Abstract
This study compared the effect of phenolics extracted from four different types of pomegranate peels for controlling the growth of Salmonella enterica and enterohemorrhagic Escherichia coli. Cells of the two bacterial cultures (5 log CFU/mL) were treated in tryptic soy broth containing 0, 1, or 2% ethanolic extracts of four pomegranate peels for 5, 10, or 24 h at 25 °C. The surviving cells were recovered on a general-purpose agar and a selective medium. The extracts of three products were more effective (p ≤ 0.05) against E. coli than the extract of the remaining product, which had a similar activity to the extracts of two of the products used against S. enterica. Longer treatment time and higher extract concentration resulted in greater pathogen population reductions. The bacterial strains used had varied susceptibility to the extracts. Reductions in cell population positively correlated with the total contents of hydrolyzable tannins in the treatment systems. These findings suggested that ethanolic extracts of evaluated pomegranate peels had inhibitory activities against the two bacterial pathogens. This highlights the potential of pomegranate peels as a promising natural alternative to conventional antimicrobials for controlling foodborne pathogens like S. enterica and E. coli.
1. Introduction
Foodborne enteric infections caused by bacterial pathogens such as Salmonella enterica and enterohemorrhagic Escherichia coli have posed significant public health concerns worldwide [1]. According to the Centers for Disease Control and Prevention (CDC), approximately 1.35 million cases of salmonellosis are estimated to occur annually, resulting in 420 fatalities in the United States [2]. Over 63,000 illnesses and more than 2100 hospitalizations and 61 deaths are caused by E. coli O157:H7 [3]. These two Gram-negative pathogens, belonging to the family of Enterobacteriaceae, can cause severe gastrointestinal symptoms, including nausea, vomiting, diarrhea, and other severe manifestations [4].
The primary niche of S. enterica and pathogenic E. coli in food animals largely contributes to their perpetuating existence in the food chain [5]. The expanding scale of the food industry, particularly that involving animal products, has resulted in the inappropriate use of antimicrobials. The extended application of antibiotics as animal growth promoters has led to the emergence of antimicrobial-resistant strains of bacterial pathogens [6]. Consequently, there is a pressing need to develop alternative antimicrobial agents aimed at controlling the spread of infectious etiologies within the food supply chain. Natural products such as fruits and vegetables are potentially rich sources of these alternative antimicrobial agents [7]. Pomegranate (Punica granatum L.) is a fruit that has been used for medicinal purposes for centuries [8]. It is well known to have anti-inflammatory and antioxidant activities that have been proposed to have practical applications in preventing a range of diseases, including cancer, cardiovascular disease, and diabetes [9]. In addition to those remedial benefits, studies have explored the antimicrobial activities of different parts of pomegranates, with the peels showing the most potent activity against a broad spectrum of microorganisms [10,11]. Ellagitannins, specifically hydrolyzable tannins, from the class of polyphenols are the primary groups of phytochemicals abundant in pomegranate peels that are responsible for their antibacterial characteristics [12]. These compounds exhibited activities against a broad spectrum of pathogens, including Gram-positive bacteria such as Staphylococcus aureus and Bacillus subtilis as well as Gram-negative bacteria like Pseudomonas aerogenosa. Ellagitannins are believed to exert their antimicrobial effects by causing oxidative damage, disrupting bacterial cell membranes, chelating irons, and interfering with cell metabolic processes [13].
For an extended period, India’s favorable climate has fostered the cultivation of pomegranates, which are now widely grown for both domestic consumption and export [14]. The pomegranate industry in India generates significant revenue, and pomegranate products are exported to several countries worldwide [15]. In comparison, pomegranate is a relatively new crop in Georgia, U.S., although the warm climate and suitable soil conditions in South Georgia have enabled its growth. Exploring the additional value of the by-products such as pomegranate peels could potentially increase profits for this burgeoning industry. The present study is designed to investigate and compare the antibacterial activity of ethanolic extracts of four pomegranate peels originating from India and Georgia, U.S. against two foodborne pathogens: S. enterica and enterohemorrhagic E. coli.
2. Materials and Methods
2.1. Preparation of Pomegranate Peel Extracts
Four powdered pomegranate peel products were selected in this study. Products I, II, and III, peels of unknown cultivars from India, were purchased from eSutras superfood, Bixa, and Nature Vibe, respectively, through Amazon.com. Product IV was a peel of a Georgia-grown cultivar sourced locally (Alma, GA, USA), which was dried and powdered by Dr. Kevin Mis Solval’s laboratory at the University of Georgia. All products were stored at refrigeration temperature until use.
A 100 g sample of each pomegranate peel powder product was mixed with 900 mL of 70% ethanol at 25 °C for 48 h under continuous agitation conditions on an orbital platform shaker (Bellco Biotechnology, East Lyme, CT, USA). The resulting mixture was centrifuged at 10,000× g for 10 min at 5 °C, and the obtained pellet was re-extracted using the same process. The supernatants collected from both extractions were combined and placed into a Pyrex glass container (Pyrex, Corning Inc. Corning, NY, USA) and evaporated to dryness at 37 °C for approximately 5 h. Each dried extract (10 g) was subsequently dissolved in 100 mL of 30% dimethyl sulfoxide (DMSO; Thermo Fisher Scientific, Waltham, MA, USA) to achieve a final concentration of 10% (w/v). The solution was sterilized using a sterile nylon membrane Corning disposable filtration and storage system (0.22 µm; Fisher Scientific, Suwanee, GA, USA). Given the high freezing point of DMSO (18.5 °C), the sterile extract was stored in a sealed container at 25 °C for further analysis.
2.2. Quantification of Hydrolyzable Tannins in Peel Extracts
An Agilent 1100 series high-performance liquid chromatography (HPLC) system (Agilent Technologies, Waldbronn, Germany) equipped with a binary high-pressure pump, a Diode-Array Detector, a thermostatted column compartment, and an autosampler was used for the analysis. Agilent OpenLAB ChemStation C.01.09 software was used to process the collected data. A ZORBAX Eclipse XDB-Phenyl column (3 × 150 mm, 5 µm particle size, Agilent Technologies) equipped with a guard column (4.6 × 12.5 mm, 5 µm particle size, Agilent Technologies) was used for the chromatographic runs. The parameters selected for the HPLC analysis conditions and mobile phase solvents were described by Wu et al. [16]
Standard gallic acid, ellagic acid, and -punicalagin, and and -punicalin were purchased from INDOFINE Chemical Company Inc. (Hillsborough, NJ, USA) or Sigma-Aldrich (St. Louis, MO, USA) and kept at 4 °C before the experiment. The extracts and the standards were purified by passing through a 0.45 µm Whatman syringeless filter device (Cytiva, Marlborough, MA, USA). The filtered samples were subsequently diluted using HPLC-grade methanol to achieve concentrations within the detection limits. The hydrolyzable tannins in analyzed samples were quantified by comparing the retention time between the standards and extract samples. Calibration curves generated using a series concentration of the standards were used to calculate the concentrations of identified hydrolyzable tannins in extract samples [16].
2.3. Targeted Bacterial Strains
Strains of S. enterica and enterohemorrhagic E. coli O157:H7 were selected as the target organisms in the study. S. enterica subsp. enterica serotype Tennessee (the peanut butter outbreak strain) and Enteritidis (the almond outbreak strain) were provided by Dr. Larry Beuchat’s laboratory at the University of Georgia. E. coli O157:H7 F4546 and K4492, which had been previously implicated in outbreaks related to sprouts and leafy green vegetables, respectively, were obtained from Dr. Mark Harrison’s laboratory at the same institution.
2.4. Antibacterial Activity Testing
The antibacterial activity of the four extracts against the S. enterica and E. coli strains was assessed using the plate count assay. A single colony of each bacterial strain was inoculated in tryptic soy broth (TSB; Becton Dickinson, Sparks, MD, USA) and incubated overnight at 37 °C. A ten-fold serial dilution was conducted in TSB to achieve a bacterial suspension of ca. 5 log CFU/mL. In the treatment groups, each TSB-diluted bacterial suspension was supplemented with each extract in the DMSO solution described above to achieve a concentration of 10% or 20%, equivalent to 1 or 2% dry ethanolic extracts of pomegranate peels. In contrast, bacterial suspensions not exposed to the extracts served as positive controls. The missing volume in the control groups was supplemented with 30% DMSO.
The surviving population of each bacterial strain was determined after an incubation period of 5, 10, or 24 h at 25 °C on an orbital platform shaker (Model 3520, Lab-line, IL, USA). Bacterial suspensions from both treatment and control groups were enumerated on tryptic soy agar (TSA; Becton Dickinson), as well as bismuth sulfite agar (BSA; Becton Dickinson, Sparks) for Salmonella and sorbitol MacConkey agar (SMAC; Becton Dickinson, Sparks) for E. coli. The cell populations were determined from the plates after incubation at 37 °C for 16–18 h. The inactivation capacity of pomegranate peel extracts was expressed as the reduction (log CFU/mL) in the bacterial cell population in the treatment groups compared to the cell populations in the control groups.
2.5. Statistical Analysis
Data were collected in two independent trials, and all samples were analyzed in duplicate. The results were reported as the means of reductions in S. enterica and E. coli cell populations. The independent variables included bacterial strain, phenolic extract, extract concentration, and treatment time. ANOVA of the statistical analysis software (Statistical Analysis Systems Institute, ver. 9.4, Cary, NC, USA) was used to determine the differences among the means of bacterial population reduction at a significance level of p ≤ 0.05. Fisher’s least significant difference test was used to determine statistical significance. The concentrations of individual hydrolyzable tannins in each extract were analyzed using the same statistical approach. A Pearson correlation analysis was conducted to examine the relationship between the total content of hydrolyzable tannins in each extract and the reduction in bacterial population.
3. Results
3.1. Overall Mean Reductions in Bacterial Cell Population Caused by Phenolic Treatments
The results in Table 1 and Table 2 indicated that all four independent variables were significant (p ≤ 0.05) factors influencing the E. coli counts from both growth media and Salmonella counts from BSA, and the variables except for extract concentration were also significant factors influencing the Salmonella counts from TSA. There were significant interactions between any two independent variables for E. coli counts and for Salmonella counts on TSA. However, there were no significant interactions between the type of extract and bacterial strain or extract concentration when Salmonella counts were recovered on BSA.

Table 1.
F-test of each independent variable and its interaction with the overall reduction in Salmonella enterica cell population as a dependent variable.

Table 2.
F-test of each independent variable and its interaction with the overall reduction in enterohemorrhagic Escherichia coli cell population as a dependent variable.
On average, the phenolic extracts of all four products exhibited an inhibitory effect against both bacterial pathogens, with the levels of mean reduction from 2.49 to 3.34 log CFU/mL for E. coli and 3.06–3.64 log CFU/mL for Salmonella according to the results from the selective media (Table 3). Variations in antibacterial activity were observed among the tested extracts. The extract of product II demonstrated the greatest inhibitory activity (3.64 log CFU/mL) against Salmonella cells, while that of product I exhibited the highest inhibitory effect (3.34 log CFU/mL) against E. coli cells. The extract of product IV consistently yielded the lowest levels of mean reduction in Salmonella and E. coli populations, which were 3.06 and 2.49 log CFU/mL, respectively.

Table 3.
Overall reductions in Salmonella enterica and enterohemorrhagic Escherichia coli cell population by the ethanolic extracts of four pomegranate peels.
In general, treatment systems with the higher concentration (2%) of the extracts had significantly (p ≤ 0.05) greater activities in inhibiting cell growth compared to those with the lower concentration (1%) of the extract except for Salmonella population reduction assessed on TSA (Table 3). A longer treatment time on average resulted in significantly higher levels of reduction in Salmonella and E. coli populations except when the former population was assessed on TSA at the 10 and 24 h sampling points. Furthermore, significantly different cell population reductions were perceived between the two strains of Salmonella and the two strains of E. coli. The mean level of reduction caused by extracts was higher for S. Enteritidis (4.00 log CFU/mL) than for S. Tennessee (2.66 log CFU/mL). Similarly, E. coli O157:H7 F4546 experienced a greater cell population reduction (3.62 log CFU/mL) than strain K4492 (2.30 log CFU/mL).
3.2. Effects of Individual Extracts on the Reduction in Salmonella Cell Population
The two different concentrations of the extracts prepared from the four pomegranate peels did not have a significantly (p > 0.5) different impact on the antimicrobial activity against S. enterica when enumerated on TSA (Table 4). On BSA, however, the treatments with 2% extracts of product I and III caused significant (p ≤ 0.05) Salmonella population reduction compared to the extracts of the other two products. Aside from that, the overall pattern of Salmonella population reduction was comparable to that illustrated in Table 3. In particular, the extract of product IV exhibited the lowest inhibitory activity, while the extract of product II led to the greatest reduction (7.27 log CFU/mL) in the bacterial cell population after a 24 h treatment period. A significant difference was also noted between the populations of the two Salmonella strains.

Table 4.
Reduction in Salmonella enterica cell population by the individual ethanolic extracts of four pomegranate peels.
3.3. Effects of Individual Extracts in the Reduction in E. coli Cell Population
The pattern of E. coli population reduction shown in Table 5 was similar to what is shown in Table 3. The highest reduction in cell population occurred at the 24 h sampling point, followed by the 10 h and 5 h sampling points. The antimicrobial activity of the extracts demonstrated a dose-dependent response except for product IV. The extract of product I was the most effective, resulting in the highest reduction in cell populations (6.15 log CFU/mL) at the 24 h sampling point. Furthermore, the susceptibility of E. coli O157:H7 F4546 to the extracts of products I, II, and IV was higher than that of K4492.

Table 5.
Reduction in enterohemorrhagic Escherichia coli cell population by the individual ethanolic extracts of four pomegranate peels.
3.4. Identification and Quantification of Hydrolyzable Tannins in the Extracts
Figure 1 displays the total contents, in milligrams per milliliter (mg/mL), of six hydrolyzable tannins in each extract used in the study. The hydrolyzable tannins were identified as α and β punicalin, α and β punicalagin, ellagic acid, and gallic acid. The total quantity of hydrolyzable tannins was calculated by adding up the concentrations of all six detected compounds. The results indicated that the average quantities of total hydrolyzable tannins in the four extracts varied from 21.79 to 57.62 mg/mL, with the extract of product I containing the highest concentration, followed by products II, III, and IV. Across all studied extracts, α and β punicalagins were the most abundant hydrolyzable tannins, with the concentrations ranging from 20.81 to 43.43 mg/mL. In contrast, α and β punicalins were present at lower concentrations (0.51 to 11.52 mg/mL), followed by ellagic acid with concentrations in the range of 0.44 to 2.05 mg/mL, and the concentration of gallic acid ranged from 0.05 to 0.65 mg/mL.
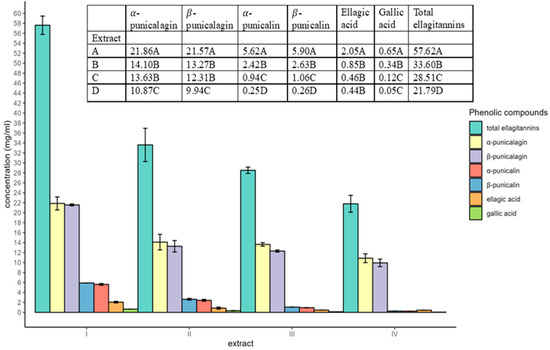
Figure 1.
The concentrations of different phenolic compounds in the extracts of four pomegranate peels, analyzed using high-performance liquid chromatography. I, II, and III: powdered India-grown cultivar of pomegranate peels purchased from Amazon, and IV: powdered pomegranate peels of Georgia-grown cultivar sourced locally.
3.5. The Analysis of Antimicrobial Activity at Different Sampling Points
The correlation coefficients shown in Table 6 revealed the relationship between the reductions in Salmonella or E. coli cell population and concentrations of total hydrolyzable tannins in the extract of each product. The reductions in the cell population positively correlated to the concentrations of hydrolyzable tannins at all sampling points. In the system containing S. Enteritidis or S. Tennessee, the R2 ranged from 0.65 to 0.98 and from 0.66 to 0.99, respectively. For E. coli, the R2 ranged from 0.57 to 0.98 and from 0.44 to 0.99, respectively, in the system with F4546 or K4492. The lowest correlation was observed in the treatment systems of both bacteria containing the extract of product IV.

Table 6.
Pearson’s correlation coefficients between the concentrations of total hydrolyzable tannins in the extracts of each pomegranate peel and the population reductions in Salmonella enterica and E. coli populations in tryptic soy broth.
4. Discussion
This reported study explored the feasibility of adding value to pomegranate production waste, which could potentially enhance the revenue of pomegranate growers and subsequently improve the sustainability of the pomegranate industry. It also provides a practical alternative to replacing antibiotic use in animal food production. This potential application is not only economically feasible but also has public health significance.
The ethanolic extracts of four pomegranate peel products tested in the study inhibited the growth of two common foodborne bacterial pathogens, S. enterica and enterohemorrhagic E. coli. These two pathogens are Gram-negative, and the hydrophilic outer membranes of Gram-negative bacteria coupled with the enzymatic machinery present in the periplasmic space provide them with an additional layer of protection against a wide range of exogenous molecules [17]. Such inherent resistance mechanisms often contribute to the reduced efficacy of various antimicrobials. For example, β-lactams such as penicillin and cephalosporins are susceptible to the hydrolysis by β-lactamases produced by the resistant bacteria in the periplasmatic space [18]. Large hydrophobic molecules, including glycopeptides, lipopeptides, and macrolides, have poor permeability through the narrow hydrophilic porins of the outer cell membrane [19]. However, the results of the current study revealed that the extracts of all four pomegranate peels exerted inhibitory activities against both evaluated bacterial pathogens, and higher levels of cell population reduction were observed with Salmonella (2.66–4.00 log CFU/mL) than E. coli (2.30–2.61 log CFU/mL) (Table 3).
In a prior study [20], different natural phenolic compounds were used to treat various strains of E. coli and Salmonella using the agar diffusion method. The findings aligned with the current research indicated that the strains of E. coli O157:H7 were less sensitive to the compounds compared to those of Salmonella. The results of the current study are also in agreement with another previous study [21] which reported that 26 strains of Salmonella were more susceptible to four evaluated hydrolyzable tannins than 23 strains of E. coli based on their average minimum inhibitory concentration values. Furthermore, this study also found notable disparities in cell population reduction between the two individual strains of Salmonella and two strains of E. coli (Table 4 and Table 5). These observations seemed to be consistent with the finding of a previous study that involved 16 Salmonella strains that had different sensitivity to ethanolic extracts tested [22]. The disparate sensitivity of various bacterial strains to phenolic extracts could be attributed to the variation in the genetic makeup of these strains. Such discrepancies may impart the expression of certain genes in response to hydrolyzable tannin exposure. For instance, a methanolic pomegranate peel extract was found to act as an efflux pump inhibitor to E. coli ATCC strain 25922, while it did not demonstrate any inhibitory activity against E. coli KAM42 [23,24].
Variations in antimicrobial activity were noticed among the extracts of four pomegranate peels (Table 3). The extracts derived from products I and II exhibited the most potent effects against E. coli and Salmonella, respectively. Intriguingly, these two extracts contained the highest quantities of total hydrolyzable tannins determined using HPLC (Figure 1). Since higher concentrations of extracts resulted in greater inhibition in bacterial growth (Table 1), hydrolyzable tannins highly likely acted as bactericides against the pathogens in a dose-dependent manner.
In general, greater quantities of hydrolyzable tannins were identified in the extracts of products I, II, and III in comparison to the extract of product IV (Figure 1). The content of hydrolyzable tannins in pomegranate peels can be influenced by multiple factors. Environmental conditions, including atmosphere temperature, were reported to have a great impact on hydrolyzable tannin content in pomegranate peels [25]. The occurrence of phenolic compounds in fruit peels is believed to be a protective plant response triggered by temperature-induced oxidative stress [26]. Fruits in India may experience a higher average temperature throughout the year compared to those in Georgia, U.S. [27]. As a result, pomegranate peels of Indian cultivars are likely to have higher levels of hydrolyzable tannins than their Georgian counterparts. Other than geographic influence, variations in hydrolyzable tannins were also observed in pomegranates growing in the same region due to hereditary characteristics [28].
Figure 1 shows that punicalagins were the major hydrolyzable tannins in all four extract samples; this observation is consistent with the findings of several previous studies [29,30,31]. Hence, the antimicrobial activity of extracts used in the present study can be largely attributed to punicalagin. Punicalagins extracted from pomegranate peels displayed a wide range of inhibitory activity against various microorganisms, including Pseudomonas aeruginosa and E. coli, as well as yeasts [29]. At a minimum inhibitory concentration of 0.25 mg/mL, punicalagin elicited substantial morphological alternations and disrupted biofilms formed by S. aureus [32]. The observed antimicrobial activity of punicalagin is likely contributed by the 16 hydroxyl functional groups attached to the molecule since this moiety has been suggested to be a critical determinant of molecular interactions, owing to its distinct polarity and oxidation characteristics [33].
Even though the extract from product I had a significantly higher content of hydrolyzable tannins (Figure 1), the extract from product II on average demonstrated greater antibacterial efficacy against the Salmonella strains used in the study (Table 3). This observation could be attributed to other antimicrobial components such as chelating agents present in the extracts. A previous study demonstrated that the antibacterial activities of a mango seed extract were comparable to those of tannic acid, despite significant differences in their total phenolic contents [34]. This was primarily due to mangiferin, a chelating agent that acted synergistically with the gallotannins in the mango kernel extract. Therefore, other antimicrobials such as some chelating agents may exist in extract II which work in harmony with the hydrolyzable tannins in inhibiting the growth of Salmonella. Furthermore, corilagin, a dominant compound in pomegranate peel extract was reported in a previous study to act synergistically with hydrolyzable tannins in Combretum extracts to inhibit the growth of Mycobacterium [35,36].
The current study showed that the reduction in bacterial cell population was influenced by treatment time, with the highest cell population reduction occurring at the 24 h sampling point, which suggested that prolonged exposure to pomegranate peel extracts may be necessary to maximize their antimicrobial activity (Table 1, Table 2 and Table 3). A comparable result was reported by a previous study [20] which investigated the antimicrobial activity of a few phenolic compounds against S. enterica and E. coli during a treatment period of 60 h. The phenolics were effective against both bacterial pathogens, and peak antibacterial activity was observed between 24 and 36 h of the treatments.
It is noteworthy that a greater decline in bacterial populations was observed on selective media as compared to the non-selective TSA, following the treatment with pomegranate peel extracts for 5, 10, and 24 h (Table 3, Table 4 and Table 5). This observation can be partially attributed to the selective action of phenolic extracts, which targeted susceptible bacterial cells while allowing tolerant bacterial cells to persist and proliferate. Furthermore, selective media enable the growth of bacterial cells that can withstand the selective agents in the growth media following the phenolic treatments. This speculation could be supported by a prior study where direct plating onto SMAC led to diminished recovery of stressed E. coli O157:H7 cells, as compared to their growth on TSA [37].
5. Conclusions
The antimicrobial efficacy observed in the current study was influenced by extract concentration, treatment duration, and the specific bacterial strain, indicating a complex interaction between the phenolic compounds and the pathogens. Hydrolyzable tannins, particularly punicalagins, were identified as key contributors to the observed antibacterial effects, although other bioactive compounds such as chelating agents may also play a synergistic role. The variation in bacterial susceptibility, even among strains of the same species, addresses the importance of understanding genetic and physiological differences when developing natural antimicrobial strategies. Additionally, the study revealed that prolonged exposure to pomegranate peel extracts is critical for maximizing bacterial population reductions, suggesting that time-dependent effects are an essential consideration in practical applications. Overall, these findings highlight the potential of pomegranate peel extracts as a natural alternative to conventional antimicrobials, offering a viable and economical option for controlling foodborne pathogens and ensuring food safety. Further research is warranted to optimize extract formulations and explore their applicability across food systems.
Author Contributions
Conceptualization, J.C.; methodology, J.C. and W.W.; formal analysis, W.W.; resources, J.C. and K.M.S.; data curation, W.W.; writing—original draft preparation, W.W.; writing—review and editing, J.C. and K.M.S.; supervision, J.C. and K.M.S.; project administration, J.C.; funding acquisition, J.C. and K.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this project was made possible by a grant/cooperative agreement (Federal Award Identification Number AM190100XXXXG033) from the U.S. Department of Agriculture (USDA) Marketing Service. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the USDA.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available upon request.
Acknowledgments
We would like to offer special thanks to the Georgia Department of Agriculture for its partnership during the administration of this award.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Bintsis, T. Foodborne Pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef]
- CDC. Preventing Salmonella Infection. (Salmonellosis). Available online: https://www.cdc.gov/salmonella/prevention/index.html (accessed on 6 December 2024).
- Fatima, R.; Aziz, M. Enterohemorrhagic Escherichia coli. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ramirez, D.; Giron, M. Enterobacter Infections. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Winfield, M.D.; Groisman, E.A. Role of Nonhost Environments in the Lifestyles of Salmonella and Escherichia coli. Appl. Environ. Microbiol. 2003, 69, 3687–3694. [Google Scholar] [CrossRef]
- Samtiya, M.; Matthews, K.R.; Dhewa, T.; Puniya, A.K. Antimicrobial Resistance in the Food Chain: Trends, Mechanisms, Pathways, and Possible Regulation Strategies. Foods 2022, 11, 2966. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Rosas-Domínguez, C.; Vega-Vega, V.; González-Aguilar, G.A. Antioxidant Enrichment and Antimicrobial Protection of Fresh-Cut Fruits Using Their Own Byproducts: Looking for Integral Exploitation. J. Food Sci. 2010, 75, R175–R181. [Google Scholar] [CrossRef]
- Howell, A.B.; D′Souza, D.H. The Pomegranate: Effects on Bacteria and Viruses That Influence Human Health. Evid. Based Complement. Altern. Med. 2013, 2013, 606212. [Google Scholar] [CrossRef]
- Eghbali, S.; Askari, S.F.; Avan, R.; Sahebkar, A. Therapeutic Effects of Punica granatum (Pomegranate): An Updated Review of Clinical Trials. J. Nutr. Metab. 2021, 2021, 5297162. [Google Scholar] [CrossRef]
- Dahham, S.; Ali, M.N.; Tabassum, H.; Khan, M. Studies on Antibacterial and Antifungal Activity of Pomegranate (Punica granatum L.). Am. Eurasian J. Agric. Environ. Sci. 2010, 9, 273–281. [Google Scholar]
- Wu, W.; Mis Solval, K.; Chen, J. Inhibitory Activity of Aqueous Extracts of Pomegranate Peel Products and Juice Powder against Salmonella enterica. LWT 2022, 155, 112934. [Google Scholar] [CrossRef]
- Manso, T.; Lores, M.; de Miguel, T. Antimicrobial Activity of Polyphenols and Natural Polyphenolic Extracts on Clinical Isolates. Antibiotics 2022, 11, 46. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as Antimicrobial Agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Jain, K.; Desai, N. Pomegranate the Cash Crop of India: A Comprehensive Review on Agricultural Practices and Diseases. Int. J. Health Sci. 2018, 8, 315–336. [Google Scholar]
- Khan, N.; Fahad, S.; Naushad, M.; Faisal, S. Pomegrantes Economics and Medicinal Aspects in the World; Social Science Research Network: Rochester, NY, USA, 2020. [Google Scholar] [CrossRef]
- Wu, W.; Mis Solval, K.; Chen, J. Ellagitannin Content and Anti-Enterohemorrhagic Escherichia coli Activity of Aqueous Extracts Derived from Commercial Pomegranate Products. Heliyon 2024, 10, e29700. [Google Scholar] [CrossRef]
- Miller, S.I.; Salama, N.R. The Gram-Negative Bacterial Periplasm: Size Matters. PLOS Biol. 2018, 16, e2004935. [Google Scholar] [CrossRef]
- Bush, K.; Jacoby, G.A. Updated Functional Classification of Beta-Lactamases. Antimicrob. Agents Chemother. 2010, 54, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Delcour, A.H. Outer Membrane Permeability and Antibiotic Resistance. Biochim. Biophys. Acta 2009, 1794, 808–816. [Google Scholar] [CrossRef]
- Cetin-Karaca, H.; Newman, M.C. Antimicrobial Efficacy of Plant Phenolic Compounds against Salmonella and Escherichia coli. Food Biosci. 2015, 11, 8–16. [Google Scholar] [CrossRef]
- Taguri, T.; Tanaka, T.; Kouno, I. Antimicrobial Activity of 10 Different Plant Polyphenols against Bacteria Causing Food-Borne Disease. Biol. Pharm. Bull. 2004, 27, 1965–1969. [Google Scholar] [CrossRef]
- Choi, J.-G.; Kang, O.-H.; Lee, Y.-S.; Chae, H.-S.; Oh, Y.-C.; Brice, O.-O.; Kim, M.-S.; Sohn, D.-H.; Kim, H.-S.; Park, H.; et al. In Vitro and In Vivo Antibacterial Activity of Punica granatum Peel Ethanol Extract against Salmonella. Evid. Based Complement. Altern. Med. 2011, 2011, 690518. [Google Scholar] [CrossRef]
- Dey, D.; Debnath, S.; Hazra, S.; Ghosh, S.; Ray, R.; Hazra, B. Pomegranate Pericarp Extract Enhances the Antibacterial Activity of Ciprofloxacin against Extended-Spectrum β-Lactamase (ESBL) and Metallo-β-Lactamase (MBL) Producing Gram-Negative Bacilli. Food Chem. Toxicol. 2012, 50, 4302–4309. [Google Scholar] [CrossRef]
- Tariq, A.; Sana, M.; Shaheen, A.; Ismat, F.; Mahboob, S.; Rauf, W.; Mirza, O.; Iqbal, M.; Rahman, M. Restraining the Multidrug Efflux Transporter STY4874 of Salmonella Typhi by Reserpine and Plant Extracts. Lett. Appl. Microbiol. 2019, 69, 161–167. [Google Scholar] [CrossRef]
- Schwartz, E.; Tzulker, R.; Glazer, I.; Bar-Ya’akov, I.; Wiesman, Z.; Tripler, E.; Bar-Ilan, I.; Fromm, H.; Borochov-Neori, H.; Holland, D.; et al. Environmental Conditions Affect the Color, Taste, and Antioxidant Capacity of 11 Pomegranate Accessions’ Fruits. J. Agric. Food Chem. 2009, 57, 9197–9209. [Google Scholar] [CrossRef]
- Gautier, H.; Diakou-Verdin, V.; Bénard, C.; Reich, M.; Buret, M.; Bourgaud, F.; Poëssel, J.L.; Caris-Veyrat, C.; Génard, M. How Does Tomato Quality (Sugar, Acid, and Nutritional Quality) Vary with Ripening Stage, Temperature, and Irradiance? J. Agric. Food Chem. 2008, 56, 1241–1250. [Google Scholar] [CrossRef]
- Climate of India. Available online: https://en.wikipedia.org/wiki/Climate_of_India#:~:text=India%20is%20home%20to%20an,Himalayas%20and%20the%20Thar%20Desert (accessed on 11 November 2024).
- Abid, M.; Yaich, H.; Cheikhrouhou, S.; Khemakhem, I.; Bouaziz, M.; Attia, H.; Ayadi, M.A. Antioxidant Properties and Phenolic Profile Characterization by LC–MS/MS of Selected Tunisian Pomegranate Peels. J. Food Sci. Technol. 2017, 54, 2890–2901. [Google Scholar] [CrossRef]
- Gosset-Erard, C.; Zhao, M.; Lordel-Madeleine, S.; Ennahar, S. Identification of Punicalagin as the Bioactive Compound behind the Antimicrobial Activity of Pomegranate (Punica granatum L.) Peels. Food Chem. 2021, 352, 129396. [Google Scholar] [CrossRef]
- Parashar, A.; Gupta, C.; Gupta, S.K.; Kumar, A. Antimicrobial Ellagitannin From Pomegranate (Punica granatum) Fruits. Int. J. Fruit Sci. 2009, 9, 226–231. [Google Scholar] [CrossRef]
- Seeram, N.; Lee, R.; Hardy, M.; Heber, D. Rapid Large Scale Purification of Ellagitannins from Pomegranate Husk, a by-Product of the Commercial Juice Industry. Sep. Purif. Technol. 2005, 41, 49–55. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, C.; Wu, Q.; Zheng, Z.; Liu, P.; Li, G.; Peng, X.; Xia, X. Antimicrobial Activity of Punicalagin Against Staphylococcus aureus and Its Effect on Biofilm Formation. Foodborne Pathog. Dis. 2017, 14, 282–287. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Martins, C.; Pereira, C.; Silvestre, A.J.D.; Rocha, S.M. Current Challenges and Perspectives for the Use of Aqueous Plant Extracts in the Management of Bacterial Infections: The Case-Study of Salmonella enterica Serovars. Int. J. Mol. Sci. 2019, 20, 940. [Google Scholar] [CrossRef]
- Widsten, P.; Cruz, C.D.; Fletcher, G.C.; Pajak, M.A.; McGhie, T.K. Tannins and Extracts of Fruit Byproducts: Antibacterial Activity against Foodborne Bacteria and Antioxidant Capacity. J. Agric. Food Chem. 2014, 62, 11146–11156. [Google Scholar] [CrossRef]
- Salih, E.Y.A.; Julkunen-Tiitto, R.; Luukkanen, O.; Fahmi, M.K.M.; Fyhrquist, P. Hydrolyzable Tannins (Ellagitannins), Flavonoids, Pentacyclic Triterpenes and Their Glycosides in Antimycobacterial Extracts of the Ethnopharmacologically Selected Sudanese Medicinal Plant Combretum hartmannianum Schweinf. Biomed. Pharmacother. 2021, 144, 112264. [Google Scholar] [CrossRef]
- Man, G.; Xu, L.; Wang, Y.; Liao, X.; Xu, Z. Profiling Phenolic Composition in Pomegranate Peel From Nine Selected Cultivars Using UHPLC-QTOF-MS and UPLC-QQQ-MS. Front. Nutr. 2022, 8, 807447. [Google Scholar] [CrossRef]
- Baylis, C.L.; MacPhee, S.; Robinson, A.J.; Griffiths, R.; Lilley, K.; Betts, R.P. Survival of Escherichia coli O157:H7, O111:H− and O26:H11 in Artificially Contaminated Chocolate and Confectionery Products. Int. J. Food Microbiol. 2004, 96, 35–48. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).