal efficacy of albedo extract of Punica granatum on Candida albicans—An in vitro study. J. Oral Maxillofac. Pathol. 2024, 28, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Farhat, G.; Cheng, L.; Al-Dujaili, E.A.S.; Zubko, M. Antimicrobial Potential of Pomegranate and Lemon Extracts Alone or in Combination with Antibiotics against Pathogens. Int. J. Mol. Sci. 2024, 25, 6943. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Chang, Y.L.; Yang, Y.L.; Chen, Y.L. Natural alkaloid tryptanthrin exhibits novel anticryptococcal activity. Med. Mycol. 2020, 21, myaa074. [Google Scholar] [CrossRef]
- Cruz, M.C.; Santos, P.O.; Barbosa, A.M., Jr.; de Mélo, D.L.; Alviano, C.S.; Antoniolli, A.R.; Alviano, D.S.; Trindade, R.C. Antifungal activity of Brazilian medicinal plants involved in popular treatment of mycoses. J. Ethnopharmacol. 2007, 111, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.R.; Gouveia, L.F.; Taylor, E.L.; Resende-Stoianoff, M.A.; Pianetti, G.A.; César, I.C.; Santos, D.A. Dynamic interaction between fluconazole and amphotericin B against Cryptococcus gattii. Antimicrob. Agents Chemother. 2012, 56, 2553–2558. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Khan, A.; Azam, M.; Allemailem, K.S.; Alrumaihi, F.; Almatroudi, A.; AAlhumaydhi, F.; Azam, F.; Khan, S.H.; Zofair, S.F.F.; et al. Liposomal Ellagic Acid Alleviates Cyclophosphamide-Induced Toxicity and Eliminates the Systemic Cryptococcus neoformans Infection in Leukopenic Mice. Pharmaceutics 2021, 13, 882. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.P.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.A.; Gaertner, A.A.E.; Henriquez, S.A.; Fang, D.; Colon-Reyes, R.J.; Brumaghim, J.L.; Kozubowski, L. Fluconazole induces ROS in Cryptococcus neoformans and contributes to DNA damage in vitro. PLoS ONE 2018, 13, e0208471. [Google Scholar] [CrossRef]
- da Silva, A.R.; de Andrade Neto, J.B.; da Silva, C.R.; de Campos, R.S.; Costa Silva, R.A.; Freitas, D.D.; do Nascimento, F.B.; de Andrade, L.N.; Sampaio, L.S.; Grangeiro, T.B.; et al. Berberine Antifungal Activity in Fluconazole-Resistant Pathogenic Yeasts: Action Mechanism Evaluated by Flow Cytometry and Biofilm Growth Inhibition in Candida spp. Antimicrob. Agents Chemother. 2016, 60, 3551–3557. [Google Scholar] [CrossRef] [PubMed]
- Folly, M.L.C.; Ferreira, G.F.; Salvador, M.R.; Sathler, A.A.; da Silva, G.F.; Santos, J.C.B.; Dos Santos, J.R.A.; Nunes Neto, W.R.; Rodrigues, J.F.S.; Fernandes, E.S.; et al. Evaluation of in vitro Antifungal Activity of Xylosma prockia (Turcz.) Turcz. (Salicaceae) Leaves Against Cryptococcus spp. Front. Microbiol. 2020, 10, 3114. [Google Scholar] [CrossRef] [PubMed]
| Minimum Inhibitory Concentration (MIC) | |||
|---|---|---|---|
| Isolates | P. granatum (mg/mL) | Fluconazole (mg/mL) | Amphotericin B (mg/mL) |
| Cryptococcus neoformans 24067 ATCC | 0.06 | 0.016 | 0.0005 |
| Cryptococcus neoformans 62066 | >16 | 0.004 | 0.00025 |
| Cryptococcus gattii 196L | 1 | 0.032 | 0.0125 |
| Cryptococcus gattii 23109 | 0.03 | 0.016 | 0.00025 |
| Isolated | FIC Fluconazole | Interaction | FIC Amphotericin B | Interaction |
|---|---|---|---|---|
| C. n 24067 ATCC | 2.65 | Indifferent | 0.99 | Indifferent |
| C. g 196L | 0.18 | Synergism | 1.16 | Indifferent |
| C. g 23109 1 | 1.10 | Indifferent | 1.16 | Indifferent |
| C. n 62066 | 2.83 | Indifferent | 1.49 | Indifferent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.R.A.; Motta, B.L.A.; Furtado, H.L.A.; Macedo, A.T.d.; Carvalho Junior, A.R.; Lima Neto, L.G.; Pinheiro, A.J.M.C.R.; Moreira, C.R.d.S.C.; da Silva, L.C.N.; Holanda, R.A. Hydroethanolic Extract of Punica granatum Inhibits Cryptococcus by Depolarising Mitochondrial Membranes. Microbiol. Res. 2025, 16, 49. https://doi.org/10.3390/microbiolres16020049
Santos JRA, Motta BLA, Furtado HLA, Macedo ATd, Carvalho Junior AR, Lima Neto LG, Pinheiro AJMCR, Moreira CRdSC, da Silva LCN, Holanda RA. Hydroethanolic Extract of Punica granatum Inhibits Cryptococcus by Depolarising Mitochondrial Membranes. Microbiology Research. 2025; 16(2):49. https://doi.org/10.3390/microbiolres16020049
Chicago/Turabian StyleSantos, Julliana Ribeiro Alves, Brenda Letícia Araujo Motta, Haryne Lizandrey Azevedo Furtado, Alessandra Teixeira de Macedo, Alexsander Rodrigues Carvalho Junior, Lídio Gonçalves Lima Neto, Aruanã Joaquim Matheus Costa Rodrigues Pinheiro, Cibelle Raphaela da Silva Cavalcante Moreira, Luís Cláudio Nascimento da Silva, and Rodrigo Assuncao Holanda. 2025. "Hydroethanolic Extract of Punica granatum Inhibits Cryptococcus by Depolarising Mitochondrial Membranes" Microbiology Research 16, no. 2: 49. https://doi.org/10.3390/microbiolres16020049
APA StyleSantos, J. R. A., Motta, B. L. A., Furtado, H. L. A., Macedo, A. T. d., Carvalho Junior, A. R., Lima Neto, L. G., Pinheiro, A. J. M. C. R., Moreira, C. R. d. S. C., da Silva, L. C. N., & Holanda, R. A. (2025). Hydroethanolic Extract of Punica granatum Inhibits Cryptococcus by Depolarising Mitochondrial Membranes. Microbiology Research, 16(2), 49. https://doi.org/10.3390/microbiolres16020049









