Germination and Culturability after UV Irradiation of Metarhizium anisopliae Native from Soils of Tropical Cattle Farms
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Obtaining Entomopathogenic Fungi
2.3. Production of Conidia and Preparation of Inoculum
2.4. Experimental Design
2.5. Ultraviolet Irradiation
2.6. Germination and Culturability Evaluation
2.7. Statistical Analysis
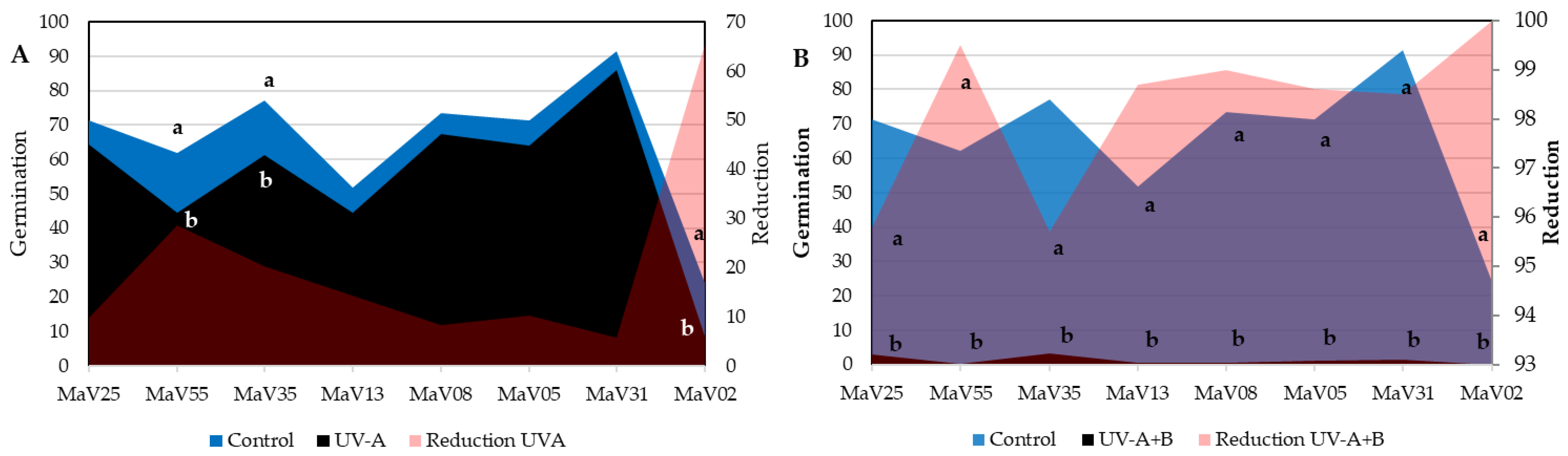
3. Results
3.1. Germination of Conidia
3.2. Culturability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodríguez-Vivas, R.I.; Hodgkinson, J.E.; Trees, A.J. Acaricide Resistance in Rhipicephalus (Boophilus) Microplus: Current Status and Mechanisms of Resistance. Rev. Mex. Cienc. Pecu. 2012, 3, 9–25. [Google Scholar]
- Alonso-Díaz, M.A.; Rodríguez-Vivas, R.I.; Fragoso-Sánchez, H.; Rosario-Cruz, R. Resistencia de La Garrapata Boophilus Microplus a Los Ixodicidas. Arch. Med. Vet. 2006, 38, 105–113. [Google Scholar] [CrossRef]
- Rangel, D.E.N.; Braga, G.U.L.; Fernandes, É.K.K.; Keyser, C.A.; Hallsworth, J.E.; Roberts, D.W. Stress Tolerance and Virulence of Insect-Pathogenic Fungi Are Determined by Environmental Conditions during Conidial Formation. Curr. Genet. 2015, 61, 383–404. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Díaz, M.A.; Fernández-Salas, A. Entomopathogenic Fungi for Tick Control in Cattle Livestock from Mexico. Front. Fungal Biol. 2021, 2, 657694. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Díaz, M.A.; García, L.; Galindo-Velasco, E.; Lezama-Gutierrez, R.; Angel-Sahagún, C.A.; Rodríguez-Vivas, R.I.; Fragoso-Sánchez, H. Evaluation of Metarhizium Anisopliae (Hyphomycetes) for the Control of Boophilus Microplus (Acari: Ixodidae) on Naturally Infested Cattle in the Mexican Tropics. Vet. Parasitol. 2007, 147, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Díaz, M.Á.; Fernández-Salas, A.; Galindo-Velasco, E.; Lezama-Gutiérrez, R.; Contreras-Lara, D. Effect of Metarhizium Anisopliae (Ma14 Strain) against Rhipicephalus Microplus on Cattle Infested Naturally. Southwest. Entomol. 2022, 47, 285–290. [Google Scholar] [CrossRef]
- Fernandes, É.K.K.; Bittencourt, V.R.E.P.; Roberts, D.W. Perspectives on the Potential of Entomopathogenic Fungi in Biological Control of Ticks. Exp. Parasitol. 2012, 130, 300–305. [Google Scholar] [CrossRef]
- Couceiro, J.d.C.; Fatoretto, M.B.; Demétrio, C.G.B.; Meyling, N.V.; Delalibera, Í. UV-B Radiation Tolerance and Temperature-Dependent Activity Within the Entomopathogenic Fungal Genus Metarhizium in Brazil. Front. Fungal Biol. 2021, 2, 645737. [Google Scholar] [CrossRef] [PubMed]
- Braga, G.U.L.; Flint, S.D.; Miller, C.D.; Anderson, A.J.; Roberts, D.W. Both Solar UVA and UVB Radiation Impair Conidial Culturability and Delay Germination in the Entomopathogenic Fungus Metarhizium Anisopliae. Photochem. Photobiol. 2001, 74, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Braga, G.U.L.; Flint, S.D.; Miller, C.D.; Anderson, A.J.; Roberts, D.W. Variability in Response to UV-B among Species and Strains of Metarhizium Isolated from Sites at Latitudes from 61°N to 54°S. J. Invertebr. Pathol. 2001, 78, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Rangel, D.E.N.; Braga, G.U.L.; Anderson, A.J.; Roberts, D.W. Influence of Growth Environment on Tolerance to UV-B Radiation, Germination Speed, and Morphology of Metarhizium Anisopliae Var. Acridum Conidia. J. Invertebr. Pathol. 2005, 90, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Salas, A.; Alonso-Morales, A.R.; Alonso-Díaz, M.Á. Distribution of Entomopathogenic Fungi in Soils of Cattle Farms and Factors Associated with Their Presence in the Mexican Tropics. Trop. Subtrop. Agroecosyst. 2020, 23. [Google Scholar] [CrossRef]
- Fernández-Salas, A.; Alonso-Díaz, M.A.; Alonso-Morales, R.A.; Lezama-Gutiérrez, R.; Rodríguez-Rodríguez, J.C.; Cervantes-Chávez, J.A. Acaricidal Activity of Metarhizium Anisopliae Isolated from Paddocks in the Mexican Tropics against Two Populations of the Cattle Tick Rhipicephalus Microplus. Med. Vet. Entomol. 2017, 31, 36–43. [Google Scholar] [CrossRef] [PubMed]
- García de Miranda, E. Modificaciones al Sistema de Clasificación Climática de Köppen, 5th ed.; Libros UNAM: Ciudad de México, Mexico, 2004; ISBN 9070-32-1010-4. [Google Scholar]
- Cañedo, V.; Ames, T. Manual de Laboratorio Para El Manejo de Hongos Entomopatógenos; Centro Internacional de la Papa (CIP): Lima, Peru, 2004; ISBN 92-9060-238-4. [Google Scholar]
- Braga, G.U.L.; Flint, S.D.; Messias, C.L.; Anderson, A.J.; Roberts, D.W. Effects of UVB Irradiance on Conidia and Germinants of the Entomopathogenic Hyphomycete Metarhizium Anisopliae: A Study of Reciprocity and Recovery. Photochem. Photobiol. 2001, 73, 140–146. [Google Scholar] [CrossRef]
- Vega, F.E.; Kaya, H.K. Insect Pathology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 9780123849847. [Google Scholar]
- Yao, S.L.; Ying, S.H.; Feng, M.G.; Hatting, J.L. In Vitro and in Vivo Responses of Fungal Biocontrol Agents to Gradient Doses of UV-B and UV-A Irradiation. BioControl 2010, 55, 413–422. [Google Scholar] [CrossRef]
- Griffiths, H.R.; Mistry, P.; Herbert, K.E.; Lunec, J. Molecular and Cellular Effects of Ultraviolet Light-Induced Genotoxicity. Crit. Rev. Clin. Lab. Sci. 1998, 35, 189–237. [Google Scholar] [CrossRef]
- Braga, G.U.L.; Flint, S.D.; Messias, C.L.; Anderson, A.J.; Roberts, D.W. Effect of UV-B on Conidia and Germlings of the Entomopathogenic Hyphomycete Metarhizium Anisopliae. Mycol Res 2001, 105, 874–882. [Google Scholar] [CrossRef]
- Rangel, D.E.N.; Braga, G.U.L.; Flint, S.D.; Anderson, A.J.; Roberts, D.W. Variations in UV-B Tolerance and Germination Speed of Metarhizium Anisopliae Conidia Produced on Insects and Artificial Substrates. J. Invertebr. Pathol. 2004, 87, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Beys da Silva, W.O.; Santi, L.; Schrank, A.; Vainstein, M.H. Metarhizium Anisopliae Lipolytic Activity Plays a Pivotal Role in Rhipicephalus (Boophilus) Microplus Infection. Fungal Biol. 2010, 114, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, É.; Da Silva, S.H.; Dos Reis Marques, E.; Roberts, D.W.; Braga, G.U.L. Quantification of Cyclobutane Pyrimidine Dimers Induced by UVB Radiation in Conidia of the Fungi Aspergillus Fumigatus, Aspergillus Nidulans, Metarhizium Acridum and Metarhizium Robertsii. Photochem. Photobiol. 2010, 86, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.T.; Bateman, R.P.; Prior, C.; Leather, S.R. Effects of Simulated Solar Radiation on Conidial Germination of Metarhizium Anisopliae in Different Formulations. Crop. Prot. 1998, 17, 675–679. [Google Scholar] [CrossRef]
- Braga, G.U.L.; Rangel, D.E.N.; Flint, S.D.; Anderson, A.J.; Roberts, D.W. Conidial Pigmentation Is Important to Tolerance against Solar-Simulated Radiation in the Entomopathogenic Fungus Metarhizium Anisopliae. Photochem. Photobiol. 2006, 82, 418–422. [Google Scholar] [CrossRef]
- Acheampong, M.A.; Hill, M.P.; Moore, S.D.; Coombes, C.A. UV Sensitivity of Beauveria Bassiana and Metarhizium Anisopliae Isolates under Investigation as Potential Biological Control Agents in South African Citrus Orchards. Fungal Biol. 2020, 124, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bravo, M.; Flores-León, A.; Calero-López, S.; Gutiérrez-Sánchez, F.; Valverde-García, P.; Quesada-Moraga, E. UV-B Radiation-Related Effects on Conidial Inactivation and Virulence against Ceratitis Capitata (Wiedemann) (Diptera; Tephritidae) of Phylloplane and Soil Metarhizium Sp. Strains. J. Invertebr. Pathol. 2017, 148, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, I.M.W.; Forim, M.R.; da Silva, M.F.G.F.; Fernandes, J.B.; Filho, A.B. Effect of Ultraviolet Radiation on Fungi Beauveria Bassiana and Metarhizium Anisopliae, Pure and Encapsulated, and Bio-Insecticide Action on Diatraea Saccharalis. Adv. Entomol. 2016, 4, 151–162. [Google Scholar] [CrossRef]
- Bidochka, M.J.; Kamp, A.M.; Lavender, T.M.; Dekoning, J.; De Croos, J.N.A. Habitat Association in Two Genetic Groups of the Insect-Pathogenic Fungus Metarhizium Anisopliae: Uncovering Cryptic Species? Appl. Environ. Microbiol. 2001, 67, 1335. [Google Scholar] [CrossRef]
- Corval, A.R.C.; Mesquita, E.; Corrêa, T.A.; Silva, C.d.S.R.; de Bitencourt, R.O.B.; Fernandes, É.K.K.; Bittencourt, V.R.E.P.; Roberts, D.W.; Gôlo, P.S. UV-B Tolerances of Conidia, Blastospores, and Microsclerotia of Metarhizium Spp. Entomopathogenic Fungi. J. Basic Microbiol. 2021, 61, 15–26. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso-Díaz, M.Á.; Lozano-Velázquez, M.d.L.; García-Galicia, I.A.; Fernández-Salas, A. Germination and Culturability after UV Irradiation of Metarhizium anisopliae Native from Soils of Tropical Cattle Farms. Microbiol. Res. 2024, 15, 1326-1333. https://doi.org/10.3390/microbiolres15030089
Alonso-Díaz MÁ, Lozano-Velázquez MdL, García-Galicia IA, Fernández-Salas A. Germination and Culturability after UV Irradiation of Metarhizium anisopliae Native from Soils of Tropical Cattle Farms. Microbiology Research. 2024; 15(3):1326-1333. https://doi.org/10.3390/microbiolres15030089
Chicago/Turabian StyleAlonso-Díaz, Miguel Ángel, María de Lourdes Lozano-Velázquez, Iván Adrián García-Galicia, and Agustín Fernández-Salas. 2024. "Germination and Culturability after UV Irradiation of Metarhizium anisopliae Native from Soils of Tropical Cattle Farms" Microbiology Research 15, no. 3: 1326-1333. https://doi.org/10.3390/microbiolres15030089
APA StyleAlonso-Díaz, M. Á., Lozano-Velázquez, M. d. L., García-Galicia, I. A., & Fernández-Salas, A. (2024). Germination and Culturability after UV Irradiation of Metarhizium anisopliae Native from Soils of Tropical Cattle Farms. Microbiology Research, 15(3), 1326-1333. https://doi.org/10.3390/microbiolres15030089






