Research on the Influence of Enterobius vermicularis on the Composition and Quality of the Intestinal Microbiota, and the Susceptibility to Co-Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Period and Design
2.1.1. Individuals Infected with E. vermicularis
2.1.2. Control Group of Individuals with No Data on Enterobiasis or Other Parasitic Infection
2.2. Parasitological Diagnosis
2.3. Microbiological Diagnosis
2.4. Statistical Analysis
3. Results
3.1. Individuals Infected with E. vermicularis
3.1.1. Clinical Characteristics, Stage of the Parasite (Eggs/Adults) Found in Clinical Samples and Parasite Burden Grade
3.1.2. Bacterial Co-Infections
3.1.3. Statistical Analysis
3.2. Control Group
4. Discussion
4.1. Epidemiology
4.2. Co-Infections
4.3. Microbiota and Immunity
4.4. Clinical Implications
4.5. Limitations of the Study
4.6. Future Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| E. vermicularis | Enterobius vermicularis |
| E. coli | Escherichia coli |
| NCIPD | National Centre of Infectious and Parasitic Diseases |
| DPTM | Department of Parasitology and Tropical Medicine |
References
- Burkhart, C.; Burkhart, C. Assessment of frequency, transmission, and genitourinary complications of enterobiasis (pinworms). Int. J. Dermatol. 2005, 44, 837–840. [Google Scholar] [CrossRef]
- Kim, B.; Lee, B.; Chung, H.; Lee, K.; Chung, H.; Ock, M. Egg positive rate of Enterobius vermicularis of primary school children in Geoge Island. Korean J. Parasitol. 2003, 4, 75–77. [Google Scholar] [CrossRef]
- Paniker, C. Textbook of Medical Parasitology, 7th ed.; Jaypee Brothers Medical Publisher Ltd.: New Delhi, India, 2013; pp. 190–194. [Google Scholar]
- Cranston, I.; Potgieter, N.; Mathebula, S.; Ensink, J. Transmission of Enterobius vermicularis eggs through hands of school children in rural South Africa. Acta Trop. 2015, 150, 94–96. [Google Scholar] [CrossRef]
- Cook, G. Enterobius vermicularis infection. Gut 1994, 35, 1159–1162. [Google Scholar] [CrossRef]
- Ibarra, J. Threadworms: A starting point for family hygiene. Br. J. Community Nurs. 2001, 6, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Otu-Bassey, I.; Ejezie, G.; Epoke, J.; Useh, M. Enterobiasis and its relationship with anal itching and enuresis among school-age children in Calabar, Nigeria. Ann. Trop. Med. Parasitol. 2005, 99, 611–616. [Google Scholar] [CrossRef]
- Tornieporth, N.; Disko, R.; Brandis, A.; Barutzki, D. Ectopic Enterobiasis: A case report and review. J. Infect. 1992, 24, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Ok, U.; Ertan, P.; Limoncu, E.; Ece, A.; Ozbakkaloglu, B. Relationship between pinworm and urinary tract infections in young girls. APMIS 1999, 107, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Taghipour, A.; Olfatifar, M.; Javanmard, E.; Norouzi, M.; Mirjalali, H.; Zali, M. The neglected role of Enterobius vermicularis in appendicitis: A systematic review and meta-analysis. PLoS ONE 2020, 15, e02322143. [Google Scholar] [CrossRef]
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Moreira de Gouveia, M.; Donadille, A.; Jubelin, G. Enterobacteriaceae in the human gut: Dinamics and ecological roles in health and disease. Biology 2024, 13, 142. [Google Scholar] [CrossRef]
- Kupritz, J.; Angelova, A.; Nutman, T.B.; Gazzinelli-Guimaraes, P.H. Helminth-Induced Human Gastrointestinal Dysbiosis: A Systematic Review and Meta-Analysis Reveals Insights into Altered Taxon Diversity and Microbial Gradient Collapse. mBio 2021, 12, e02890-21. [Google Scholar] [CrossRef] [PubMed]
- Chakarova, B. Enterobiasis. In Parasitology (Local and Tropical Parasitosis), 2nd ed.; Boeva-Bangyozova, V., Vutova, K., Eds.; Arso: Sofia, Bulgaria, 2008; pp. 244–249. (In Bulgarian) [Google Scholar]
- Dudlová, A.; Juriš, P.; Jarčuška, P.; Vasilková, Z.; Vargová, V.; Sumková, M.; Krčméry, V. The incidence of pinworm (Enterobius vermicularis) in pre-school and school aged children in the Eastern Slovakia. Helminthologia 2018, 55, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Berrilli, F.; Di Cave, D.; Cavallero, S.; D’Amelio, S. Interactions between parasites and microbial communities in the human gut. Fron. Cell. Infect. Microbiol. 2012, 16, 141. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Yen, C.; Hwang, K.; Wang, L. Enterobius vermicularis infection and its risk factors among pre-school children in Taipei, Taiwan. J. Microbiol. Immunol. Infect. 2018, 51, 559–564. [Google Scholar] [CrossRef]
- Dutto, M.; Montù, D.; Raineri, G. Enterobiasis in pediatric subjects in north-western Italy: A study of home remedies. Ann. Ig. 2012, 24, 81–84. (In Italian) [Google Scholar] [PubMed]
- Fan, C.K.; Chuang, T.W.; Huang, Y.C.; Yin, A.W.; Chou, C.M.; Hsu, Y.T.; Kios, R.; Hsu, S.L.; Wang, Y.T.; Wu, M.S.; et al. Enterobius vermicularis infection: Prevalence and risk factors among preschool children in kindergarten in the capital area, Republic of the Marshall Islands. BMC Infect. Dis. 2019, 19, 536. [Google Scholar] [CrossRef]
- Friesen, J.; Bergmann, C.; Neuber, R.; Fuhrmann, J.; Wenzel, T.; Durst, A.; Müller, M.; Ignatius, R. Detection of Enterobius vermicularis in greater Berlin, 2007–2017: Seasonality and increased frequency of detection. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 719–723. [Google Scholar] [CrossRef]
- Vargas-Arzola, J.; Segura-Salvador, A.; Hernández-Osorio, L.; Santos-Hernández, N.G.; Vidal-López, D.G.; Moreno-Rodríguez, A.; De Fuentes-Vicente, J.A. Enterobius vermicularis infection in a child population with evidence of vulvovaginitis and bacterial coinfection in girls in Oaxaca, Mexico. Adv. Public Health 2024, 2024, 8408028. [Google Scholar] [CrossRef]
- Lee, S.C.; Tang, M.S.; Lim, Y.A.; Choy, S.H.; Kurtz, Z.D.; Cox, L.M.; Gundra, U.M.; Cho, I.; Bonneau, R.; Blaser, M.J.; et al. Correction: Helminth colonization is associated with increased diversity of the gut microbiota. PLoS Negl. Trop. Dis. 2021, 15, e0009325. [Google Scholar] [CrossRef]
- Kurdova-Mincheva, R. Pathology of the gastrointestinal tract. In Clinical Parasitology and Tropical Medicine; Petrov, P., Kurdova-Mincheva, R., Eds.; East-West: Sofia, Bulgaria, 2016; pp. 79–85. (In Bulgarian) [Google Scholar]
- Korpe, P.S.; Ravdin, J.I.; Petri, W.A.; Bennett, J. Introduction to Protozoal Diseases. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 8th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2015; pp. 3045–3046. [Google Scholar] [CrossRef]
- Hamdy, D.; El Wahab, W.; Senosy, S.; Mabrouk, A. Blastocystis spp. and Giardia intestinalis co-infection profile in children suffering from acute diarrhea. J. Parasit. Dis. 2020, 44, 88–98. [Google Scholar] [CrossRef]
- Zali, M.R.; Mehr, A.J.; Rezaian, M.; Meamar, A.R.; Vaziri, S.; Mohraz, M. Prevalence of intestinal parasitic pathogens among HIV positive individuals in Iran. Jpn. J. Infect. Dis. 2004, 57, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Azarinoosh, S.A.; Nahrevanian, H.; Assmar, M.; Esfandiary, B.; Amirkhani, A. Simultaneous prevalence of Blastocystis hominis in patients with giardiasis from Tonekabon city, Mazandaran province. Iran. J. Biol. Sci. 2010, 3, 1–7. [Google Scholar]
- Izvekova, G. Parasitic infections and intestinal microbiota: A review. Biol. Bull. Russ. Acad. Sci. 2022, 49, 323–332. [Google Scholar] [CrossRef]
- Palmas, C.; Gabriele, F.; Conchedda, M.; Bortoletti, G.; Ecca, A. Causality or coincidence: May the slow disappearance of helminths be responsible for the imbalances in immune control mechanisms? J. Helminthol. 2017, 77, 147–153. [Google Scholar] [CrossRef]
- Blagova, N.; Hudoyan, Z. Enterobioz; YaGMA: Yaroslavl, Russia, 2017; p. 31. (In Russian) [Google Scholar]
- Marchenko, V.; Stepanchenko, K. Condition of immune status in adults with enterobiasis invasion. World Med. Biol. 2020, 74, 93–97. [Google Scholar] [CrossRef]
- Akoolo, L.; Rocha, S.; Parveen, N. Protozoan co-infections and paraite influence on the efficacy of vaccines against bacterial and viral pathogens. Front. Microbiol. 2022, 13, 1020029. [Google Scholar] [CrossRef] [PubMed]
- Dahal, R.H.; Kim, S.; Kim, Y.K.; Kim, E.S.; Kim, J. Insight into gut dysbiosis of patients with inflammatory bowel disease and ischemic colitis. Front. Microbiol. 2023, 14, 1174832. [Google Scholar] [CrossRef]
- Song, H.J.; Cho, C.H.; Kim, J.S.; Choi, M.H.; Hong, S.T. Prevalence and risk factors for enterobiasis among preschool children in a metropolitan city in Korea. Parasitol. Res. 2003, 91, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.L.; Darling, A.E.; Eisen, J.A. Metagenomic Sequencing of an In Vitro-Simulated Microbial Community. PLoS ONE 2010, 5, e10209. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Gender | Age | ||
|---|---|---|---|---|
| Male Number (%) | Female Number (%) | Children and Adolescents Number (%) | Adults Number (%) | |
| asymptomatic with symptoms | 45 (59%) 31 (41%) | 42 (45%) 51 (55%) | 85(52%) 77 (48%) | 2 (29%) 5 (71%) |
| no presence of adult forms in clinical samples with the presence of adult forms in clinical samples | 62 (82%) 14 (18%) | 67 (73%) 25 (27%) | 124 (77%) 37 (23%) | 5 (71%) 2 (28%) |
| low parasite burden high parasite burden | 27 (36%) 49 (64%) | 29 (31%) 64 (69%) | 53 (33%) 109 (67%) | 3 (43%) 4 (57%) |
| Gender | Age (Year) | Clinical Symptom | Isolated Intestinal Pathogen |
|---|---|---|---|
| male | 5 | bruxism | E. coli 0139 |
| female | 3 | asymptomatic | E. coli 075 |
| male | 4 | asymptomatic | E. coli 0146 |
| female | 18 | abdominal pain | E. coli 018 |
| male | 11 | bruxism | E. coli 018 |
| female | 7 | asymptomatic | E. coli 018 |
| male | 10 | asymptomatic | E. coli 0142 |
| female | 10 | perianal itching | E. coli 0158 |
| female | 6 | asymptomatic | E. coli 0146 |
| female | 6 | tenesmus | E. coli 018 |
| male | 13 | diarrhea | Salmonella arizonae |
| female | 7 | diarrhea | Citrobacter gillenii |
| Distribution of Cases by Gender (Number of Cases/%) | Species of Isolated Opportunistic Pathogen | ||||
|---|---|---|---|---|---|
| Klebsiella pneumoniae | Enterobacter spp. | Proteus mirabilis | Citrobacter freundii | Pseudomonas aeruginosa, Enterococcus fecalis, Morganella morganii | |
| Males | 9 (47.4%) | 5 (26.3%) | 2 (10.5%) | 2 (10.5%) | 1 (5.3%) |
| Females | 11 (50%) | 4 (18.2%) | 3 (13.6%) | 2 (9.1%) | 2 (9.1%) |
| Proportion of all cases with opportunistic bacterial pathogens | 20 (48.8%) | 9 (22%) | 5 (12.2%) | 4 (9.8%) | 3 (7.3%) |
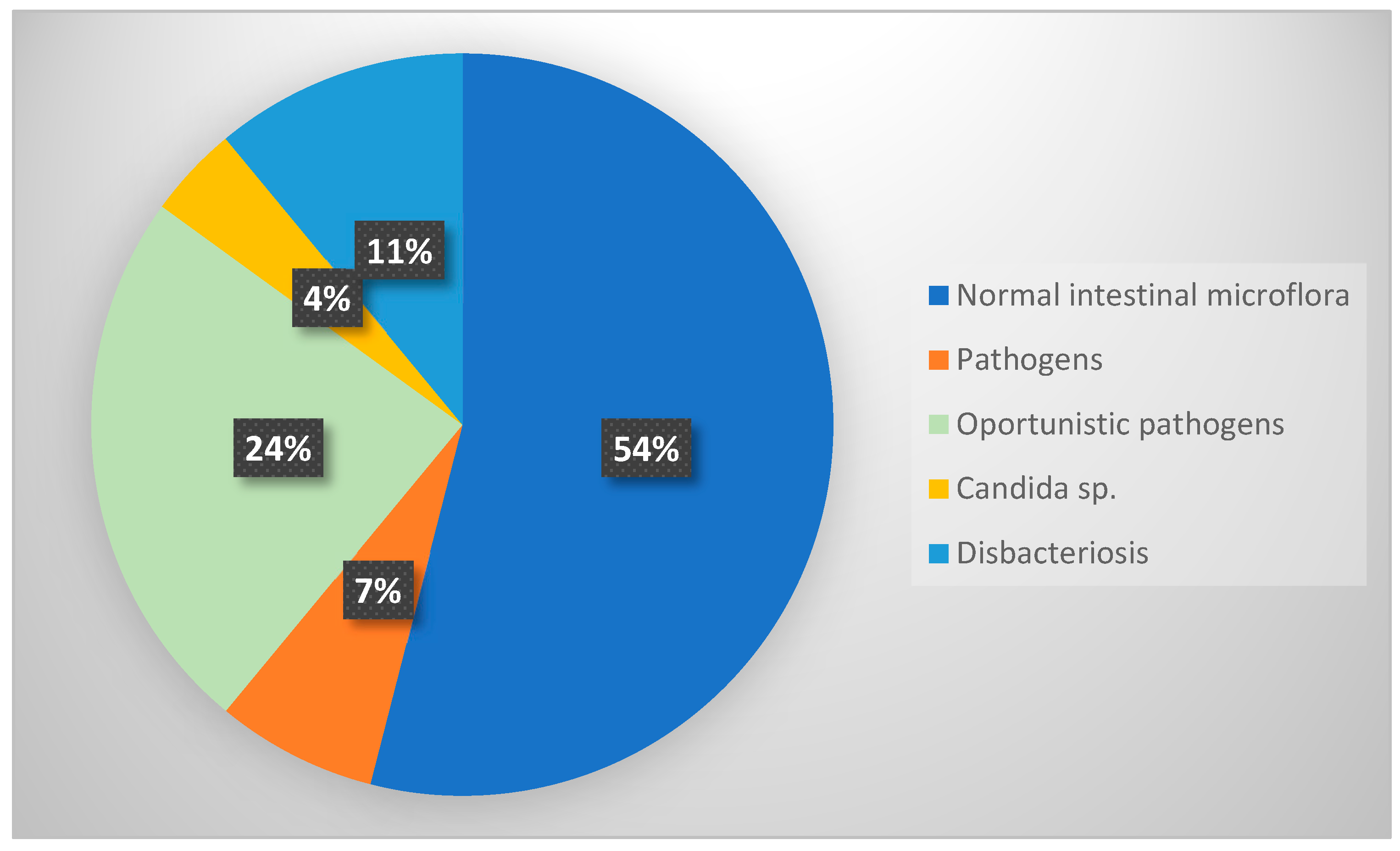
| Normal Intestinal Flora Number (%) | Pathogenic Flora Number (%) | Opportunistic Pathogens Number (%) | Candida spp. Number (%) | Lack of Commensal Flora Number (%) | Chi-Square Test p Value | |
|---|---|---|---|---|---|---|
| Gender male (76) female (93) | 39 (23%) 52 (31%) | 5 (3%) 7 (4%) | 19 (11%) 22 (13%) | 3 (2%) 3 (2%) | 10 (6%) 9 (5%) | 0.944 |
| Children’s age groups 1–3 (n = 25) 4–6 (n = 77) 7–9 (n = 36) 10–12 (n = 20) 13–15 (n = 2) 16–18 (n = 2) | 17 (10%) 42 (26%) 18 (11%) 8 (5%) 1 (1%) 0 (0%) | 1 (1%) 4 (2%) 2 (1%) 3 (2%) 1 (1%) 1 (1%) | 3 (2%) 25 (15%) 7 (4%) 6 (4%) 0 (0%) 0 (0%) | 1 (1%) 0 (0%) 2 (1%) 2 (1%) 0 (0%) 0 (0%) | 3 (2%) 6 (4%) 7 (4%) 1 (1%) 0 (0%) 1 (1%) | 0.031 |
| asymptomatic (n = 87) with clinical symptoms (n = 82) | 49 (29%) 42 (25%) | 5 (3%) 7 (4%) | 22 (13%) 19 (11%) | 2 (1%) 4 (2%) | 9 (5%) 10 (12%) | 0.797 |
| Only E. vermicularis eggs in the clinical samples (n = 129) Presence of adult forms in clinical samples (n = 39) | 76 (45%) 15 (9%) | 8 (5%) 4 (2%) | 31 (19%) 9 (5%) | 5 (3%) 1 (1%) | 9 (5%) 10 (6%) | 0.015 |
| Low parasitic burden (n = 56) High parasitic burden (113) | 37 (22%) 54 (32%) | 4 (2%) 8 (5%) | 10 (6%) 31 (18%) | 1 (1%) 5 (3%) | 4 (2%) 15 (9%) | 0.220 |
| Gender | Age | Reason for Examination | Isolated Bacterial Species |
|---|---|---|---|
| male | 5 | bruxism | E. coli 0139 |
| female | 3 | prophylactic examination | E. coli 075 |
| male | 4 | prophylactic examination | E. coli 0146 |
| female | 18 | abdominal pain | E. coli 018 |
| male | 11 | bruxism | E. coli 018 |
| female | 7 | prophylactic examination | E. coli 018 |
| male | 10 | prophylactic examination | E. coli 0142 |
| female | 10 | perianal itching | E. coli 0158 |
| female | 6 | prophylactic examination | E. coli 0146 |
| female | 6 | tenesmus | E. coli 018 |
| male | 13 | diarrhea | Salmonella arizonae |
| female | 7 | diarrhea | Citrobacter galineii |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaneva, E.; Harizanov, R.; Pavlova, M.; Velcheva, D.; Tsvetkova, N.; Ivanova, A.; Videnova, M.; Borisova, R.; Alexiev, I.; Dimitrova, R. Research on the Influence of Enterobius vermicularis on the Composition and Quality of the Intestinal Microbiota, and the Susceptibility to Co-Infections. Microbiol. Res. 2025, 16, 215. https://doi.org/10.3390/microbiolres16100215
Kaneva E, Harizanov R, Pavlova M, Velcheva D, Tsvetkova N, Ivanova A, Videnova M, Borisova R, Alexiev I, Dimitrova R. Research on the Influence of Enterobius vermicularis on the Composition and Quality of the Intestinal Microbiota, and the Susceptibility to Co-Infections. Microbiology Research. 2025; 16(10):215. https://doi.org/10.3390/microbiolres16100215
Chicago/Turabian StyleKaneva, Eleonora, Rumen Harizanov, Maria Pavlova, Desislava Velcheva, Nina Tsvetkova, Aleksandra Ivanova, Mihaela Videnova, Raina Borisova, Ivailo Alexiev, and Reneta Dimitrova. 2025. "Research on the Influence of Enterobius vermicularis on the Composition and Quality of the Intestinal Microbiota, and the Susceptibility to Co-Infections" Microbiology Research 16, no. 10: 215. https://doi.org/10.3390/microbiolres16100215
APA StyleKaneva, E., Harizanov, R., Pavlova, M., Velcheva, D., Tsvetkova, N., Ivanova, A., Videnova, M., Borisova, R., Alexiev, I., & Dimitrova, R. (2025). Research on the Influence of Enterobius vermicularis on the Composition and Quality of the Intestinal Microbiota, and the Susceptibility to Co-Infections. Microbiology Research, 16(10), 215. https://doi.org/10.3390/microbiolres16100215







