In Vitro and In Vivo Wide-Spectrum Dual Antimycetomal Activity of Eight Essential Oils Coupled with Chemical Composition and Metabolomic Profiling
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Extraction of Essential Oils
2.3. Strains Used in This Study
2.4. In Vitro Antifungal Assay of Essential Oils
2.5. In Vitro Antibacterial Assay of Essential Oils
2.6. In Vivo Studies in Galleria mellonella Model
Galleria mellonella Larvae
2.7. In Vivo Toxicity Studies of Essential Oils in Galleria mellonella Model
2.8. In Vivo Efficacy of Essential Oils in M. mycetomatis Galleria mellonella Model
2.9. In Vivo Efficacy of Essential Oils in A. madurae Galleria mellonella Model
2.10. Statistical Analysis
2.11. Measurement of Free Radical Scavenging Activity
2.12. GC–MS Analysis of the Oils
2.13. Chemometric Analysis
3. Results
3.1. In Vitro Antifungal and Antibacterial Activity of Essential Oils
3.2. Toxicity Studies in an Uninfected Galleria mellonella Model
3.3. Efficacy of M. fragrans and P. anisum EOs in G. mellonella Larvae Infected with M. mycetomatis
3.4. Efficacy of M. fragrans and P. anisum EOs in G. mellonella Larvae Infected with A. madurae
3.5. Antioxidant Activity of Essential Oils
3.6. Chemical Profiles of the Essential Oils
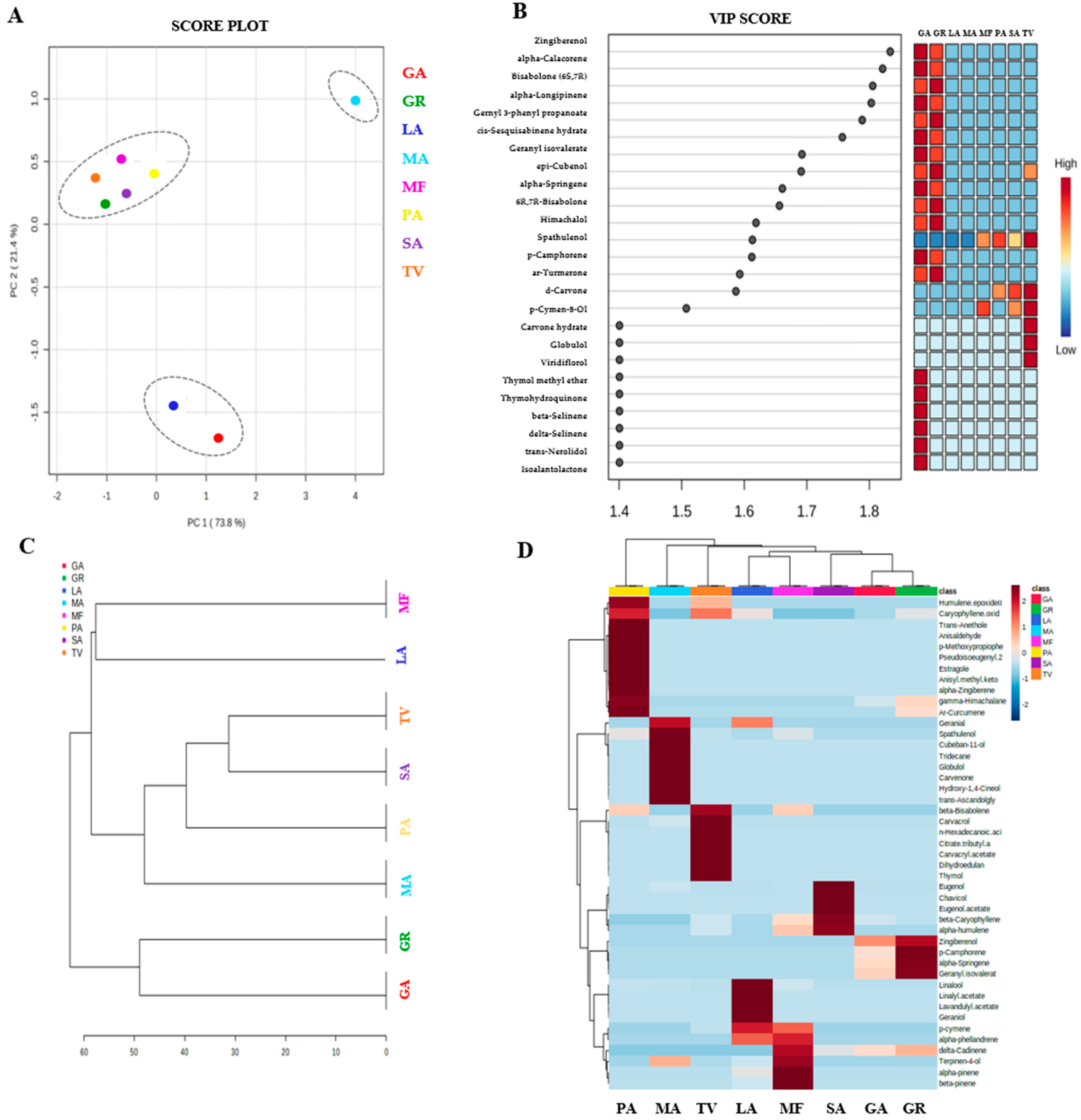
3.7. Discrimination of Oil Samples by Chemometric Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Relhan, V.; Mahajan, K.; Agarwal, P.; Garg, V.K. Mycetoma: An update. Indian J. Dermatol. 2017, 62, 332. [Google Scholar] [CrossRef] [PubMed]
- Van de Sande, W.W.J.; Fahal, A.H.; Goodfellow, M.; Mahgoub, E.l.S.; Welsh, O.; Zijlstra, E.E. Merits and pitfalls of the currently used diagnostic tools in mycetoma. PLoS Negl. Trop. Dis. 2014, 8, e2918. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Melse, Y.; Konings, M.; Duong, H.P.; Eadie, K.; Laleu, B.; Perry, B.; Todd, M.H.; Ioset, J.-R.; van de Sande, W.W.J. Addressing the most neglected diseases through an open research model: The discovery of fenarimols as novel drug candidates for eumycetoma. PLoS Negl. Trop. 2018, 12, e0006437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Welsh, O.; Vera-Cabrera, L.; Welsh, E.; Salinas, M.C. Actinomycetoma and advances in its treatment. Clin. Dermatol. 2012, 30, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Elkheir, L.Y.M.; Haroun, R.; Mohamed, M.A.; Fahal, A.H. Madurella mycetomatis causing eumycetoma medical treatment: The challenges and prospects. PLoS Negl. Trop. Dis. 2020, 14, e0008307. [Google Scholar] [CrossRef] [PubMed]
- Zein, H.A.M.; Fahal, A.H.; Mahgoub, E.S.; El Hassan, T.A.; Abdel-Rahman, M.E. Predictors of cure, amputation and follow-up dropout among patients with mycetoma seen at the Mycetoma Research Centre, University of Khartoum, Sudan. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Vera-Cabrera, L.; Daw-Garza, A.; Said-Fernández, S.; Lozano-Garza, H.G.; de Torres, N.W.; Rocha, N.C.; Ocampo-Candiani, J.; Choi, S.-H.; Welsh, O. Therapeutic effect of a novel oxazolidinone, DA-7867, in BALB/c mice infected with Nocardia brasiliensis. PLoS Negl. Trop. Dis. 2008, 2, e289. [Google Scholar] [CrossRef]
- Vera-Cabrera, L.; Gomez-Flores, A.; Escalante-Fuentes, W.G.; Welsh, O. In vitro activity of PNU-100766 (linezolid), a new oxazolidinone antimicrobial, against Nocardia brasiliensis. Antimicrob. Agents Chemother. 2011, 45, 3629–3630. [Google Scholar] [CrossRef]
- Mahmoud, A.B.; Abd Algaffar, S.; van de Sande, W.; Khalid, S.; Kaiser, M.; Mäser, P. Niclosamide Is Active In Vitro against Mycetoma Pathogens. Molecules 2021, 26, 4005. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Kong, W.; Zhao, G.; Yang, M. Mechanisms of antifungal and anti-aflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L.) on Aspergillus flavus. Food Chem. 2007, 220, 1–8. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential Oils: Sources of Antimicrobials and Food Preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef]
- Prakash, B.; Singh, P.; Kedia, A.; Dubey, N.K. Assessment of some essential oils as food preservatives based on antifungal, antiaflatoxin, antioxidant, activities and in vivo efficacy in food system. Food Res. Int. 2012, 49, 201–208. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Rikke, L.M. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R. Quorum sensing and phytochemicals. Int. J. Mol. Sci. 2013, 14, 12607–12619. [Google Scholar] [CrossRef]
- Siehnel, R.; Traxler, B.; An, D.D.; Parsek, M.R.; Schaefer, A.L.; Singh, P.K. A unique regulator controls the activation threshold of quorum-regulated genes in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2010, 107, 7916–7921. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. GOST 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods-A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines. 2016, 22, 25. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid. Based Complement. Alternat Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef]
- Tariqa, S.; Wania, S.; Rasoola, W.; Shafia, K.; Bhata, M.A.; Prabhakarb, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef]
- van de Sande, W.W.; Fahal, A.H.; Riley, T.V.; Verbrugh, H.; van Belkum, A. In vitro susceptibility of Madurella mycetomatis, prime agent of Madura foot, to tea tree oil and artemisinin. J. Antimicrob. Chemother. 2007, 59, 553–555. [Google Scholar] [CrossRef] [PubMed]
- Elfadil, H.; Fahal, A.; Kloezen, W.; Ahmed, E.M.; van de Sande, W. The in vitro antifungal activity of Sudanese medicinal plants against Madurella mycetomatis, the eumycetoma major causative agent. PLoS Negl. Trop. Dis. 2015, 9, e0003488. [Google Scholar] [CrossRef]
- Konings, M.; Eadie, K.; Lim, W.; Fahal, A.H.; Mouton, J.; Tesse, N.; van de Sande, W.W. The synthetic synergistic cinnamon oil CIN-102 is active against Madurella mycetomatis, the most common causative agent of mycetoma. PLoS Negl. Trop. Dis. 2022, 15, e0009488. [Google Scholar] [CrossRef]
- Stojković, D.; Soković, M.; Glamočlija, J.; Džamić, A.; Ristić, M.; Fahal, A.; Khalid, S.; Djuic, I.; Petrovic, S. Susceptibility of three clinical isolates of Actinomodura madurae to α-Pinene, the bioactive agent of Pinus pinaster turpentine oil. Arch. Biol. Sci. 2008, 60, 697–701. [Google Scholar] [CrossRef]
- Abd Algaffar, S.O.; Verbon, A.; vande Sande, W.W.J.; Khalid, S.A. Development and validation of an in vitro resazurin-based susceptibility assay against Madurella mycetomatis. Antimicrob. Agents Chemother. 2021, 65, e01338-20. [Google Scholar] [CrossRef]
- CLSI. Susceptibility Testing of Mycobacteria, Nocardia spp., and Other Aerobic Actinomycetes, 1st ed.; M24; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Abd Algaffar, S.O.; Verbon, A.; Khalid, S.A.; van de Sande, W.W.J. Development and validation of a resazurin assay for in vitro susceptibility testing of Actinomadura madurae: A common causative agent of actinomycetoma. J. Antimicrob. Chemother. 2022, 23, 155–160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eadie, K.; Parel, F.; Helvert-van Poppel, M.; Fahal, A.; van de Sande, W. Combining two antifungal agents does not enhance survival of Galleria mellonella larvae infected with Madurella mycetomatis. Trop. Med. Int. Health 2017, 22, 696–702. [Google Scholar] [CrossRef]
- Rosas-Taraco, A.G.; Perez-Liñan, A.R.; Bocanegra-Ibarias, P.; Perez-Rivera, L.I.; Salinas-Carmona, L.C. Nocardia brasiliensis induces an immunosuppressive microenvironment that favours chronic infection in BALB/c mice. Infect. Immun. 2012, 80, 2493–2499. [Google Scholar] [CrossRef]
- Sarker, S.; Nahar, L. Natural Products Isolation, 3rd ed.; Humana Press: London, UK, 2012. [Google Scholar]
- Powers, C.N.; Satyal, P.; Mayo, J.A.; McFeeters, H.; McFeeters, R.L. Bigger Data Approach to Analysis of Essential Oils and Their Antifungal Activity against Aspergillus niger, Candida albicans, and Cryptococcus neoformans. Molecules 2019, 24, 2868. [Google Scholar] [CrossRef]
- Ashmawy, N.S.; Hamoda, A.M.; Gad, H.A.; El-Keblawy, A.A.; Soliman, S.S. Newly-sprouted leaves at the stem base differ anatomically and histochemically from the crown leaves in Ficus johann. Bot. Lett. 2023, 170, 591–599. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Shammout, O.D.; Ashmawy, N.S.; Shakartalla, S.B.; Altaie, A.M.; Semreen, M.H.; Omar, H.A.; Soliman, S.S.M. Comparative sphingolipidomic analysis reveals significant differences between doxorubicin-sensitive and-resistance MCF-7 cells. PLoS ONE 2021, 16, e0258363. [Google Scholar] [CrossRef] [PubMed]
- Kanda, D.; Kaur, S.; Koul, O. A comparative study of monoterpenoids and phenylpropanoids from essential oils against stored grain insects: Acute toxins or feeding deterrents. J. Pest. Sci. 2016, 90, 531–545. [Google Scholar] [CrossRef]
- Alansari, R.M.; Seleem, A.A.; Hussein, B.H.M. Recording insect death and essential oil composition of Ferula communis L. flowers in Al Ula, Kingdom of Saudi Arabia. Kuwait J. Sci. 2024, 51, 100171. [Google Scholar] [CrossRef]
- Kosalec, I.; Pepeljnjak, S.; Kuatrak, D. Antifungal activity of fluid extract and essential oil from anise fruits (Pimpinella anisum L., Apiaceae). Acta Pharm. 2005, 55, 377–385. [Google Scholar]
- Ozcan, M.M.; Chalchat, J.C. Chemical composition and antifungal effect of anise (Pimpinella anisum L.) fruit oil at ripening stage. Ann. Microbiol. 2006, 56, 353–358. [Google Scholar] [CrossRef]
- Hu, F.; Tu, X.F.; Thakur, K.; Hu, F.; Li, X.L.; Zhang, Y.S. Comparison of antifungal activity of essential oils from different plants against three fungi. Food Chem. Toxicol. 2019, 134, 110821. [Google Scholar] [CrossRef]
- Al-Bayati; Firas, A.Y. Synergistic antibacterial activity between Thymus vulgaris and Pimpinella anisum essential oils and methanol extracts. J. Ethnopharmacol. 2008, 116, 403–406. [Google Scholar] [CrossRef]
- Topuz, O.K.; Özvural, E.B.; Zhao, Q.; Huang, Q.; Chikindas, M.; Gölükçü, M. Physical and antimicrobial properties of anise oil loaded nanoemulsions on the survival of foodborne pathogens. Food Chem. 2016, 203, 117–123. [Google Scholar] [CrossRef]
- Shojaii, A.; Fard, M.A. Review of Pharmacological Properties and Chemical Constituents of Pimpinella anisum. ISRN Pharm. 2012, 2012, 510795. [Google Scholar] [CrossRef]
- Singh, G.; Kapoor, I.P.S.; Singh, P.; de Heluani, C.S.; Catalan, C.A.N. Chemical composition and antioxidant potential of essential oil and oleoresins from anise seeds (Pimpinella anisum L.). Int. J. Essen. Oil Ther. 2008, 2, 122–130. [Google Scholar]
- Esfandyari-Manesh, M.; Ghaedi, Z.; Asemi, M.; Khanavi, M.; Manayi, A.; Jamalifar, H.; Atyabi, F.; Dinarvand, R. Study of antimicrobial activity of anethole and carvone loaded PLGA nanoparticles. J. Pharm. Res. 2013, 7, 290–295. [Google Scholar] [CrossRef]
- Yutani, M.; Hashimoto, Y.; Ogita, A.; Kubo, I.; Tanaka, T.; Fujita, K.I. Morphological Changes of the Filamentous Fungus Mucor mucedo and Inhibition of Chitin Synthase Activity Induced by Anethole. Phytother. Res. 2011, 25, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Wojda, I.; Staniec, B.; Sułek, M.; Kordaczuk, J. The greater wax moth Galleria mellonella: Biology and use in immune studies. Pathog. Dis. 2020, 23, ftaa057. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sungur, I.; Muluk, N.B.; Vejselova Sezer, C.V.; Kutlu, H.M.; Cingi, C. Efficacy and toxicity of anise oil as a potential topical wound healer: A cell culture study. Eur. Rev. Med. Pharmacol. Sci. 2023, 27 (Suppl. 2), 14–20. [Google Scholar]
- Coelho-de-Souza, A.N.; Lahlou, S.; Barreto, J.E.; Yum, M.E.; Oliveira, A.C.; Oliveira, H.D.; Celedônio, N.R.; Feitosa, R.G.F.; Duarte, G.P.; Santos, C.F.; et al. Essential oil of Croton zehntneri and its major constituent anethole display gastroprotective effect by increasing the face mucous layer. Fundam. Clin. Pharmacol. 2013, 27, 288–298. [Google Scholar] [CrossRef]
- Yu, C.; Wang, T.; Yang, Z. Effects of dietary supplementation of trans-anethole on the intestinal antioxidant status, immune function, and liver lipid metabolism in broilers. Ital. J. Anim. Sci. 2022, 21, 729–736. [Google Scholar] [CrossRef]
- Iannarelli, R.; Marinelli, O.; Morelli, M.B.; Santoni, G.; Amantini, C.; Nabissi, M.; Maggi, F. Aniseed (Pimpinella anisum L.) essential oil reduces pro-inflammatory cytokines and stimulates mucus secretion in primary airway bronchial and tracheal epithelial cell lines. Ind. Crops Prod. 2018, 114, 81–86. [Google Scholar] [CrossRef]
- Olajide, O.A.; Makinde, J.M.; Awe, S.O. Evaluation of the pharmacological properties of nutmeg oil in rats and mice. Pharm. Biol. 2000, 38, 385–390. [Google Scholar] [CrossRef]
- da Silveira Sá, R.C.; Andrade, L.N.; de Oliveira, R.R.B.; de Sousa, D.P. A Review on Anti-Inflammatory Activity of Phenylpropanoids Found in Essential Oils. Molecules 2014, 19, 1459–1480. [Google Scholar] [CrossRef]
- Nikolic, V.; Nikolic, L.; Dinic, A.; Gajic, I.; Urosevic, M.; Stanojevic, L.; Danilovic, B. Chemical Composition, Antioxidant and Antimicrobial Activity of Nutmeg (Myristica fragrans Houtt.) Seed Essential Oil. J. Essent. Oil-Bear. Plants 2021, 24, 218–227. [Google Scholar] [CrossRef]
- Din, M.U.; Ali, A.; Yasir, M.; Jilani, M.I.; Shoaib, S.; Latif, M.; Ahmad, A.; Naz, S.; Aslam, F.; Iqbal, M.; et al. Chemical Composition and in vitro Evaluation of Cytotoxicity, Antioxidant and Antimicrobial Activities of Essential Oil Extracted from Myristica fragrans Houtt. Pol. J. Environ. Stud. 2021, 30, 1585–1590. [Google Scholar] [CrossRef] [PubMed]
- Sandner, G.; Heckmann, M.; Weghuber, J. Immunomodulatory activities of selected essential oils. Biomolecules 2020, 10, 1139. [Google Scholar] [CrossRef] [PubMed]
- Grazul, M.; Kwiatkowski, P.; Hartman, K.; Kilanowicz, A.; Sienkiewicz, M. How to Naturally Support the Immune System in Inflammation—Essential Oils as Immune Booster. Biomedicines 2023, 11, 2381. [Google Scholar] [CrossRef]
- Chen, X.; Shang, S.; Yan, F.; Jiang, H.; Zhao, G.; Tian, S.; Chen, R.; Chen, D.; Dang, Y. Antioxidant Activities of Essential Oils and Their Major Components in Scavenging Free Radicals, Inhibiting Lipid Oxidation and Reducing Cellular Oxidative Stress. Molecules 2023, 28, 4559. [Google Scholar] [CrossRef]
- Dupont, B.; Datry, A.; Poirée, S.; Canestri, A.; Boucheneb, S.; Fourniols, E. Role of a NSAID in the apparent cure of a fungal mycetoma. J. Mycol. Med. 2016, 26, 2. [Google Scholar] [CrossRef]
- Diallo, B.; Barro-Traoré, F.; Bamba, S.; Sanou-Lamien, A.; Traoré, S.S.; Andonaba, J.B.; Konaté, I.; Niamba, P.; Traoré, A.; Guiguemdé, T. Multifocal extrapodal actinomycotic mycetoma: Good response to treatment with the combination of cotrimoxazole and NSAIDs. Med. Mycol. J. 2015, 25, 297–302. [Google Scholar] [CrossRef]
- Cakir, A.; Kordali, S.; Zengin, H.; Izumi, S.; Hirata, T. Composition and antifungal activity of essential oils isolated from Hypericum hyssopifolium and Hypericum heterophyllum. Flavour. Fragr. J. 2004, 19, 62–68. [Google Scholar] [CrossRef]
- Cavaleiro, C.; Gonçalves, M.J.; Serra, D.; Santoro, G.; Tomi, F.; Bighelli, A.; Salgueiro, L.; Casanova, J. Composition of a volatile extract of Eryngium duriaei subsp. juresianum (M. Laínz) M. Laínz, signalised by the antifungal activity. J. Pharm. Biomed. Anal. 2011, 54, 619–622. [Google Scholar] [CrossRef]
- Sobrinho, A.C.N.; de Morais, S.M.; de Souza, E.B.; Albuquerque, M.R.J.R.; dos Santos, H.S.; Cavalcante, C.S.d.P.; de Sousa, H.A.; Fontenelle, R.O.d.S. Antifungal and antioxidant activities of Vernonia chalybaea Mart. ex DC. essential oil and their major constituent β-caryophyllene. Braz. Arch. Biol. Technol. 2020, 63, e20190177. [Google Scholar] [CrossRef]
- Tan, N.; Satana, D.; Sen, B.; Tan, E.; Altan, H.B.; Demirci, B.; Uzun, M. Antimycobacterial and antifungal activities of selected four Salvia species. Rec. Nat. Prod. 2016, 10, 593–603. [Google Scholar]
- Mashigo, M.; Combrinck, S.; Regnier, T.; Du Plooy, W.; Augustyn, W.; Mokgalaka, N. Chemical variations, trichome structure and antifungal activities of essential oils of Helichrysum splendidum from South Africa. S. Afr. J. Bot. 2015, 96, 78–84. [Google Scholar] [CrossRef]
- Houicher, A.; Hamdi, M.; Hechachna, H.; Özogul, F. Chemical composition and antifungal activity of Anacyclus valentinus essential oil from Algeria. Food Biosci. 2018, 25, 28–31. [Google Scholar] [CrossRef]
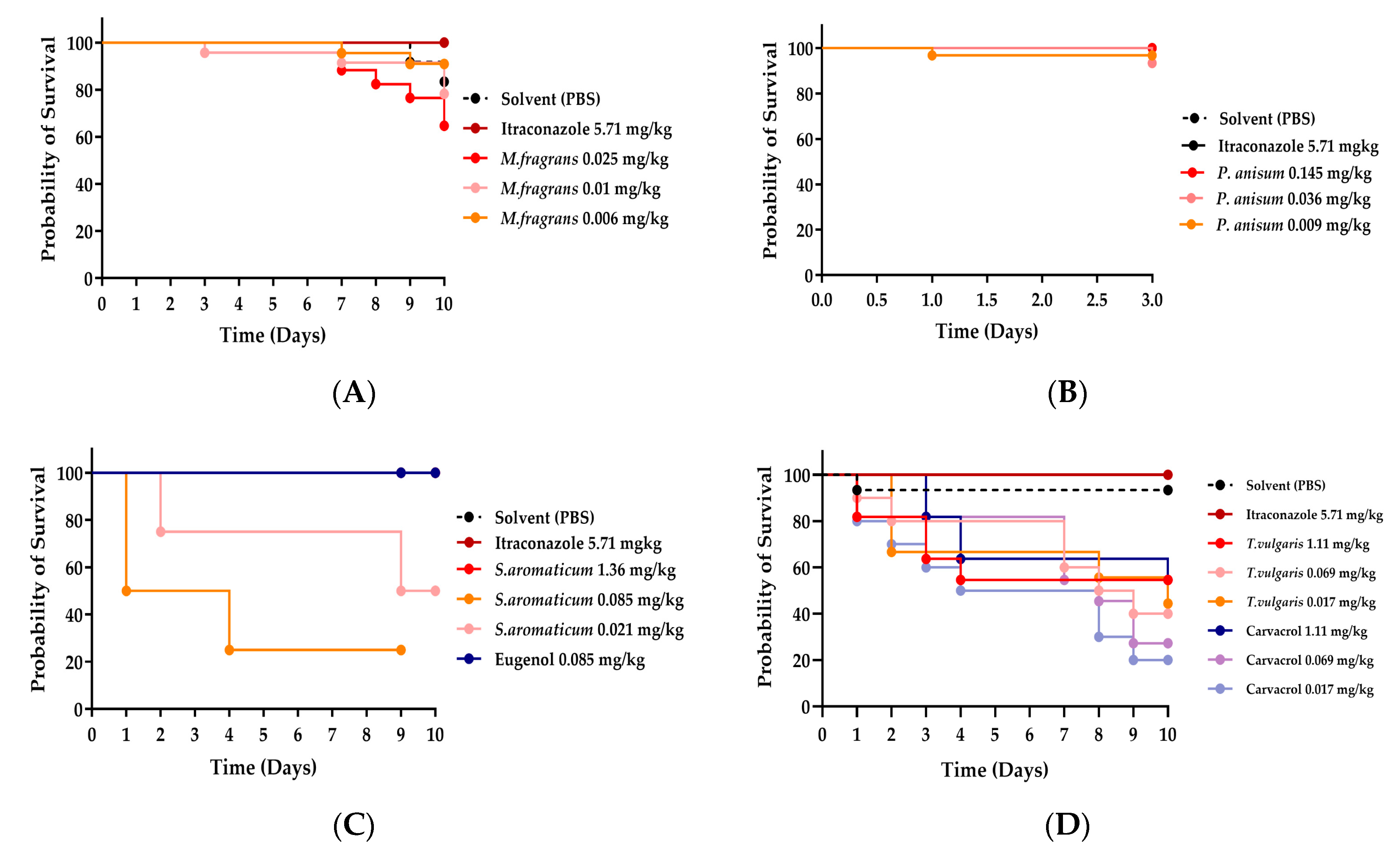
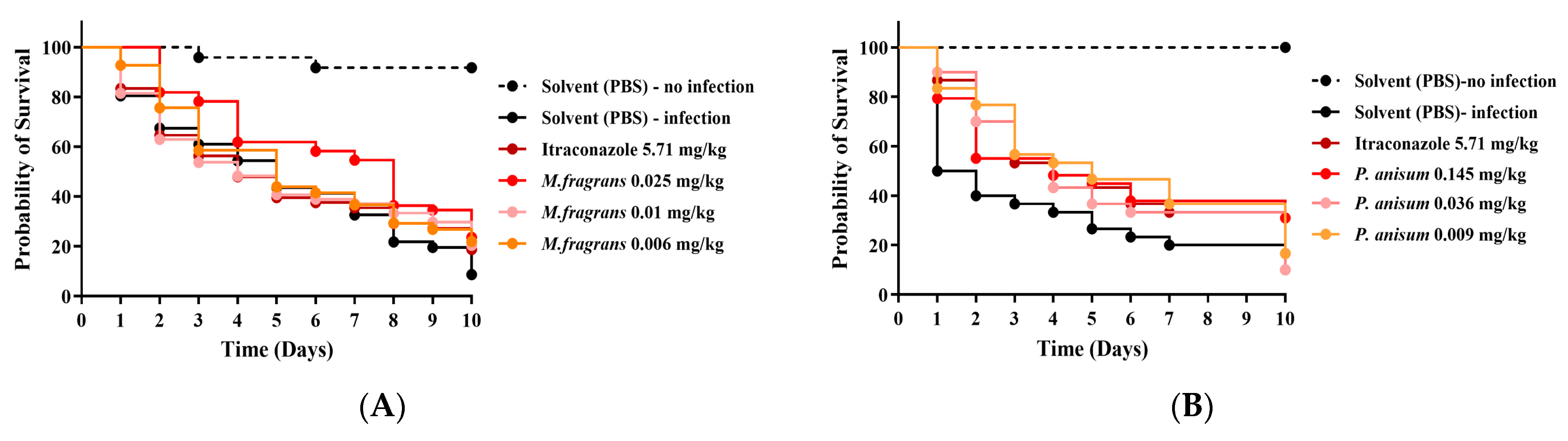
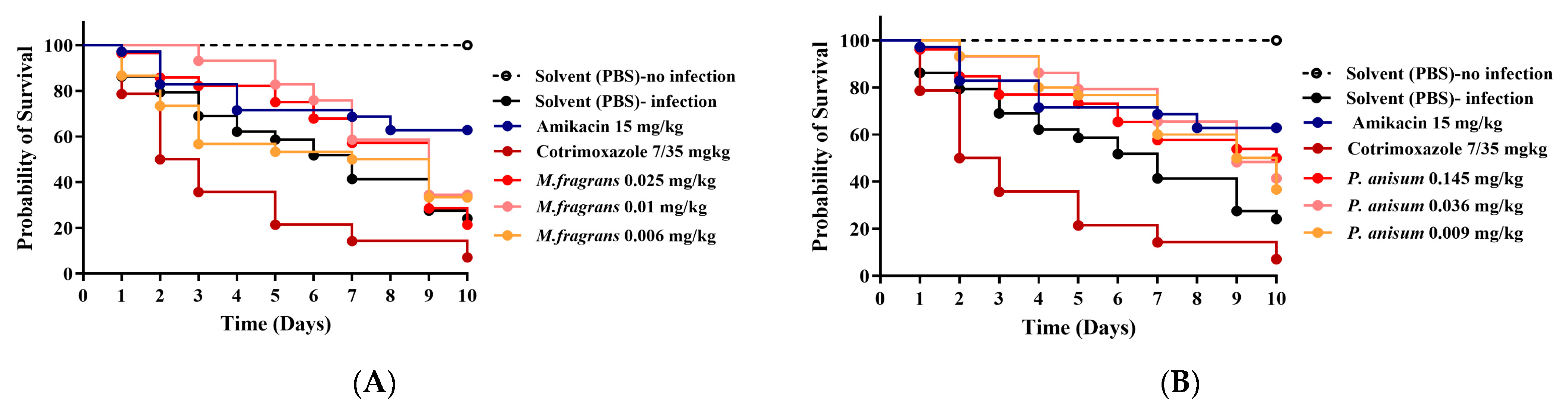
| Essential Oil/Standard | Max MIC | Eumycetoma (MIC/MFC) | Actinomycetoma (MIC/MBC) | DPPH % RSA ± SD | ||||
|---|---|---|---|---|---|---|---|---|
| SO1 | AL1 | t606931 | SAK-A03 | DSM 43236 | DSM 44005 | |||
| Geigeria alata DC. roots | 0.016 | 0.016/0.016 | <0.008/<0.008 | 0.016/0.16 | 0.004/0.004 | 0.004/0.008 | 0.008/0.008 | 95.90 ± 0.005 |
| Thymus vulgaris L. | 0.031 | 0.016/0.016 | 0.004/0.016 | 0.008/0.008 | 0.004/0.008 | 0.031/0.063 | 0.016/0.016 | 90.60 ± 0.003 |
| Myristica fragrans Houtt. | 0.031 | 0.031/0.031 | 0.008/0.016 | 0.031/0.063 | 0.008/0.016 | 0.016/0.031 | 0.008/0.016 | 90.30 ± 0.008 |
| Syzigum aromaticum L. | 0.063 | 0.031/0.063 | <0.008/<0.008 | 0.063/0.063 | 0.004/0.008 | 0.004/0.008 | 0.008/0.008 | 83.40 ± 0.021 |
| Pimpinella anisum L. | 0.125 | 0.063/0.125 | 0.008/0.031 | 0.125/0.12 | 0.004/0.008 | 0.004/0.016 | 0.004/0.016 | 98.00 ± 0.003 |
| Lavandula officinalis Mill. | 0.25 | 0.016/0.016 | <0.008/<0.008 | 0.25/0.125 | 0.063/0.125 | 0.031/0.063 | 0.031/0.063 | 83.60 ± 0.006 |
| Geigeria alata DC. aerial part | 0.25 | 0.031/0.063 | 0.016/0.016 | 0.25/0.125 | 0.125/0.25 | 0.063/0.125 | 0.125/0.125 | 60.10 ± 0.004 |
| Melaleuca alternifolia Cheel. | >0.25 | 0.125/>0.25 | >0.25 | >0.25 | >0.125 | >0.125 | 0.125/0.25 | 20.41 ± 0.002 |
| Itraconazole (1 µg/mL) | - | 0.063/0.25 | 0.016/0.031 | 0.031/0.125 | - | - | - | - |
| Amikacin (0.5 µg/mL) | - | - | - | - | 0.016/0.031 | 0.031/0.063 | 0.016/0.016 | - |
| Cotrimoxazole (4/76 µg/mL) | - | - | - | - | 0.25/4.78/0.25/4.78 | 0.25/4.78/0.5/9.5 | 1/19/1/19 | - |
| Ascorbic acid (0.5 mM) | - | - | - | - | - | - | - | 96.42 ± 0.008 |
| Propyl gallate (100 µM) | - | - | - | - | - | - | - | 91.18 ± 0.002 |
| NO | Component | Peak Area % | |||||||
|---|---|---|---|---|---|---|---|---|---|
| GR | TV | MF | SA | PA | LA | GA | MA | ||
| 1. | Myrcene | _ | _ | 0.68 | _ | _ | _ | _ | _ |
| 2. | Limonene | _ | _ | 0.98 | _ | _ | 1.83 | _ | _ |
| 3. | α-Pinene | _ | _ | 6.67 | _ | 0.01 | 0.94 | _ | _ |
| 4. | β-Pinene | _ | _ | 6.67 | _ | 0.02 | 0.34 | _ | _ |
| 5. | Sabinene | _ | _ | 19.97 | 0.01 | 0.02 | 0.22 | _ | _ |
| 6. | Linalool | 0.05 | 0.08 | 0.88 | 0.01 | 0.42 | 14.99 | _ | 0.33 |
| 7. | Terpinen-4-ol | 0.08 | 0.51 | 17.87 | 0.01 | 0.03 | 1.77 | _ | 8.10 |
| 8. | Eugenol | _ | 0.08 | 0.40 | 89.37 | 0.07 | _ | _ | 2.76 |
| 9. | Carvacrol | _ | 91.99 | _ | _ | _ | _ | _ | 4.86 |
| 10. | α-Terpineol | _ | 0.15 | 1.42 | _ | _ | 2.75 | _ | 6.64 |
| 11. | trans-Anethole | 0.19 | 0.05 | 0.15 | 0.59 | 76.87 | _ | 0.09 | 0.31 |
| 12. | Estragole | _ | _ | _ | _ | 1.23 | _ | _ | _ |
| 13. | Geraniol | _ | _ | _ | _ | _ | 5.51 | _ | _ |
| 14. | Citronellol | _ | _ | _ | _ | _ | 1.02 | _ | _ |
| 15. | p-Cymen-8-Ol | _ | 0.07 | 0.32 | _ | _ | _ | _ | 1.36 |
| 16. | Carvotanacetone | _ | 0.04 | _ | _ | _ | _ | _ | 1.75 |
| 17. | Camphor | _ | _ | _ | _ | _ | 4.75 | _ | _ |
| 18. | Phytone | 0.11 | 0.09 | _ | _ | _ | _ | 1.79 | _ |
| 19. | Anisaldehyde | _ | _ | _ | 0.02 | 10.35 | _ | _ | _ |
| 20. | Methyl eugenol | _ | _ | 1.89 | _ | _ | _ | _ | _ |
| 21. | 1,8-Cineole | _ | 0.04 | 0.22 | _ | _ | 9.07 | _ | _ |
| 22. | Hydroxy-1,4-cineole | _ | _ | _ | _ | _ | _ | _ | 13.33 |
| 23. | Linalyl acetate | _ | _ | 0.10 | _ | _ | 13.13 | _ | _ |
| 24. | Eugenol acetate | _ | _ | _ | 3.80 | _ | _ | _ | _ |
| 25. | Geranyl 3 phenyl propanoate | 2.46 | _ | _ | _ | _ | _ | 2.92 | _ |
| 26. | β-Caryophyllene | 0.53 | 0.69 | 1.44 | 4.62 | _ | 0.33 | 0.73 | _ |
| 27. | γ-Himachalane | 0.32 | _ | _ | _ | 1.07 | _ | 0.12 | _ |
| 28. | Bisabolone (6S,7R) | 27.91 | _ | _ | _ | _ | _ | 31.36 | _ |
| 29. | 6R,7R-Bisabolone | 2.1 | _ | _ | _ | _ | _ | 3.46 | _ |
| 30. | epi-Cubenol | 2.4 | _ | _ | 0.01 | _ | _ | 2.65 | 0.28 |
| 31. | α-Copaene | _ | _ | 1.19 | 0.09 | _ | _ | _ | _ |
| 32. | Spathulenol | _ | 0.10 | 0.24 | _ | 0.25 | _ | _ | 1.58 |
| 33. | Globulol | _ | _ | _ | _ | _ | _ | _ | 2.60 |
| 34. | Caryophyllene oxide | 0.4 | 1.06 | 0.45 | 0.19 | _ | _ | 1.37 | _ |
| 35. | Palmitic acid | 1.94 | _ | _ | _ | _ | _ | 36.38 | _ |
| 36. | Linolenic acid | _ | _ | _ | _ | _ | _ | 3.76 | _ |
| 37. | Myristic acid | _ | _ | _ | _ | _ | _ | 1.02 | _ |
| 38. | Myristicin | _ | _ | 13.15 | _ | _ | _ | _ | _ |
| 39. | Safrole | _ | _ | 1.98 | _ | _ | _ | _ | _ |
| 40. | Elemicin | _ | _ | 12.42 | _ | _ | _ | _ | _ |
| 41. | Cyclooctanone | _ | _ | _ | _ | _ | _ | _ | 1.60 |
| 42. | Isononyl acetate | _ | _ | _ | _ | _ | 8.16 | _ | _ |
| 43. | Lavandulyl acetate | _ | _ | _ | _ | _ | 1.01 | _ | _ |
| 44. | α-Terpinyl acetate | _ | _ | _ | _ | _ | 19.20 | _ | _ |
| 45. | Terpinyl acetate | _ | _ | _ | _ | _ | 2.87 | _ | _ |
| 46. | trans-Ascaridolglycol | _ | _ | _ | _ | _ | _ | _ | 15.31 |
| 47. | Methanetriol | _ | _ | _ | _ | _ | _ | _ | 1.46 |
| 48. | cis-9-Octadecenamide | _ | _ | _ | _ | _ | _ | 1.36 | _ |
| 49. | Pseudoisoeugenyl 2-methylbutyrate | _ | _ | _ | 0.03 | 4.76 | _ | _ | _ |
| 50. | α-Selinene | 0.54 | _ | _ | 0.02 | _ | _ | _ | _ |
| 51. | α-Longipinene | 0.53 | _ | _ | _ | _ | _ | 0.31 | _ |
| 52. | α-Springene | 2.18 | _ | _ | _ | _ | _ | 0.65 | _ |
| 53. | β-Elemene | 0.54 | _ | 0.06 | _ | 0.04 | _ | _ | _ |
| 54. | p-Camphorene | 0.68 | _ | _ | _ | _ | _ | 0.16 | _ |
| 55. | Geranyl isobutyrate | 0.51 | _ | _ | _ | _ | _ | _ | _ |
| 56. | Eudesma-4,11-dien-2-ol | 1.53 | _ | _ | _ | _ | _ | _ | _ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd Algaffar, S.O.; Satyal, P.; Ashmawy, N.S.; Verbon, A.; van de Sande, W.W.J.; Khalid, S.A. In Vitro and In Vivo Wide-Spectrum Dual Antimycetomal Activity of Eight Essential Oils Coupled with Chemical Composition and Metabolomic Profiling. Microbiol. Res. 2024, 15, 1280-1297. https://doi.org/10.3390/microbiolres15030086
Abd Algaffar SO, Satyal P, Ashmawy NS, Verbon A, van de Sande WWJ, Khalid SA. In Vitro and In Vivo Wide-Spectrum Dual Antimycetomal Activity of Eight Essential Oils Coupled with Chemical Composition and Metabolomic Profiling. Microbiology Research. 2024; 15(3):1280-1297. https://doi.org/10.3390/microbiolres15030086
Chicago/Turabian StyleAbd Algaffar, Shereen O., Prabodh Satyal, Naglaa S. Ashmawy, Annelies Verbon, Wendy W. J. van de Sande, and Sami A. Khalid. 2024. "In Vitro and In Vivo Wide-Spectrum Dual Antimycetomal Activity of Eight Essential Oils Coupled with Chemical Composition and Metabolomic Profiling" Microbiology Research 15, no. 3: 1280-1297. https://doi.org/10.3390/microbiolres15030086
APA StyleAbd Algaffar, S. O., Satyal, P., Ashmawy, N. S., Verbon, A., van de Sande, W. W. J., & Khalid, S. A. (2024). In Vitro and In Vivo Wide-Spectrum Dual Antimycetomal Activity of Eight Essential Oils Coupled with Chemical Composition and Metabolomic Profiling. Microbiology Research, 15(3), 1280-1297. https://doi.org/10.3390/microbiolres15030086







