Efficacy of an Indigenously Isolated Rice Field Methanotroph as a Potential Bio-Inoculant for Promoting Rice Plant Growth
Abstract
1. Introduction
2. Materials and Methods
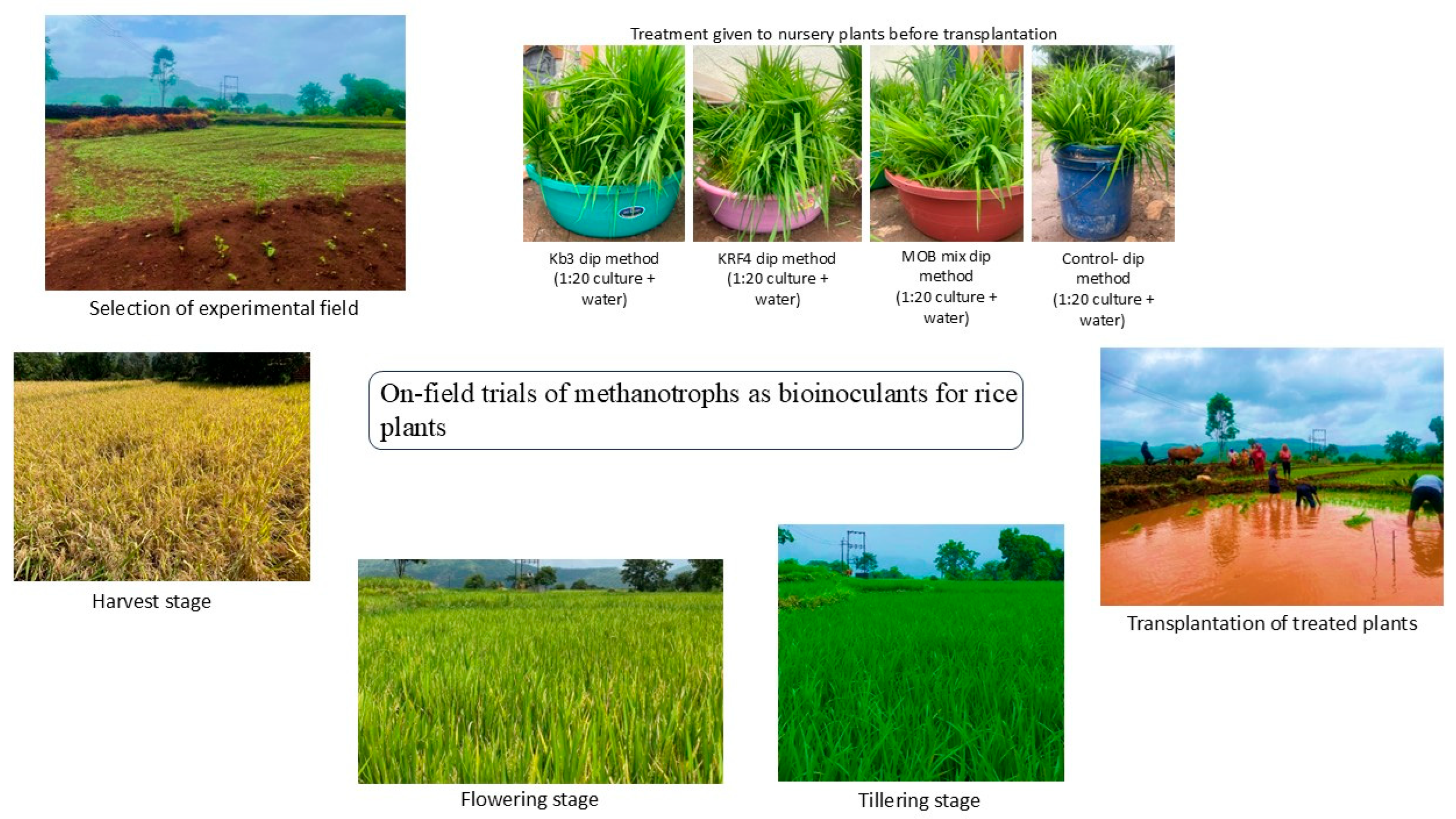
2.1. Selection of a Rice Field for the Field Experiment
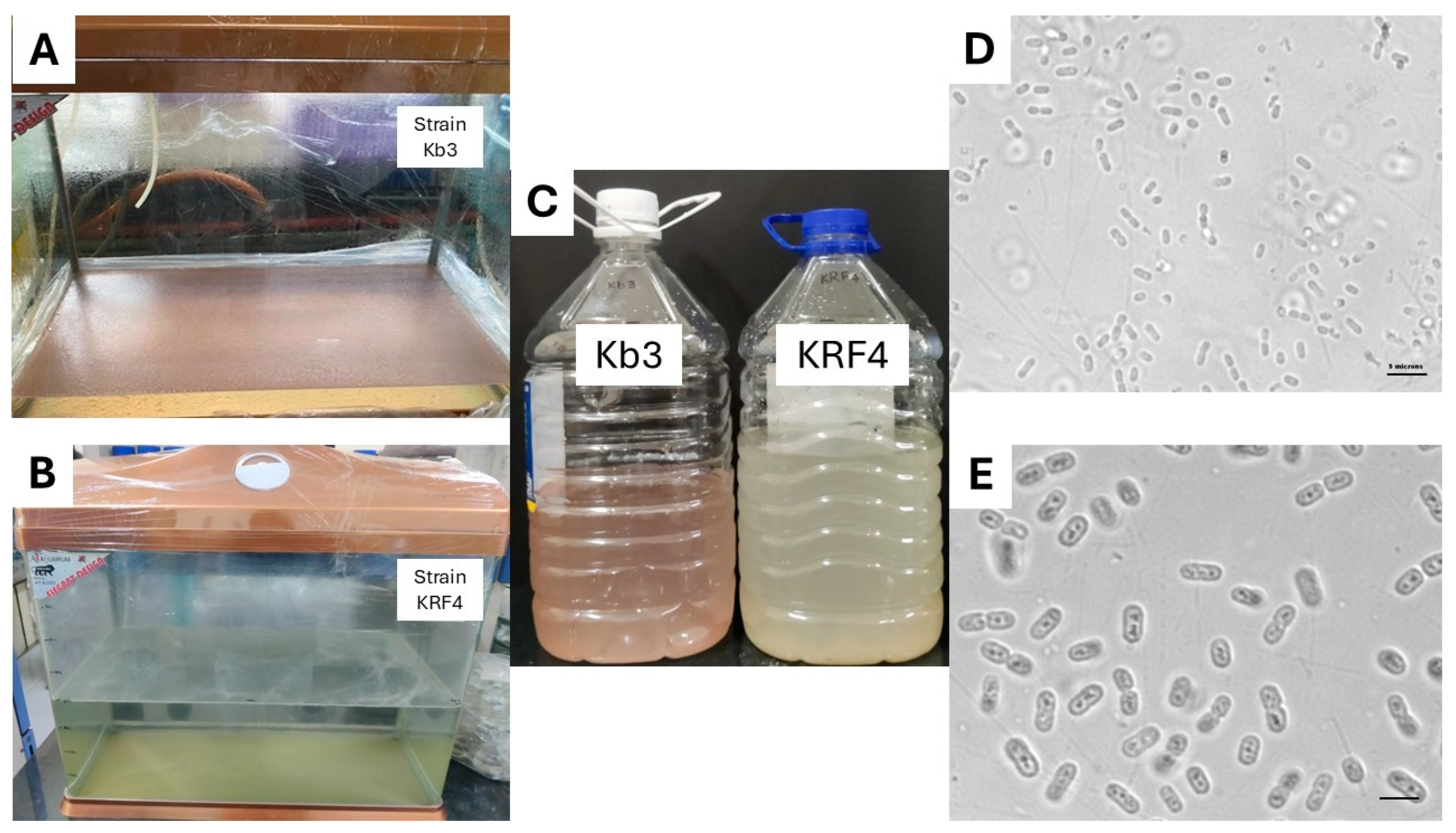
2.2. Treatment of Rice Plantlets with Selected Methanotroph Strains
2.3. Data Collection, Farm Visits, and Soil Sampling
2.4. Soil Sampling for Enrichment of Methanotrophs
2.5. Data Analysis
2.6. Comparison of Methylomonas and Methylomagnum Genomes
3. Results
3.1. Agri-Environmental Conditions
3.2. Overall Health of the Plants and Early Flowering Seen in Methylomonas Kb3-Treated Plants
3.3. Enhanced Growth Yield, Plant Height in Methylomonas Kb3-Treated Plants
3.4. Comparison of Genome Information
3.5. Re-Isolation of Methanotrophs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Methane-oxidizing bacteria | MOB |
References
- Saunois, M.; Bousquet, P.; Poulter, B.; Peregon, A.; Ciais, P.; Canadell, J.G.; Dlugokencky, E.J.; Etiope, G.; Bastviken, D.; Houweling, S.; et al. The global methane budget 2000–2012. Earth Syst. Sci. Data 2016, 8, 697–751. [Google Scholar] [CrossRef]
- Ganesan, A.; Rigby, M.; Lunt, M.F.; Parker, R.J.; Boesch, H.; Goulding, N.; Umezawa, T.; Zahn, A.; Chatterjee, A.; Prinn, R.G.; et al. Atmospheric observations show accurate reporting and little growth in India’s methane emissions. Nat. Commun. 2017, 8, 836. [Google Scholar] [CrossRef]
- Hanson, R.S.; Hanson, T.E. Methanotrophic bacteria. Microbiol. Mol. Biol. Rev. 1996, 60, 439–471. [Google Scholar] [CrossRef] [PubMed]
- Auman, A.; Stolyar, S.; Lidstrom, M.E. Molecular characterization of methanotrophic isolates from freshwater lake sediment. Appl. Environ. Microbiol. 2000, 66, 5259. [Google Scholar] [CrossRef] [PubMed]
- Rahalkar, M. Aerobic Methanotrophic Bacterial Communities from Sediments of Lake Constance. Ph.D. Thesis, University of Konstanz, Konstanz, Germany, 2007. [Google Scholar]
- Lüke, C.; Krause, S.; Cavigiolo, S.; Greppi, D.; Lupotto, E.; Frenzel, P. Biogeography of wetland rice methanotrophs. Environ. Microbiol. 2010, 12, 862–872. [Google Scholar] [CrossRef]
- Yun, J.; Ma, A.; Li, Y.; Zhuang, G.; Wang, Y.; Zhang, H. Diversity of methanotrophs in Zoige wetland soils under both anaerobic and aerobic conditions. J. Environ. Sci. 2010, 22, 1232–1238. [Google Scholar] [CrossRef]
- Pardhi, K.; Manvi, S.; Bahulikar, R.A.; Patil, Y.; Kadam, Y.; Kadam, S.; Saraf, C.; Rahalkar, M.C. Cultivation of Diverse Type I and Type II Methanotrophs from Tropical Wetlands in India, Including Rare Taxa (Methylocucumis and Methylolobus). Methane 2025, 4, 17. [Google Scholar] [CrossRef]
- Lüke, C. Molecular Ecology and Biogeography of Methanotrophic Bacteria in Wetland Rice Fields. Ph.D. Thesis, Max-Planck-Institut für Terrestrische Mikrobiologie, Marburg, Germany, 2010. [Google Scholar]
- Conrad, R. The global methane cycle: Recent advances in understanding the microbial processes involved. Environ. Microbiol. Rep. 2009, 1, 285–292. [Google Scholar] [CrossRef]
- Wu, L.; Ma, K.; Lu, Y. Rice roots select for type I methanotrophs in rice field soil. Syst. Appl. Microbiol. 2009, 32, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, B.; Frenzel, P. Rice roots and CH4 oxidation: The activity of bacteria, their distribution and the microenvironment. Soil Biol. Biochem. 1998, 30, 1903–1916. [Google Scholar] [CrossRef]
- Fazli, P.; Man, H.C.; Shah, U.K.M.; Idris, A. Characteristics of methanogens and methanotrophs in rice fields: A review. Asia-Pac. J. Mol. Biol. Biotechnol. 2013, 21, 3–17. [Google Scholar]
- Hardoim, P.R.; Andreote, F.D.; Reinhold-Hurek, B.; Sessitsch, A.; van Overbeek, L.S.; van Elsas, J.D. Rice root-associated bacteria: Insights into community structures across 10 cultivars. FEMS Microbiol. Ecol. 2011, 77, 154–164. [Google Scholar] [CrossRef]
- Sessitsch, A.; Hardoim, P.; Doering, J.; Weilharter, A.; Krause, A.; Woyke, T.; Mitter, B.; Hauberg-Lotte, L.; Friedrich, F.; Rahalkar, M.; et al. Functional characteristics of an endophyte community colonizing rice roots as revealed by metagenomic analysis. Mol. Plant-Microbe Interact. 2012, 25, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Engelhard, M.; Hurek, T.; Reinhold-Hurek, B. Preferential occurrence of diazotrophic endophytes, Azoarcus spp., in wild rice species and land races of Oryza sativa in comparison with modern races. Environ. Microbiol. 2000, 2, 131–141. [Google Scholar] [CrossRef]
- Rahalkar, M.C.; Khatri, K.; Pandit, P.S.; Dhakephalkar, P.K. A putative novel Methylobacter member (KRF1) from the globally important Methylobacter clade 2: Cultivation and salient draft genome features. Antonie van Leeuwenhoek 2019, 112, 1399–1408. [Google Scholar] [CrossRef]
- Rahalkar, M.C.; Mohite, J.A.; Pardhi, K.; Manvi, S.S.; Kadam, Y.S.; Patil, Y.V. Insights into Methylocucumis oryzae, a Large-sized, Phylogenetically Unique Type Ia Methanotroph with Biotechnological Potential. Ind. J. Microbiol. 2024, 64, 1964–1969. [Google Scholar] [CrossRef]
- Pandit, P.S.; Rahalkar, M.; Dhakephalkar, P.; Ranade, D.R.; Pore, S.; Arora, P.; Kapse, N. Deciphering community structure of methanotrophs dwelling in rice rhizospheres of an Indian rice field using cultivation and cultivation independent approaches. Microb. Ecol. 2016, 71, 634–644. [Google Scholar] [CrossRef]
- Rahalkar, M.; Khatri, K.; Pandit, P.S.; Bahulikar, R.; Mohite, J.A. Cultivation of Important Methanotrophs From Indian Rice Fields. Front. Microbiol. 2021, 12, 669244. [Google Scholar] [CrossRef]
- Mohite, J.; Khatri, K.; Pardhi, K.; Manvi, S.S.; Jadhav, R.; Rathod, S.; Rahalkar, M.C. Exploring the potential of methanotrophs for plant growth promotion in rice agriculture. Methane 2023, 2, 361–371. [Google Scholar] [CrossRef]
- Kadam, S.; Saraf, C. Experimental trials for use of selected methanotrophs as bioinoculant in rice plant (Indrayani variety). Master’s Thesis, Swami Ramanand Teerth Marathwada University, Nanded, India, 2024. [Google Scholar]
- Mohite, J.; Pardhi, K.; Rahalkar, M.C. Single-Cell Protein Using an Indigenously Isolated Methanotroph Methylomagnum ishizawai, Using Biogas. Microbiol. Res. 2025, 16, 171. [Google Scholar] [CrossRef]
- Pandit, P.S.; Ranade, D.R.; Dhakephalkar, P.K.; Rahalkar, M.C. A pmoA-based study reveals dominance of yet uncultured Type I methanotrophs in rhizospheres of an organically fertilized rice field in India. 3 Biotech 2016, 6, 135. [Google Scholar] [CrossRef] [PubMed]
- Rahalkar, M.C.; Pandit, P. Genome-based insights into a putative novel Methylomonas species (strain Kb3), isolated from an Indian rice field. Gene Rep. 2018, 13, 9–13. [Google Scholar] [CrossRef]
- Khalifa, A.; Lee, C.G.; Ogiso, T.; Ueno, C.; Dianou, D.; Demachi, T.; Katayama, A.; Asakawa, S. Methylomagnum ishizawai gen. nov., sp. nov., a mesophilic type I methanotroph isolated from rice rhizosphere. Int. J. Syst. Evol. Microbiol. 2015, 65, 3527–3534. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Paleontol. Electron. 2001, 4, 9. [Google Scholar]
- Kumar, S.R.; David, E.M.; Pavithra, G.J.; Kumar, G.S.; Subbian, E. Methane-derived microbial biostimulant reduces greenhouse gas emissions and improves rice yield. Front. Plant Sci. 2024, 15, 1432460. [Google Scholar] [CrossRef]
- Cao, W.; Cai, Y.; Bao, Z.; Wang, S.; Yan, X.; Jia, Z. Methanotrophy Alleviates Nitrogen Constraint of Carbon Turnover by Rice Root-Associated Microbiomes. Front. Microbiol. 2022, 13, 885087. [Google Scholar] [CrossRef]
- Auman, A.J.; Speake, C.C.; Lidstrom, M.E. nifH Sequences and Nitrogen Fixation in Type I and Type II Methanotrophs. Appl. Environ. Microbiol. 2001, 67, 4009–4016. [Google Scholar] [CrossRef]
- Cai, Y.; Zheng, Y.; Bodelier, P.L.E.; Conrad, R.; Jia, Z. Conventional methanotrophs are responsible for atmospheric methane oxidation in paddy soils. Nat. Commun. 2016, 7, 11728. [Google Scholar] [CrossRef] [PubMed]
- Hara, S.; Wada, N.; Hsiao, S.S.; Zhang, M.; Bao, Z.; Iizuka, Y.; Lee, D.; Sato, S.; Tang, S.; Minamisawa, K. In Vivo Evidence of Single 13C and 15N Isotope–Labeled Methanotrophic Nitrogen-Fixing Bacterial Cells in Rice Roots. mBio 2022, 13, e0125522. [Google Scholar] [CrossRef] [PubMed]
- Rahalkar, M.; Bahulikar, R. Hemerythrins are widespread and conserved for methanotrophic guilds. Gene Rep. 2018, 11, 250–254. [Google Scholar] [CrossRef]
- Shende, S.M.; Hasure, R.R.; Gajbhiye, P.N.; Gedam, V.B.; Shinde, R.H. Response of dry seeding of kharif paddy (Oryza sativa L.) Varieties to different fertilizer levels with respect growth characters. Int. J. Chem. Stud. 2020, 8, 1272–1277. [Google Scholar] [CrossRef]


| Treatment | Total Plant Height (cm) | No. of Tillers | No. of Panicles | Panicle Height (cm) | Weight of 1000 Grains g | Average Grain Weight per Hill g | Yield Quintal per ha | Increase in Yield |
|---|---|---|---|---|---|---|---|---|
| Kb3 | 106.8 ± 4.6 | 19 ± 3 | 18 ± 3 | 22.8 ± 1.5 | 20.01 | 38.1 | 61.06 | 17% |
| KRF4 | 96.0 ± 6 | 17 ± 3 | 15 ± 1 | 23.3 ± 2.2 | 22.71 | 31.6 | 50.5 | Little decrease (−3%) |
| Kb3 + KRF4 (MOB mix) | 101.8 ± 6.4 | 18 ± 3 | 16 ± 3 | 24.1 ± 1.2 | 21.59 | 37.5 | 59.99 | 15% |
| Control | 94.8 ± 6.4 | 19 ± 3 | 17 ± 3 | 23.3 ± 1.3 | 20.68 | 32.6 | 52.13 | Control yield |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manvi, S.; Pardhi, K.; Kadam, S.; Kadam, Y.; Patil, Y.; Bahulikar, R.A.; Rahalkar, M.C. Efficacy of an Indigenously Isolated Rice Field Methanotroph as a Potential Bio-Inoculant for Promoting Rice Plant Growth. Microbiol. Res. 2025, 16, 228. https://doi.org/10.3390/microbiolres16110228
Manvi S, Pardhi K, Kadam S, Kadam Y, Patil Y, Bahulikar RA, Rahalkar MC. Efficacy of an Indigenously Isolated Rice Field Methanotroph as a Potential Bio-Inoculant for Promoting Rice Plant Growth. Microbiology Research. 2025; 16(11):228. https://doi.org/10.3390/microbiolres16110228
Chicago/Turabian StyleManvi, Shubha, Kajal Pardhi, Shirish Kadam, Yash Kadam, Yukta Patil, Rahul A. Bahulikar, and Monali C. Rahalkar. 2025. "Efficacy of an Indigenously Isolated Rice Field Methanotroph as a Potential Bio-Inoculant for Promoting Rice Plant Growth" Microbiology Research 16, no. 11: 228. https://doi.org/10.3390/microbiolres16110228
APA StyleManvi, S., Pardhi, K., Kadam, S., Kadam, Y., Patil, Y., Bahulikar, R. A., & Rahalkar, M. C. (2025). Efficacy of an Indigenously Isolated Rice Field Methanotroph as a Potential Bio-Inoculant for Promoting Rice Plant Growth. Microbiology Research, 16(11), 228. https://doi.org/10.3390/microbiolres16110228







